
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (1): 32-42.DOI: 10.15541/jim20220384
Special Issue: 【信息功能】敏感陶瓷(202506)
• Topical Section: Anti-epidemic Biomaterials (Contributing Editor: YANG Yong) • Previous Articles Next Articles
LIU Yao1,2( ), YOU Xunhai1,3, ZHAO Bing1,3, LUO Xiaoying4(
), YOU Xunhai1,3, ZHAO Bing1,3, LUO Xiaoying4( ), CHEN Xing1,2,3(
), CHEN Xing1,2,3( )
)
Received:2022-07-04
Revised:2022-08-18
Published:2023-01-20
Online:2022-09-15
Contact:
CHEN Xing, professor. E-mail: xingchen@hfut.edu.cn;About author:LIU Yao (1993-), female, PhD candidate. E-mail: 18691965261@163.com
Supported by:CLC Number:
LIU Yao, YOU Xunhai, ZHAO Bing, LUO Xiaoying, CHEN Xing. Functional Nanomaterials for Electrochemical SRAS-CoV-2 Biosensors: a Review[J]. Journal of Inorganic Materials, 2023, 38(1): 32-42.
| Detection method | Time/h | Advantage | Disadvantage |
|---|---|---|---|
| Reverse transcrition-polymerase chain reaction (RT-PCR) | 4-6 | High sensitivity and reliability Low cost Versatility in sample types | Special instruments Complicated operation Time-consuming |
| Enzyme linked immunosorbent assay (ELISA) | 1-3 | Simple operation Low price Fast detection | Low specificity Suitability only for the late stage of the disease |
| Surface-enhanced Raman spectroscopy (SERS) | <1 | Simple construction Good repeatability | Specialized SERS active substrates |
| Electrochemical detection | <1 | Lower cost Simpler construction Higher specificity Relatively lower sensitivity | Lower clinical trial accuracy |
Table 1 Comparison of detection methods for SARS-CoV-2 detection
| Detection method | Time/h | Advantage | Disadvantage |
|---|---|---|---|
