
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (10): 1035-1045.DOI: 10.15541/jim20180003
• REVIEW • Next Articles
CHU Zeng-Yong, LI Gao-Lin, JIANG Zhen-Hua, WANG Chun-Hua
Received:2018-01-02
Revised:2018-03-05
Published:2018-10-20
Online:2018-09-25
Supported by:CLC Number:
CHU Zeng-Yong, LI Gao-Lin, JIANG Zhen-Hua, WANG Chun-Hua. Recent Progress in High-quality Perovskite CH3NH3PbI3 Single Crystal[J]. Journal of Inorganic Materials, 2018, 33(10): 1035-1045.
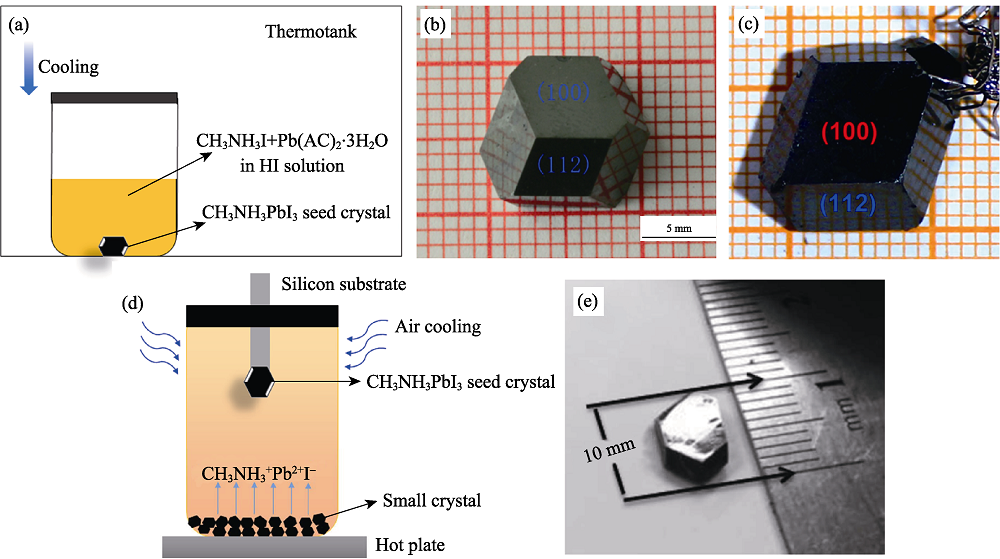
Fig. 4 Schematic diagram of solution temperature-lowering crystallization(STL) (a)-(c) Crystallization process of BSSG and images of as-prepared CH3NH3PbI3 single crystal[38, 42]; (d)-(e) Crystallization process of TSSG and image of as-prepared CH3NH3PbI3 single crystal[40]
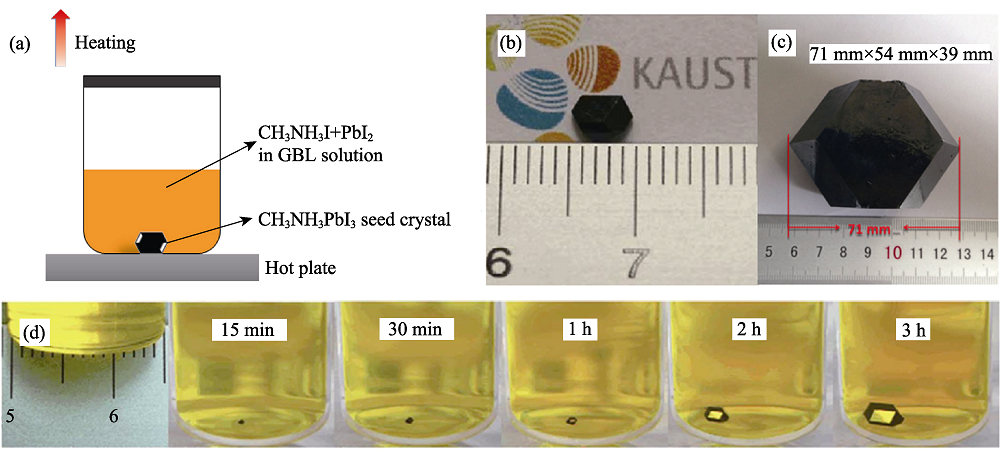
Fig. 5 Schematic diagram of inverse temperature crystallization (ITC) (a)-(c) Crystallization process of ITC and images of as-prepared CH3NH3PbI3 single crystal[41, 43]; (d) CH3NH3PbI3 crystal growth at different time intervals by ITC[41]

Fig. 6 (a)-(c) Schematic diagram of thinness- and shape- controlled growth and images of as-prepared CH3NH3PbI3 single crystal wafer; (d) Mass photodetectors based on a piece of single CH3NH3PbI3 crystal wafer[57]
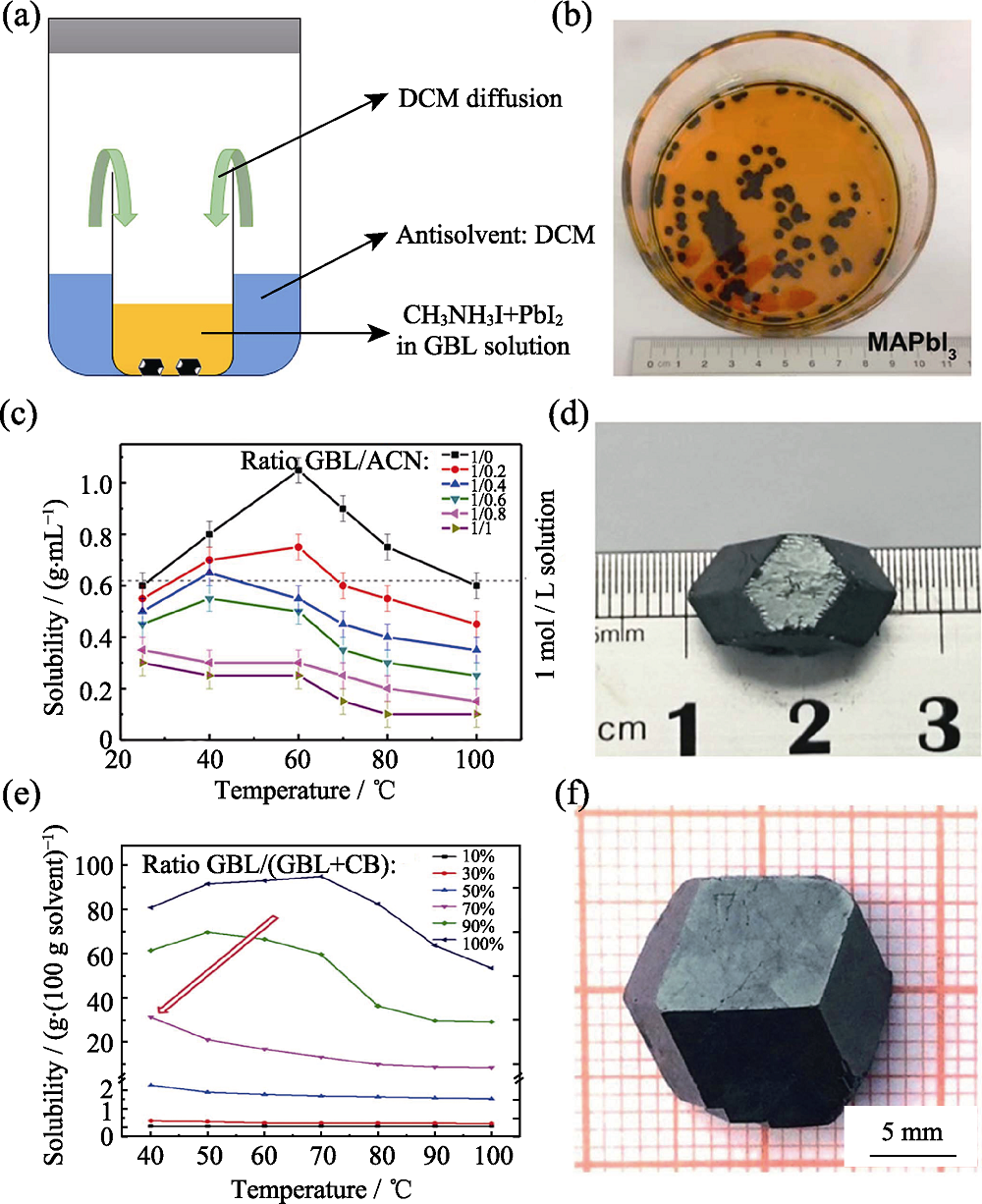
Fig. 7 Schematic diagram of solvent assisted crystallization (SAC) (a)-(b) Crystallization process of DCM assisted and images of as-prepared CH3NH3PbI3 crystals[39]; (c)-(d) Solubility of CH3NH3PbI3 at different temperatures in mixed-solvent of GBL and ACN, and image of as-prepared CH3NH3PbI3 single crystal[44]; (e)-(f) Solubility of CH3NH3PbI3 at different temperatures in mixed-solvent of GBL and CB, and image of as-prepared CH3NH3PbI3 single crystal[45]
| Growth method | Solvent | T/℃ | Size/mm | Carrier mobility/ (cm2•V-1•s-1) | Trap density/cm-3 | Bandgap/eV | Crystal system | Ref. |
|---|---|---|---|---|---|---|---|---|
| STL | HI | 65→40 | 10×10×8 | — | — | 1.48 | Tetragonal | [38] |
| HI | 100→57 | 12×12×7 | — | — | 1.48 | Tetragonal | [42] | |
| HI | 105→40 | 20×18×6 | 167±35 | (1.8±1.0)×109 | — | Tetragonal | [60] | |
| HI | 75 | 10×3 | 164±25 | 3.6×1010 | — | Tetragonal | [40] | |
| ITC | GBL | 60→110 | 5.8 | 67.2±7.3 | (1.4±0.2)×1010 | 1.51 | Tetragonal | [41] |
| GBL | 50→100 | 71×54×39 | 34 | 4.8×1010 | 1.53 | Tetragonal | [43] | |
