
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (1): 104-110.DOI: 10.15541/jim20240279
Previous Articles Next Articles
FENG Guanzheng1,2,3( ), YANG Jian1,2, ZHOU Du1,2, CHEN Qiming1,2,3, XU Wentao1,2, ZHOU Youfu1,2(
), YANG Jian1,2, ZHOU Du1,2, CHEN Qiming1,2,3, XU Wentao1,2, ZHOU Youfu1,2( )
)
Received:2024-06-07
Revised:2024-09-02
Published:2025-01-20
Online:2024-09-02
Contact:
ZHOU Youfu, professor. E-mail: yfzhou@fjirsm.ac.cnAbout author:FENG Guanzheng (1998-), male, Master candidate. E-mail: fengguanzheng01@163.com
Supported by:CLC Number:
FENG Guanzheng, YANG Jian, ZHOU Du, CHEN Qiming, XU Wentao, ZHOU Youfu. Mechanism for Hydrothermal-carbothermal Synthesis of AlN Nanopowders[J]. Journal of Inorganic Materials, 2025, 40(1): 104-110.
| Phase | Surface energy/ (J·m-2) | Ref. | Method |
|---|---|---|---|
| α-Al2O3 | 2.04 | [ | MD simulation |
| 2.64 | [ | High-temperature calorimetry | |
| 2.57 | [ | Static lattice calculation | |
| 2.03 | [ | MD simulation | |
| 4.89 | [ | Ab initio calculation | |
| γ-Al2O3 | 0.79 | [ | MD simulation |
| 1.66 | [ | High-temperature calorimetry | |
| 1.53 | [ | High-temperature calorimetry |
Table 1 Compilation of measured and calculated surface energies in literature
| Phase | Surface energy/ (J·m-2) | Ref. | Method |
|---|---|---|---|
| α-Al2O3 | 2.04 | [ | MD simulation |
| 2.64 | [ | High-temperature calorimetry | |
| 2.57 | [ | Static lattice calculation | |
| 2.03 | [ | MD simulation | |
| 4.89 | [ | Ab initio calculation | |
| γ-Al2O3 | 0.79 | [ | MD simulation |
| 1.66 | [ | High-temperature calorimetry | |
| 1.53 | [ | High-temperature calorimetry |
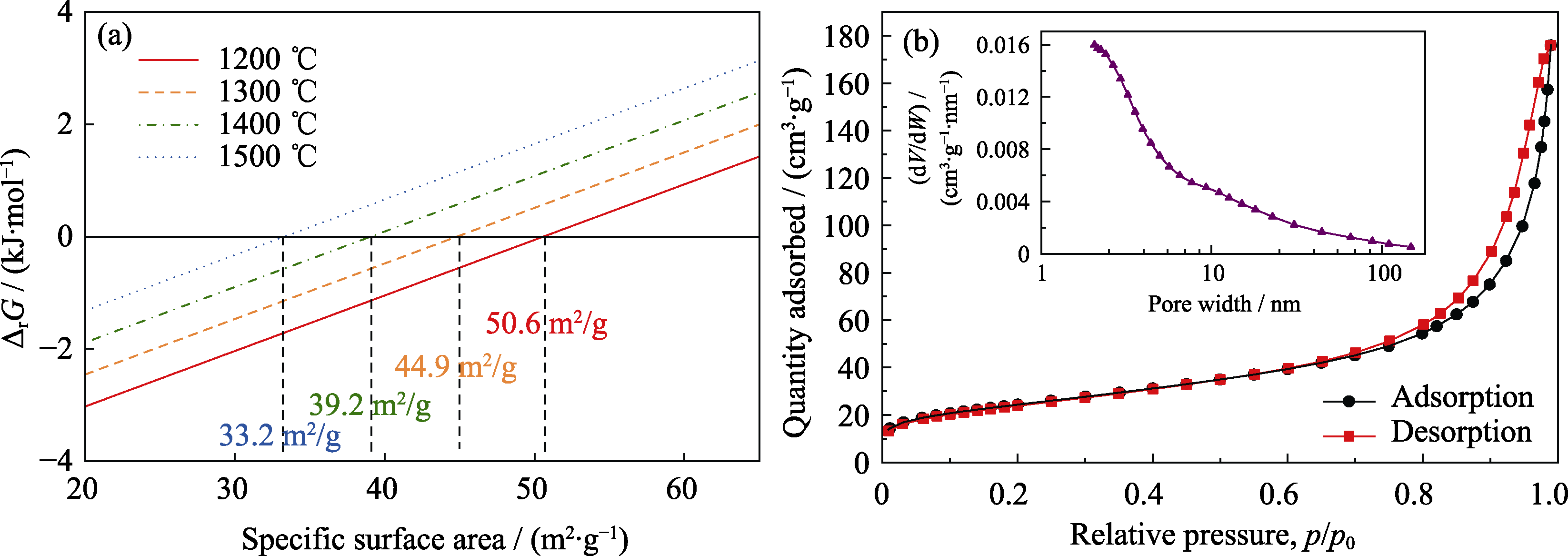