| Reverse transcrition-polymerase chain reaction (RT-PCR) | 4-6 | High sensitivity and reliability Low cost Versatility in sample types | Special instruments Complicated operation Time-consuming |
| Enzyme linked immunosorbent assay (ELISA) | 1-3 | Simple operation Low price Fast detection | Low specificity Suitability only for the late stage of the disease |
| Surface-enhanced Raman spectroscopy (SERS) | <1 | Simple construction Good repeatability | Specialized SERS active substrates |
| Electrochemical detection | <1 | Lower cost Simpler construction Higher specificity Relatively lower sensitivity | Lower clinical trial accuracy |
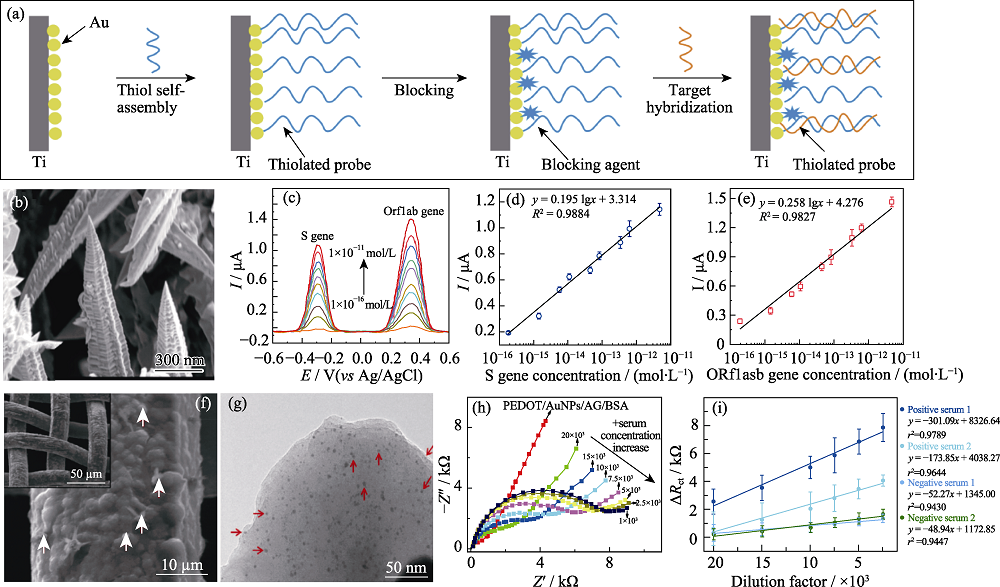
Fig. 2 Electrochemical biosensors based on gold nanomaterials for the detection of SARS-CoV-2 (a) Schematic diagram of probe DNA fixation and target nucleotide hybridization on gold electrode[46]; (b) SEM images of 3D gold nanoneedle structures[47];(c-e) Square wave stripping voltammetric response and corresponding calibration plots of 3D gold nanoneedle modified electrode toward S and ORF1ab genes[47]; (f) SEM and (g) TEM images of PEDOT/AuNPs/AG[48]; (h-i) Nyquist plots and corresponding calibration plots of the PEDOT/AuNPs/AG/BSA modified electrode toward different positive serum concentrations[48]
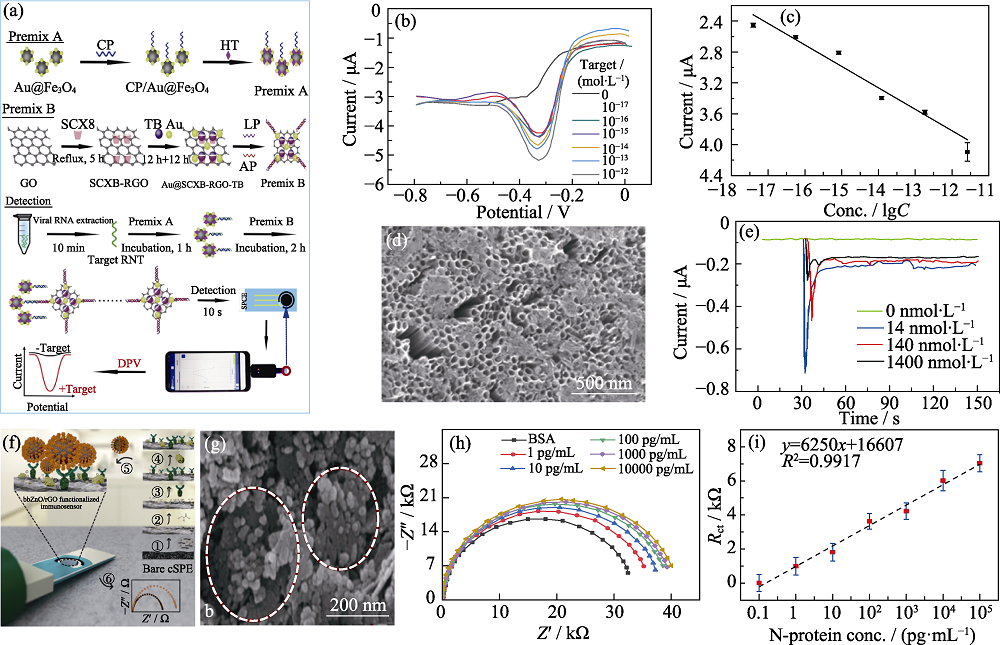
Fig. 3 Metal oxide nanomaterials used in electrochemical sensors to detect SARS-CoV-2 (a) Schematic of portable electrochemical biosensor based on probe recognition technology for the detection of SARS-CoV-2 RNA[6]; (b) DPV curves for different concentrations of artificial target for the SARS-CoV-2 biosensor[6]; (c) Resulting calibration plot for lgC vs. DPV response signals[6]; (d) SEM image of the Co-functionalized TNTs[49]; (e) Amperometry response curves