| GBL | — | 113×58×52 | 41 | 2.1×108 | — | Tetragonal | [55] | |
| GBL | 60→110 | 150 μm in thickness | 39.6 | 6.0×108 | 1.45 | Tetragonal | [56] | |
| SAC | GBL/DCM | Room temperature | Millimeters | 2.5 | (3.3±0.3)×1010 | 1.51 | Tetragonal | [39] |
| GBL/ACN | 60→70 | 17 | — | — | — | Tetragonal | [44] | |
| GBL/CB | 30→60 | 15×15×10 | — | — | — | Cubic | [45] |
Table 1 A summary of properties of CH3NH3PbI3 single crystal by different methods
| Growth method | Solvent | T/℃ | Size/mm | Carrier mobility/ (cm2•V-1•s-1) | Trap density/cm-3 | Bandgap/eV | Crystal system | Ref. |
|---|---|---|---|---|---|---|---|---|
| STL | HI | 65→40 | 10×10×8 | — | — | 1.48 | Tetragonal | [38] |
| HI | 100→57 | 12×12×7 | — | — | 1.48 | Tetragonal | [42] | |
| HI | 105→40 | 20×18×6 | 167±35 | (1.8±1.0)×109 | — | Tetragonal | [60] | |
| HI | 75 | 10×3 | 164±25 | 3.6×1010 | — | Tetragonal | [40] | |
| ITC | GBL | 60→110 | 5.8 | 67.2±7.3 | (1.4±0.2)×1010 | 1.51 | Tetragonal | [41] |
| GBL | 50→100 | 71×54×39 | 34 | 4.8×1010 | 1.53 | Tetragonal | [43] | |
| GBL | — | 113×58×52 | 41 | 2.1×108 | — | Tetragonal | [55] | |
| GBL | 60→110 | 150 μm in thickness | 39.6 | 6.0×108 | 1.45 | Tetragonal | [56] | |
| SAC | GBL/DCM | Room temperature | Millimeters | 2.5 | (3.3±0.3)×1010 | 1.51 | Tetragonal | [39] |
| GBL/ACN | 60→70 | 17 | — | — | — | Tetragonal | [44] | |
| GBL/CB | 30→60 | 15×15×10 | — | — | — | Cubic | [45] |
| [1] | KOJIMA A, TESHIMA K, SHIRAI Y,et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc., 2009, 131(17): 6050-6051. |
| [2] | YANG W S, PARK B W, JUNG E H,et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science, 2017, 356(6345): 1376-1379. |
| [3] | |
| [4] | WEBER D.CH3NH3PbX3, a Pb(II)-system with cubic perovskite structure.Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1978, 33: 1443-1445. |
| [5] | MITZI D B.Templating and structural engineering in organic- inorganic perovskites.J. Chem. Soc., Dalton Trans., 2001, 1: 1-12. |
| [6] | SIDEY V.On the effective ionic radii for ammonium.Acta Crystallogr., Sect. B: Struct. Sci., 2016, 72: 626-633. |
| [7] | NIE W, TSAI H, ASADPOUR R,et al. High-efficiency solution- processed perovskite solar cells with millimeter-scale grains. Science, 2015, 347(6221): 522-525. |
| [8] | LIU C, FAN J, LI H,et al. Highly efficient perovskite solar cells with substantial reduction of lead content. Sci. Rep., 2016, 6: 35705. |