Fig. 5 (a) Gibbs free energy of the transformation from γ-Al2O3 to α-Al2O3 at different temperatures calculated as a function of specific surface area, and (b) N2 adsorption-desorption isotherm of γ-Al2O3 with inset showing pore size distribution determined by application of BJH method to the isotherm
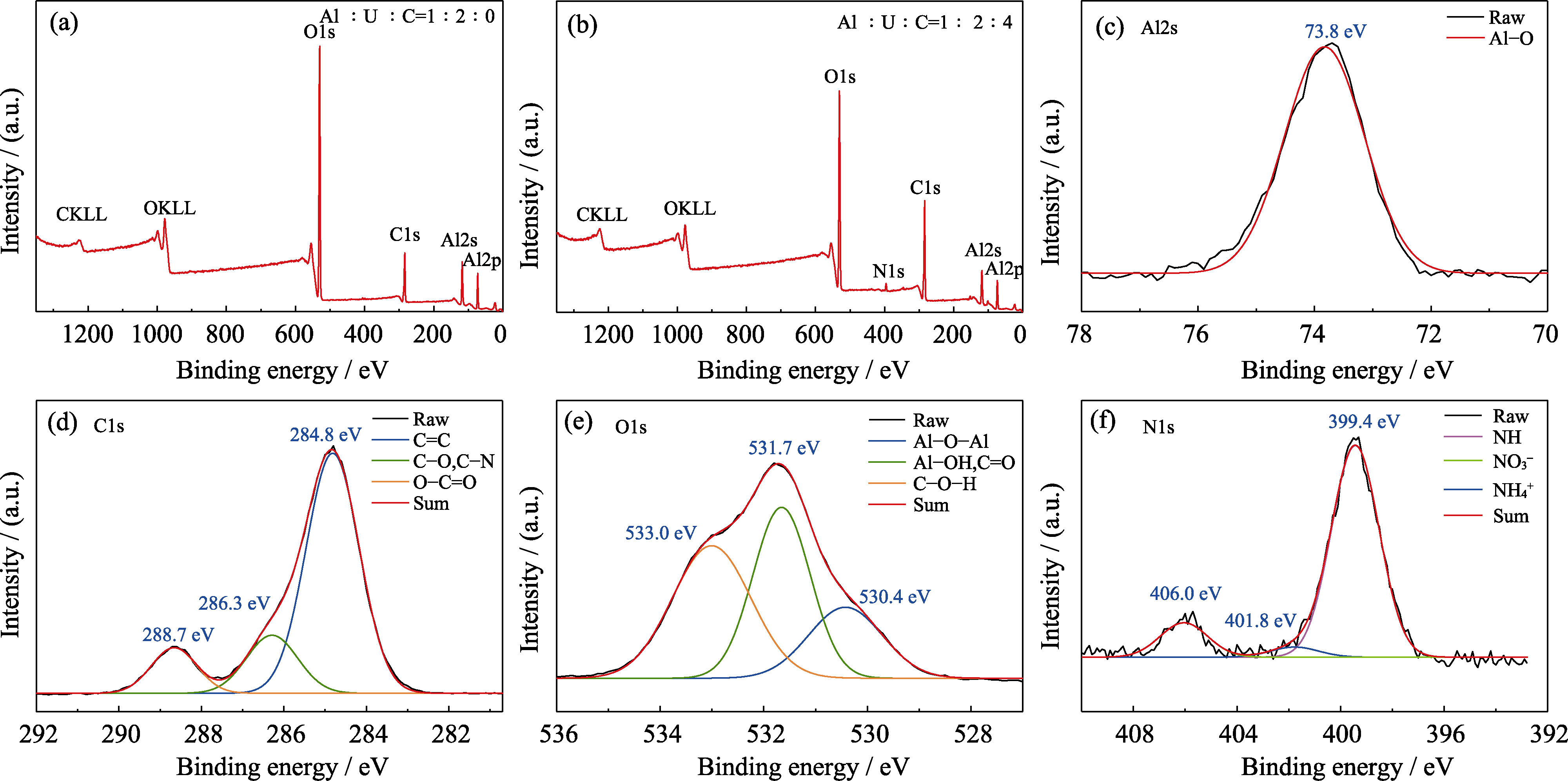
Fig. S3 (a, b) Survey spectra of the precursors at (a) Al : U : C=1 : 2 : 0 and (b) Al : U : C=1 : 2 : 4; (c-f) XPS spectra of (c) Al2s, (d) C1s, (e) O1s, and (f) N1s for the precursors at Al : U : C=1 : 2 : 4
| [1] | YIM W M, PAFF R J. Thermal expansion of AlN, sapphire, and silicon. Journal of Applied Physics, 1974, 45(3): 1456. |
| [2] | SLACK G A, TANZILLI R A, POHL R O, et al. The intrinsic thermal conductivity of AIN. Journal of Physics and Chemistry of Solids, 1987, 48(7): 641. |
| [3] | SELVADURAY G, SHEET L. Aluminium nitride: review of synthesis methods. Materials Science & Technology, 1993, 9(6): 463. |
| [4] | RUTKOWSKI P J, KATA D. Thermal properties of AlN polycrystals obtained by pulse plasma sintering method. Journal of Advanced Ceramics, 2013, 2(2): 180. |
| [5] | SHEPPARD L M. Aluminum nitride: a versatile but challenging material. American Ceramic Society Bulletin, 1990, 69(11): 1801. |
| [6] | BAIK Y, DREW R A L. Aluminum nitride: processing and applications. Key Engineering Materials, 1996, 122: 553. |
| [7] | LEE H M, BHARATHI K, KIM D K. Processing and characterization of aluminum nitride ceramics for high thermal conductivity. Advanced Engineering Materials, 2014, 16(6): 655. |
| [8] | CHIKAMI H, FUKUSHIMA J, HAYASHI Y, et al. Kinetics of microwave synthesis of AlN by carbothermal-reduction-nitridation at low temperature. Journal of the American Ceramic Society, 2018, 101(11): 4905. |
| [9] | WANG Y M, QIAO L, ZHENG J W, et al. Preparation of AlN with low agglomeration using polyethylene glycol and emulsifier to disperse the ultrafine raw powders. Ceramics International, 2023, 49(1): 1390. |