of Co-TNT on SARS-CoV-2 S protein of different concentrations[49]; (f) Amperometry response curves of Co-TNT sensor upon exposure to SARS-CoV-2 S protein of different concentrations[49]; (g) FESEM image of antibodies being deposited on ZnO/rGO[5]; (h-i) Nyquist plots and corresponding calibration curve of the ZnO/rGO modified electrode towards N-protein[5] ; Colorful figures are available on website
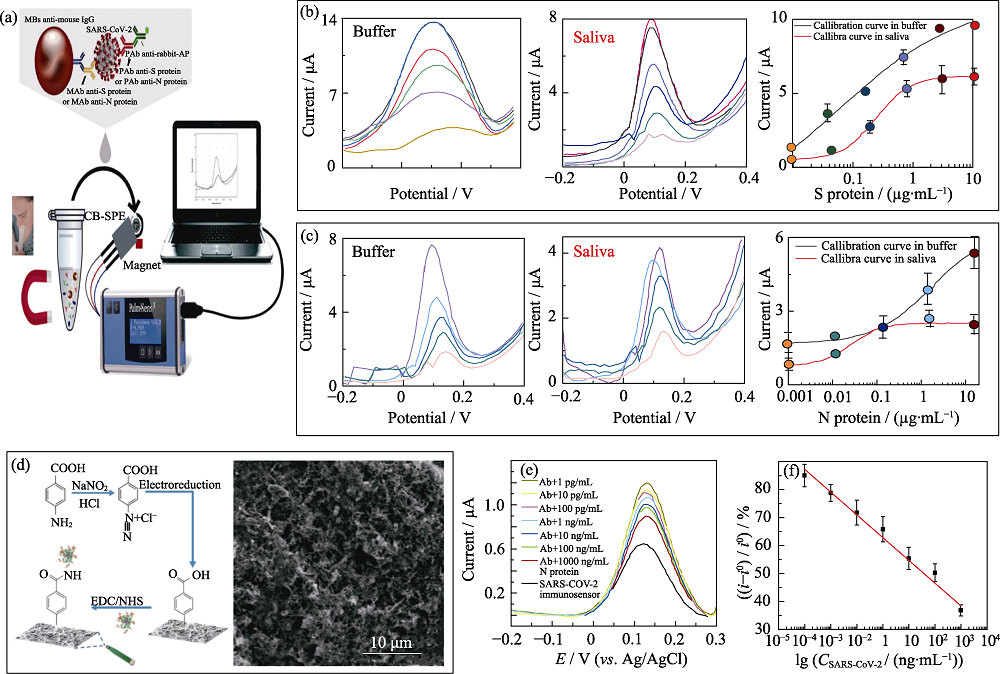
Fig. 4 Electrochemical biosensors based on carbon nanomaterials for the detection of SARS-CoV-2 (a) Schematic diagram of CBs modified SPE for SARS-CoV-2 detection[50]; (b, c) Electrochemical response signal and corresponding calibration curves of the CBs modified SPE towards S (b) and N (c) protein[50]; (d) Preparation process and SEM image of functionalized carbon nanofiber (CNF) [51]; (e, f) Square wave voltammetric respond (e) and corresponding calibration curves (f) of the the functionalized CNF modified electrode towards nucleocapsid protein at different concentrations[51]; CBs: Carbon black nanomaterials; SPE: Screen printing electrodes; EDC/NHS: 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydro/N-Hydroxy succinimide; Colorful figures are available on website
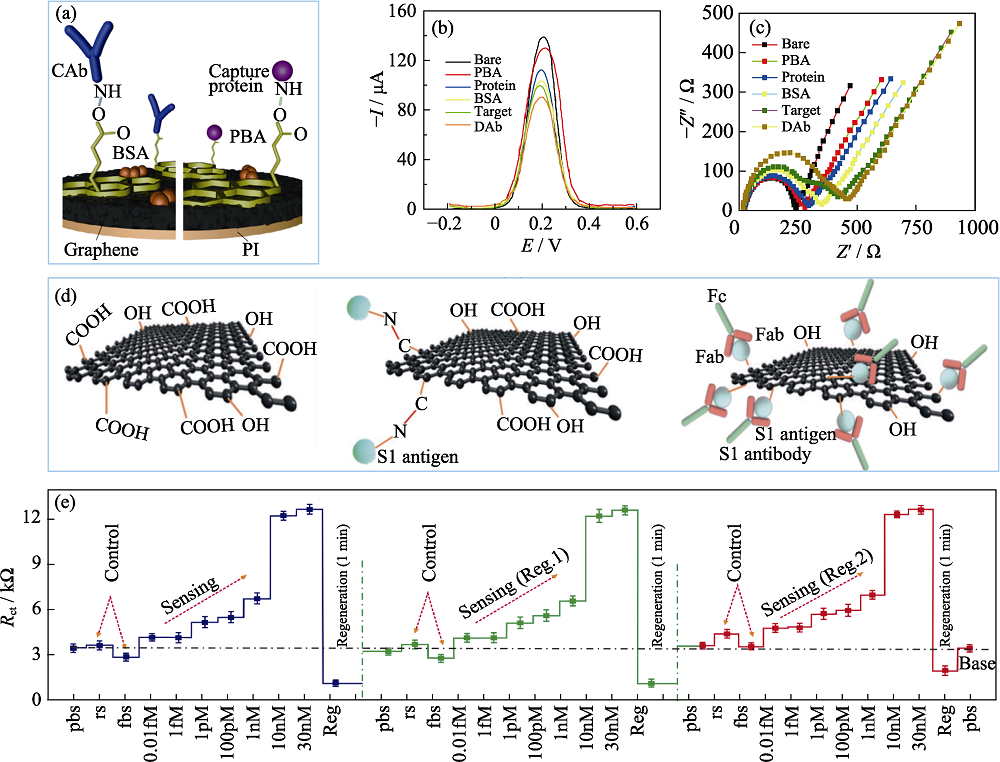
Fig. 5 Graphene nanocomposites used in electrochemical sensors to detect SARS-CoV-2 (a) Schematic diagram of functionalized graphene connected to the corresponding bioreceptors by covalent bonds[52]; (b, c) DPV respond (b) and Nyquist diagram (c) of the electrode at different steps[52]; (d) Surface modification process of reduced graphene oxide nanosheets by carboxyl functionalization[55]; (e) Continuous detection of neo-coronavirus S protein after sensor regeneration[55]. CAb: Capture antibody; DAb: Detector antibody; PI: Polyimide; BSA: Bovine serum albumin: PBA: 1-Pyrenebutyric acid; Fc: Fragment crystallizable; Fab: Fragment of antigen binding; M: mol/L; Colorful figures are available on website