| [9] | WU Y Z, ISLAM A, YANG X D,et al. Retarding the crystallization of PbI2 for highly reproducible planar-structured perovskite solar cells via sequential deposition. Energy Environ. Sci., 2014, 7(9): 2934-2938. |
| [10] | MEI A Y, LI X, LIU L F,et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science, 2014, 345(6194): 295-298. |
| [11] | SHI J, DONG J, LV S,et al. Hole-conductor-free perovskite organic lead iodide heterojunction thin-film solar cells: high efficiency and junction property. Appl. Phys. Lett., 2014, 104(6): 063901. |
| [12] | WU Y Z, YANG X D, CHEN W, et al. Perovskite solar cells with 18.21% efficiency Perovskite solar cells with 18.21% efficiency and area over 1 cm2 fabricated by heterojunction engineering. Nat. Energy, 2016, 1: 16148-1-7. |
| [13] | HU H, YAN K, PENG M,et al. Fiber-shaped perovskite solar cells with 5.3% efficiency. J. Mater. Chem. A, 2016, 4(10): 3901-3906. |
| [14] | YE T, FU W, WU J,et al. Single-crystalline lead halide perovskite arrays for solar cells. J. Mater. Chem. A, 2016, 4(4): 1214-1217. |
| [15] | YAN K, PENG M, YU X,et al. High-performance perovskite memristor based on methyl ammonium lead halides. J. Mater. Chem. C, 2016, 4(7): 1375-1381. |
| [16] | NIU G, LI W, MENG F,et al. Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A, 2013, 2(3): 705-710. |
| [17] | LIU C, DING W, ZHOU X,et al. Efficient and stable perovskite solar cells prepared in ambient air based on surface-modified perovskite layer. J. Phys. Chem. C, 2017, 121(12): 6546-6553. |
| [18] | CHATTERJEE S, PA A J.Introducing Cu2O thin films as a hole-transport layer in efficient planar perovskite solar cell structures.J. Phys. Chem. C, 2016, 120(3): 1428-1437. |
| [19] | XU W, YAO X, MENG T,et al. Perovskite hybrid solar cells with a fullerene derivative electron extraction layer. J. Mater. Chem. C, 2017, 5: 4190-4197. |
| [20] | SUN C, WU Z, YIP H L,et al. Amino-functionalized conjugated polymer as an efficient electron transport layer for high-performance planar-heterojunction perovskite solar cells. Adv. Energy Mater., 2016, 6(5): 1501534. |
| [21] | SU J, CHEN D P, LIN C T.Growth of large CH3NH3PbX3 (X=I, Br) single crystals in solution.J. Cryst. Growth, 2015, 422: 75-79. |