| [10] | KOMEYA K, MATSUKAZE N, MEGURO T. Synthesis of AlN by direct nitridation of Al alloys. Journal of the Ceramic Society of Japan, 1993, 101(1180): 1319. |
| [11] | KIMURA I, ICHIYA K, ISHII M, et al. Synthesis of fine AlN powder by a floating nitridation technique using an N2/NH3 gas mixture. Journal of Materials Science Letters, 1989, 8(3): 303. |
| [12] | WEI Z L, LI K, GE B Z, et al. Synthesis of nearly spherical AlN particles by an in-situ nitriding combustion route. Journal of Advanced Ceramics, 2021, 10(2): 291. |
| [13] | YAMAKAWA T, TATAMI J, WAKIHARA T, et al. Synthesis of AlN nanopowder from γ-Al2O3 by reduction-nitridation in a mixture of NH3-C3H8. Journal of the American Ceramic Society, 2006, 89(1): 171. |
| [14] | ZHANG Q H, GAO L. Synthesis of nanocrystalline aluminum nitride by nitridation of δ-Al2O3 nanoparticles in flowing ammonia. Journal of the American Ceramic Society, 2006, 89(2): 415. |
| [15] | KIM J K, JUNG W S. Nitridation of δ-alumina to aluminum nitride under a flow of ammonia and its mechanism. Journal of the Ceramic Society of Japan, 2011, 119(1389): 351. |
| [16] | JUNG W S. Synthesis of aluminum nitride powder from δ-alumina nanopowders under a mixed gas flow of nitrogen and hydrogen. Ceramics International, 2012, 38(1): 871. |
| [17] | YOSHIMURA M, BYRAPPA K. Hydrothermal processing of materials: past, present and future. Journal of Materials Science, 2008, 43(7): 2085. |
| [18] | WANG Q, LI H, CHEN L Q, et al. Monodispersed hard carbon spherules with uniform nanopores. Carbon, 2001, 39(14): 2211. |
| [19] |
GONG Y T, XIE L, LI H R, et al. Sustainable and scalable production of monodisperse and highly uniform colloidal carbonaceous spheres using sodium polyacrylate as the dispersant. Chemical Communications, 2014, 50(84): 12633.