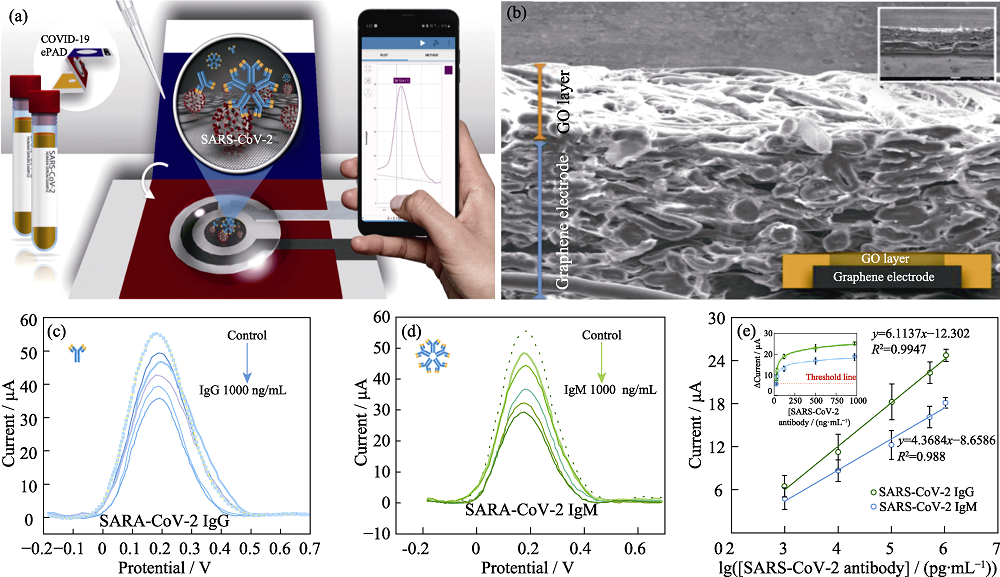
Fig. 6 Paper-based electrochemical biosensor for diagnosing COVID-19[59] (a) Schematic illustration of the detection procedure of COVID-19; (b) SEM image of the corresponding cross-sectional of GO modified paper; (c, d) Square wave stripping voltammetric responses of SARS-CoV-2 IgG (c) and IgM (d) at different concentrations; (e) linear relationship between Δ current vs logarithmic concentration of SARS-CoV-2 IgG and IgM and their corresponding relationships between Δ current and concentration of SARS-CoV-2 IgG and IgM; Colorful figures are available on website
| Material | Method | Detecting object | Limit of detection | Ref. |
|---|---|---|---|---|
| AuNPs | i-t | RNA or cDNA | N/A | [ |
| Gold nanoneedle | SWV | S gene Orf1ab gene | 5.0×10-18 g·μL-1 6.8×10-18 g·μL-1 | [ |
| AuNPs/PEDOT | EIS | Positive and negative serum sample | N/A | [ |
| Au@Fe3O4/rGO | DPV | RNA | 3×10-18 mol·L-1 | [ |
| Co-TiO2 nanotubes | i-t | RBD | 7×10-10 mol·L-1 | [ |
| ZnO/rGO | EIS | N protein antigens | 2×10-14 g·mL-1 | [ |
| Carbon black nanomaterial | LSV | S protein N protein | 1.9×10-8 g·mL-1 8×10-9 g·mL-1 | [ |
| Laser-engraved graphene | LSV | N-protein, S1-IgM S1-IgG C-reactive protein | N/A | [ |
| AuNPs/rGO | EIS | S1 protein RBD antibodies | 2.8×10-15 mol·L-1 1.69×10-14 mol·L-1 | [ |
| SiO2@UiO-66 | EIS | S protein | 1×10-13 g·mL-1 | [ |
| GO | SWV | IgG IgM | 9.6×10-10 g·mL-1 1.4×10-10 g·mL-1 | [ |
| Au@Pt/MIL-5(Al) | DPV | N-protein | 8.33×10-12 g·mL-1 | [ |