| [22] | ZHOU H, NIE Z, YIN J,et al. Antisolvent diffusion-induced growth, equilibrium behaviours in aqueous solution and optical properties of CH3NH3PbI3 single crystals for photovoltaic applications. RSC Adv., 2015, 5(104): 85344-85349. |
| [23] | RONG Y, TANG Z, ZHAO Y,et al. Solvent engineering towards controlled grain growth in perovskite planar heterojunction solar cells. Nanoscale, 2015, 7(24): 10595-10599. |
| [24] | HUANG J S, SHAO Y C, DONG Q F,et al. Organometal trihalide perovskite single crystals: a next wave of materials for 25% efficiency photovoltaics and applications beyond? J. Phys. Chem. Lett., 2015, 6(16): 3218-3227. |
| [25] | POGLITSCH A, WEBER D.Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy.J. Chem. Phys., 1987, 87(11): 6373-6378. |
| [26] | BAIKIE T, FANG Y, KADRO J M,et al. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A, 2013, 1(18): 5628-5641. |
| [27] | IM J H, LEE C R, LEE J W,et al. 6.5% Efficient perovskite quantum- dot-sensitized solar cell. Nanoscale, 2011, 3(10): 4088-4093. |
| [28] | LEE M M, TEUSCHER J, MIYASAKA T,et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science, 2012, 338(6107): 643-647. |
| [29] | KIM H S, LEE C R, IM J H,et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep., 2012, 2: 591. |
| [30] | BURSHKA J, PELLET N, MOON S J,et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature, 2013, 499(7458): 316-319. |
| [31] | KU Z, RONG Y, XU M,et al. Full printable processed mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells with carbon counter electrode. Sci. Rep., 2013, 3: 3132. |
| [32] | LIU M Z, JOHNSTON M B, SNAITH H J,et al. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature, 2013, 501(7467): 395-398. |
| [33] | ZHOU H, CHEN Q, LI G,et al. Interface engineering of highly efficient perovskite solar cells. Science, 2014, 345(6196): 542-546. |
| [34] | ZHANG W, PATHAK S, SAKAI N, et al. Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells. Nat. Commun , 2015, 6: 10030-1-9. |
| [35] | YANG W S, NOH J H, JEON N J,et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science, 2015, 348(6240): 1234-1237. |
| [36] | STOUMPOS C C, MALLIAKAS C D, KANATZIDIS M G,et al. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem., 2013, 52(15): 9019-9038. |
| [37] | PISONI A, JACIMOVIC J, BARISIC O S,et al. Ultra-low thermal conductivity in organic-inorganic hybrid perovskite CH3NH3PbI3. J. Phys. Chem. Lett., 2014, 5(14): 2488-2492. |