DOI PMID |
| [20] | CHEN W, LI D, TIAN L, et al. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chemistry, 2018, 20(19): 4438. |
| [21] | XIANG M, ZHOU Y, XU W, et al. Hydrothermal-carbothermal synthesis of highly sinterable AlN nanopowders. Journal of the American Ceramic Society, 2017, 100(6): 2482. |
| [22] | XIANG M, ZHOU Y F, XU W T, et al. Transparent AlN ceramics sintered from nanopowders produced by the wet chemical method. Journal of the Ceramic Society of Japan, 2018, 126(4): 241. |
| [23] | YANG J, CONG Y, LING J R, et al. Preparation of transparent AlON from powders synthesized by novel CRN method. Journal of the European Ceramic Society, 2022, 42(3): 935. |
| [24] | FANKHÄNEL J, SILBERNAGL D, GHASEM Z K M, et al. Mechanical properties of boehmite evaluated by atomic force microscopy experiments and molecular dynamic finite element simulations. Journal of Nanomaterials, 2016, 2016(1): 5017213. |
| [25] | WU J, XU W, DONG T, et al. Self-assembly of graphene reinforced ZrO2 composites with deformation-sensing performance. Ceramics International, 2022, 48(21): 32131. |
| [26] | ANSI V A, SREELAKSHMI P, RAVEENDRAN P, et al. Table sugar derived carbon dot—a promising green reducing agent. Materials Research Bulletin, 2021, 139: 111284. |
| [27] | YANG J, WANG L H, JIANG X X, et al. AlN nanoparticles prepared through a gelation-polymerization process. Ceramics International, 2020, 46(11): 17486. |
| [28] | HE Q, QIN M L, HUANG M, et al. Mechanism and kinetics of combustion-carbothermal synthesis of AlN nanopowders. Ceramics International, 2017, 43(12): 8755. |
| [29] | MAO X X, XU Y G, MAO X J, et al. Synthesis of fine AlN powders by foamed precursor-assisted carbothermal reduction- nitridation method. Journal of Inorganic Materials, 2019, 34(10): 1123. |
| [30] | CARSTENS S, MEYER R, ENKE D. Towards macroporous α-Al2O3—routes, possibilities and limitations. Materials, 2020, 13(7): 1787. |
| [31] | CESTEROS Y, SALAGRE P, MEDINA F, et al. Several factors affecting faster rates of gibbsite formation. Chemistry of Materials, 1999, 11(1): 123. |
| [32] | MCHALE J M, NAVROTSKY A, PERROTTA A J. Effects of increased surface area and chemisorbed H2O on the relative stability of nanocrystalline γ-Al2O3 and α-Al2O3. The Journal of Physical Chemistry B, 1997, 101(4): 603. |
| [33] | MCHALE J M, AUROUX A, PERROTTA A J, et al. Surface energies and thermodynamic phase stability in nanocrystalline aluminas. Science, 1997, 277(5327): 788. |
| [34] | BLONSKI S, GAROFALINI S H. Molecular dynamics simulations of α-alumina and γ-alumina surfaces. Surface Science, 1993, 295(1/2): 263. |
| [35] | TASKER P W. Surfaces of magnesia and alumina. Advances in Ceramics, 1984, 10: 176. |
| [36] | MACKRODT W C, DAVEY R J, BLACK S N, et al. The morphology of α-Al2O3 and α-Fe2O3: the importance of surface relaxation. Journal of Crystal Growth, 1987, 80(2): 441. |
| [37] | CAUSÀ M, DOVESI R, PISANI C, et al.Ab initio characterization of the (0001) and (101̄0) crystal faces of α-alumina. Surface Science, 1989, 215(1/2): 259. |
| [38] | CASTRO R H R, USHAKOV S V, GENGEMBRE L, et al. Surface energy and thermodynamic stability of γ-alumina: effect of dopants and water. Chemistry of Materials, 2006, 18(7): 1867. |
| [39] | TSUGE A, INOUE H, KASORI M, et al. Raw material effect on AIN powder synthesis from Al2O3 carbothermal reduction. Journal of Materials Science, 1990, 25(5): 2359. |