Table 2 Comparison of SARS-CoV-2 detection performance of electrochemical sensors constructed from different nanomaterials
| Material | Method | Detecting object | Limit of detection | Ref. |
|---|---|---|---|---|
| AuNPs | i-t | RNA or cDNA | N/A | [ |
| Gold nanoneedle | SWV | S gene Orf1ab gene | 5.0×10-18 g·μL-1 6.8×10-18 g·μL-1 | [ |
| AuNPs/PEDOT | EIS | Positive and negative serum sample | N/A | [ |
| Au@Fe3O4/rGO | DPV | RNA | 3×10-18 mol·L-1 | [ |
| Co-TiO2 nanotubes | i-t | RBD | 7×10-10 mol·L-1 | [ |
| ZnO/rGO | EIS | N protein antigens | 2×10-14 g·mL-1 | [ |
| Carbon black nanomaterial | LSV | S protein N protein | 1.9×10-8 g·mL-1 8×10-9 g·mL-1 | [ |
| Laser-engraved graphene | LSV | N-protein, S1-IgM S1-IgG C-reactive protein | N/A | [ |
| AuNPs/rGO | EIS | S1 protein RBD antibodies | 2.8×10-15 mol·L-1 1.69×10-14 mol·L-1 | [ |
| SiO2@UiO-66 | EIS | S protein | 1×10-13 g·mL-1 | [ |
| GO | SWV | IgG IgM | 9.6×10-10 g·mL-1 1.4×10-10 g·mL-1 | [ |
| Au@Pt/MIL-5(Al) | DPV | N-protein | 8.33×10-12 g·mL-1 | [ |
| [1] |
CHU D K W, PAN Y, CHENG S M S, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clinical Chemistry, 2020, 66(4): 549.
DOI PMID |
| [2] |
OROOJI Y, SOHRABI H, HEMMAT N, et al. An overview on SARS-CoV-2 (COVID-19) and other human coronaviruses and their detection capability via amplification assay, chemical sensing, biosensing, immunosensing, and clinical assays. Nano-Micro Letters, 2020, 13(1): 18.
DOI URL |
| [3] | SAMSON R, NAVALE G R, DHARNE M S, et al. Biosensors: frontiers in rapid detection of COVID-19. Biotech, 2020, 10(9): 385. |
| [4] |
ALAFEEF M, DIGHE K, MOITRA P, et al. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano, 2020, 14(12): 17028.