| [38] | DANG Y, LIU Y, SUN Y,et al. Bulk crystal growth of hybrid perovskite material CH3NH3PbI3. CrystEngComm, 2015, 17(3): 665-670. |
| [39] | SHI D, ADINOLFI V, COMIN R,et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science, 2015, 347(6221): 519-522. |
| [40] | DONG Q, FANG Y, SHAO Y,et al. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science, 2015, 347(6225): 967-970. |
| [41] | SAIDAMINOV M I, ABDELHADY A L, MURALI B, et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun., 2015, 6: 7586-1-6. |
| [42] | LIAN Z, YAN Q, LV Q,et al. High-performance planar-type photodetector on (100) facet of MAPbI3 single crystal. Sci. Rep., 2015, 5: 16563. |
| [43] | LIU Y, YANG Z, CUI D,et al. Two-inch-sized perovskite CH3NH3PbX3(X = Cl, Br, I) crystals: growth and characterization. Adv. Mater., 2015, 27(35): 5176-5183. |
| [44] | KU Z, TIEP N H, WU B,et al. Solvent engineering for fast growth of centimetric high-quality CH3NH3PbI3perovskite single crystals. New J. Chem., 2016, 40(9): 7261-7264. |
| [45] | LUAN M, SONG J, WEI X,et al. Controllable growth of bulk cubic-phase CH3NH3PbI3 single crystal with exciting room temperature stability. CrystEngComm, 2016, 18(28): 5257-5261. |
| [46] | LI G.Preparation and Characterization of Organic-inorganic Hybrid Perovskite Materials. Changsha: National University of Defense Technology Bachelor Dissertation, 2016. |
| [47] | XIAO Z, DONG Q, BI C,et al. Solvent annealing of perovskite-induced crystal growth for photovoltaic-device efficiency enhancement. Adv. Mater., 2014, 26(37): 6503-6509. |
| [48] | DONG Q, SONG J, FANG Y,et al. Lateral-structure single-crystal hybrid perovskite solar cells via piezoelectric poling. Adv. Mater., 2016, 28(14): 2816-2821. |
| [49] | ZHOU Y Y, LI C M, WANG Y, et al. Preparation and Characterization of High-quality Perovskite CH3NH3PbX3(I, Br) Single Crystal. 1st International Conference on New Material and Chemical Industry, SanYa, 2017, 167: 012019. |
| [50] | QIN X, YAO Y, DONG H,et al. Perovskite photodetectors based on CH3NH3PbI3 single crystals. Chem. Asian J., 2016, 11(19): 2675-2679. |
| [51] | DANG Y, JU D, WANG L,et al. Recent progress in the synthesis of hybrid halide perovskite single crystals. CrystEngComm, 2016, 18(24): 4476-4484. |
| [52] | LEGUY A M A, HU Y, CAMPOY M,et al. Reversible hydration of CH3NH3PbI3in films, single crystals, and solar cells. Chem. Mater., 2015, 27(9): 3397-3407. |