| [1] | YU Shengyang, SU Haijun, JIANG Hao, YU Minghui, YAO Jiatong, YANG Peixin. A Review of Pore Defects in Ultra-high Temperature Oxide Ceramics by Laser Additive Manufacturing: Formation and Suppression [J]. Journal of Inorganic Materials, 2025, 40(9): 944-956. |
| [2] | LI Fuping, CHU Jiabao, QIU Haibo, DANG Wei, LI Chenxi, ZHAO Kang, TANG Yufei. Compressive Resilience Mechanism of SiO2 Nanofibre Aerogels [J]. Journal of Inorganic Materials, 2025, 40(9): 981-988. |
| [3] | LIU Jiangping, GUAN Xin, TANG Zhenjie, ZHU Wenjie, LUO Yongming. Research Progress on Catalytic Oxidation of Nitrogen-containing Volatile Organic Compounds [J]. Journal of Inorganic Materials, 2025, 40(9): 933-943. |
| [4] | LI Ronghui, QIAN Jun. Nanocrystalline CeO2-ZrO2 Solid Solution: One-step Alcohothermal Synthesis and Arsenic Removal Performance [J]. Journal of Inorganic Materials, 2025, 40(9): 989-996. |
| [5] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [6] | YU Yiping, XIAO Peng, ZHAO Changhao, XU Mengdi, YAO Lidong, LI Wei, WANG Song. Ablation Behavior of High-temperature Laminated Ta/Ta0.5Hf0.5C Cermets under High-frequency Plasma Wind Tunnel Test [J]. Journal of Inorganic Materials, 2025, 40(7): 790-798. |
| [7] | SUN Jing, LI Xiang, MAO Xiaojian, ZHANG Jian, WANG Shiwei. Effect of Lauric Acid Modifier on the Hydrolysis Resistance of Aluminum Nitride Powders [J]. Journal of Inorganic Materials, 2025, 40(7): 826-832. |
| [8] | TANG Ying, LI Jie, XIANG Huaicheng, FANG Weishuang, LIN Huixing, YANG Junfeng, FANG Liang. Rattling Effect: A New Mechanism Affecting the Resonant Frequency Temperature Coefficient of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 656-666. |
| [9] | GUO Ziyu, ZHU Yunzhou, WANG Li, CHEN Jian, LI Hong, HUANG Zhengren. Effect of Zn2+ Catalyst on Microporous Structure of Porous Carbon Prepared from Phenolic Resin/Ethylene Glycol [J]. Journal of Inorganic Materials, 2025, 40(5): 466-472. |
| [10] | LI Ziwei, GONG Weilu, CUI Haifeng, YE Li, HAN Weijian, ZHAO Tong. (Zr, Hf, Nb, Ta, W)C-SiC Composite Ceramics: Preparation via Precursor Route and Properties [J]. Journal of Inorganic Materials, 2025, 40(3): 271-280. |
| [11] | YI Guogang, WU Yaoying, ZU Xihong. Non-solvent and Low-temperature Preparation of Porous Silicon-carbon Anodes for Enhanced Lithium Storage [J]. Journal of Inorganic Materials, 2025, 40(12): 1379-1386. |
| [12] | YUAN Long, JIA Ru, YUAN Meng, ZHANG Jian, DUAN Yu, MENG Xiangdong. Mechanism and Application of X-ray Induced Photochromic Materials: A Review [J]. Journal of Inorganic Materials, 2025, 40(10): 1097-1110. |
| [13] | ZHANG Li, GUAN Haoyang, ZHENG Qining, HONG Zhiliang, WANG Jiaxuan, XING Ning, LI Mei, LIU Yongsheng, ZHANG Chengyu. Creep Properties and Damage Mechanisms of SiCf/SiC-SiYBC Prepared by Melt Infiltration [J]. Journal of Inorganic Materials, 2025, 40(1): 23-30. |
| [14] | WANG Wenting, XU Jingjun, MA Ke, LI Meishuan, LI Xingchao, LI Tongqi. Oxidation Behavior at 1000-1300 ℃ in air of Ti2AlC-20TiB2 Synthesized by in-situ Reaction/Hot Pressing [J]. Journal of Inorganic Materials, 2025, 40(1): 31-38. |
| [15] | HUANG Jie, WANG Liuying, WANG Bin, LIU Gu, WANG Weichao, GE Chaoqun. Research Progress on Modulation of Electromagnetic Performance through Micro-nanostructure Design [J]. Journal of Inorganic Materials, 2024, 39(8): 853-870. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||