DOI PMID |
| [5] |
HAGHAYEGH F, SALAHANDISH R, HASSANI M, et al. Highly stable buffer-based zinc oxide/reduced graphene oxide nanosurface chemistry for rapid immunosensing of SARS-CoV-2 antigens. ACS Appl. Mater. Interfaces, 2022, 14(8): 10844.
DOI URL |
| [6] |
ZHAO H, LIU F, XIE W, et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sensors and Actuators B Chemical, 2021, 327: 128899.
DOI URL |
| [7] |
FALSEY A R, WALSH E E. Novel coronavirus and severe acute respiratory syndrome. Lancet, 2003, 361(9366): 1312.
DOI PMID |
| [8] |
ZAKI A M, VANBOHEEMEN S, BESTEBROER T M, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New England Journal of Medicine, 2012, 367: 1814.
DOI URL |
| [9] |
ZHU N, ZHANG D, WANG W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 2020, 382(8): 727.
DOI URL |
| [10] |
YAO H, SONG Y, CHEN Y, et al. Molecular architecture of the SARS-CoV-2 virus. Cell, 2020, 183(3): 730.
DOI PMID |
| [11] |
CHOUDHRY N, ZHAO X, XU D, et al. Chinese therapeutic strategy for fighting COVID-19 and potential small-molecule inhibitors against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Journal of Medicinal Chemistry, 2020, 63(22): 13205.
DOI URL |
| [12] |
THOMS M, BUSCHAUER R, AMEISMEIER M, et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science, 2020, 369(6508): 1249.
DOI PMID |
| [13] |
FENG W, NEWBIGGING A M, LE C, et al. Molecular diagnosis of COVID-19: challenges and research needs. Analytical Chemistry, 2020, 92: 10196.
DOI PMID |
| [14] |
XIE C B, JIANG L X, HUANG G, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. International Journal of Infectious Diseases, 2020, 93: 264.
DOI PMID |
| [15] |
SADIGHBAYAN D, HASANZADEH M, GHAFAR-ZADEH E. Biosensing based on field-effect transistors (FET): recent progress and challenges. Trac-Trends in Analytical Chemistry, 2020, 133: 116067.
DOI URL |
| [16] | LIU W, LIU L, KOU G, et al. Evaluation of nucleocapsid and spike protein-based ELISAs for detecting antibodies against SARS-CoV-2. Journal of Clinical Microbiology, 2020, 58(6): e0461. |
| [17] |
PENG Y, LIN C, LI Y, et al. Identifying infectiousness of SARS-CoV-2 by ultra-sensitive SnS2 SERS biosensors with capillary effect. Matter, 2022, 5(2): 694.
DOI URL |
| [18] |
SITJAR J, LIAO J D, LEE H, et al. Challenges of SERS technology as a non-nucleic acid or -antigen detection method for SARS-CoV-2 virus and its variants. Biosensors & Bioelectronics, 2021, 181: 113153.
DOI URL |
| [19] |
YANG Y, PENG Y, LIN C, et al. Human ACE2-functionalized gold "virus-trap" nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano-Micro Letters, 2021, 13(1): 109.
DOI PMID |
| [20] |
CHAIBUN T, PUENPA J, NGAMDEE T, et al. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nature Communications, 2021, 12(1): 802.
DOI PMID |
| [21] |
KUDR J, MICHALEK P, ILIEVA L, et al. COVID-19: a challenge for electrochemical biosensors. TrAC Trends in Analytical Chemistry, 2021, 136: 116192.
DOI URL |
| [22] |
TRAN V V, TRAN N H T, HWANG H S, et al. Development strategies of conducting polymer-based electrochemical biosensors for virus biomarkers: potential for rapid COVID-19 detection. Biosensors & Bioelectronics, 2021, 182: 113192.
DOI URL |
| [23] |
EJAZI S A, GHOSH S, ALI N. Antibody detection assays for COVID-19 diagnosis: an early overview. Immunology and Cell Biology, 2020, 99(1): 21.
DOI URL |
| [24] | MATHEW D, GILES J R, BAXTER A E, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science, 2020, 369(6508): 8511. |
| [25] |
ONG D, FRAGKOU P C, SCHWEITZER V A, et al. How to interpret and use COVID-19 serology and immunology tests. Clinical Microbiology and Infection, 2021, 27(7): 981.