| [53] | VINCENT B R, ROBERTSON K N, CAMERON T S,et al. Alkylammonium lead halides. Part 1. Isolated PbI64- ions in (CH3NH3)4PbI6·2H2O. Can. J. Chem., 1987, 65: 1042-1046. |
| [54] | KADRO J M, NONOMURA K, GACHET D,et al. Facile route to freestanding CH3NH3PbI3 crystals using inverse solubility. Sci. Rep., 2015, 5: 11654. |
| [55] | KATZ E A.High quality large single crystals of metal halide perovskites for optoelectronic applications.Sci. Chi. Chem., 2017, 60(10): 1326-1328. |
| [56] | LIU Y C, REN X D, ZHANG J,et al. 120 millimeter single-crystalline perovskite and wafers: towards viable applications. Sci. Chi. Chem., 2017, 60(10): 1367-1376. |
| [57] | LIU Y, ZHANG Y, YANG Z,et al. Thinness- and shape-controlled growth for ultrathin single-crystalline perovskite wafers for mass production of superior photoelectronic devices. Adv. Mater., 2016, 28(41): 9204-9209. |
| [58] | NAYAK P K, MOORE D T, WENGER B,et al. Mechanism for rapid growth of organic-inorganic halide perovskite crystals. Nat. Commun., 2016, 7: 13303. |
| [59] | KAWAMURA Y, MASHIYAMA H, HASEBE, K. structural study on cubic-tetragonal transition of CH3NH3PbI3.J. Phys. Soc. Jpn., 2002, 71(7): 1694-1697. |
| [60] | LIAN Z, YAN Q, GAO T,et al. Perovskite CH3NH3PbI3(Cl) single crystals: rapid solution growth, unparalleled crystalline quality, and low trap density toward 108 cm-3. J. Am. Chem. Soc., 2016, 138(30): 9409-9412. |
| [61] | YANG B, KEUM J, OVCHINNIKOVA O S,et al. Deciphering halogen competition in organometallic halide perovskite growth. J. Am. Chem. Soc., 2016, 138(15): 5028-5035. |
| [62] | ZHANG Y, HUANG F Q, MI Q X,et al. Preferential facet growth of methylammonium lead halide single crystals promoted by halide coordination. Chem. Lett., 2016, 45(8): 1030-1032. |
| [63] | DING J, DU S, ZHAO Y,et al. High-quality inorganic-organic perovskite CH3NH3PbI3 single crystals for photo-detector applications. J. Mater. Sci., 2017, 52(1): 276-284. |
| [64] | XING G, MATHEWS N, LIM S S,et al. Low-temperature solution- processed wavelength-tunable perovskites for lasing. Nat. Mater., 2014, 13(5): 476-480. |
| [65] | DOU L, YANG Y, YOU J, et al. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun., 2014, 5: 5404-1-6. |
| [66] | WEI H, FANG Y, MULLIGAN P,et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nature Photon., 2016, 10: 333-339. |
| [67] | TAN Z K, MOGHADDAM R S, LAI M L,et al. Bright light-emitting diodes based on organometal halide perovskite. Nature Nanotech., 2014, 9(9): 687-692. |
| [68] | STRANKS S D, EPERON G E, GRANCINI G,et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science, 2013, 342(6156): 341-344. |
| [69] | KIM H S, MORA-SERO I, GONZALEZ V,et al. Mechanism of carrier accumulation in perovskite thin-absorber solar cells. Nat. Commun., 2013, 4: 2242. |
| [70] | CHEN Y S, MANSER J S, KAMAT P V.All solution-processed lead halide perovskite-BiVO4 tandem assembly for photolytic solar fuels production.J. Am. Chem. Soc., 2015, 137(2): 974-981. |
| [71] | XU Q, WEI H, WEI W,et al. Detection of charged particles with a methylammonium lead tribromide perovskite single crystal. Nucl. Instrum. Methods Phys. Res., Sect. A, 2017, 848: 106-108. |
| [72] | SEMONIN O E, ELBAZ G A, STRAUS D B,et al. Limits of carrier diffusion in n-type and p-type CH3NH3PbI3 perovskite single crystals. J. Phys. Chem. Lett., 2016, 7(17): 3510-3518. |
| [73] | WANGYANG P H, SUN H, ZHU X H, et al.Solution-processable methyl ammonium lead iodide single crystal photodetectors for visible light and X-ray. Phys. Status Solidi A, 2017, 214(11): 1700538-1-5. |
| [74] | DING J, YAN Q F.Progress in organic-inorganic hybrid halide perovskite single crystal: growth techniques and applications.Sci. Chi. Mater., 2017, 60: 1063-1078. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | WANG Xiaobo, ZHU Yuliang, XUE Wenchao, SHI Ruchuan, LUO Bofeng, LUO Chengtao. Effect of PbTiO3 Content Variation on High-power Performance of PMN-PT Single Crystal [J]. Journal of Inorganic Materials, 2025, 40(7): 840-846. |
| [3] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [4] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [5] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [6] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [7] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [10] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [11] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [12] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [13] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [14] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [15] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||