DOI URL |
| [26] |
KIMMEL D W, LEBLANC G, MESCHIEVITZ M E, et al. Electrochemical sensors and biosensors. Analytical Chemistry, 2012, 84(2): 685.
DOI PMID |
| [27] | FREW J E, HILL H A. Electrochemical biosensors. Analytical Chemistry, 2010, 39(5): 1747. |
| [28] | BALKOURANI G, BROUZGOU A, ARCHONTI M, et al. Emerging materials for the electrochemical detection of COVID-19. Journal of Electroanalytical Chemistry, 2021, 893: 115285. |
| [29] |
ANTIOCHIA R. Developments in biosensors for CoV detection and future trends. Biosensors and Bioelectronics, 2020, 173: 112777.
DOI URL |
| [30] |
ERDEN P E, KILIÇ E. A review of enzymatic uric acid biosensors based onamperometric detection. Talanta, 2013, 107: 312.
DOI URL |
| [31] |
BRETT C M A, OLIVEIRA B A M. Electrochemical sensing in solution-origins, applications and future perspectives. Journal of Solid State Electrochemistry, 2011, 15(7/8): 1487.
DOI URL |
| [32] |
GUTH U, VONAU W, ZOSEL J. Recent developments in electrochemical sensor application and technology-a review. Measurement Science and Technology, 2009, 20(4): 042002.
DOI URL |
| [33] |
KARIMI-MALEH H, OROOJI Y, KARIMI F, et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosensors & Bioelectronics, 2021, 184: 113252.
DOI URL |
| [34] | CHAROENKITAMORN K, TUE PT, CHIKAE M, et al. Gold nanoparticle-labeled electrochemical immunoassay using open circuit potential for human chorionic gonadotropin detection. Electroanalysis, 2018, 30(8): 1766. |
| [35] |
RASHED M Z, KOPECHEK J A, PRIDDY M C, et al. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance- based detector. Biosensors & Bioelectronics, 2021, 171: 112709.
DOI URL |
| [36] |
LASSERRE P, BALANSETHUPATHY B, VEZZA V J, et al. SARS-CoV-2 aptasensors based on electrochemical impedance spectroscopy and low-cost gold electrode substrates. Analytical Chemistry, 2022, 94(4): 2126.
DOI PMID |
| [37] |
XU H, ZHENG J, LIANG H, et al. Electrochemical sensor for cancer cell detection using calix 8 arene/polydopamine/phosphorene nanocomposite based on host-guest recognition. Sensors and Actuators B-Chemical, 2020, 317: 128193.
DOI URL |
| [38] |
SEO G, LEE G, MI J K, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano, 2020, 14: 5135.
DOI PMID |
| [39] |
MOKHTARZADEH A, EIVAZZADEH-KEIHAN R, PASHAZADEH P, et al. Nanomaterial-based biosensors for detection of pathogenic virus. Trends in Analytical Chemistry, 2017, 97: 445.
DOI URL |
| [40] |
YUAN F, XIA Y, LU Q, et al. Recent advances in inorganic functional nanomaterials based flexible electrochemical sensors. Talanta, 2022, 244: 123419.
DOI URL |
| [41] |
ZHONG C, YANG B, JIANG X, et al. Current progress of nanomaterials in molecularly imprinted electrochemical sensing. Critical Reviews in Analytical Chemistry, 2018, 48(1): 15.
DOI PMID |
| [42] |
CHOI H K, LEE M J, SANG N L, et al. Noble metal nanomaterial-based biosensors for electrochemical and optical detection of viruses causing respiratory illnesses. Frontiers in Chemistry, 2021, 9: 672739.
DOI URL |
| [43] |
REZAEI B, BOROUJENI MK, ENSAFI A A. Fabrication of DNA, o-phenylenediamine, and gold nanoparticle bioimprinted polymer electrochemical sensor for the determination of dopamine. Biosensors & Bioelectronics, 2015, 66: 490.
DOI URL |
| [44] |
XIAO T, HUANG J, WANG D, et al. Au and Au-based nanomaterials: synthesis and recent progress in electrochemical sensor applications. Talanta, 2020, 206: 120210.
DOI URL |
| [45] |
JANS H, HUO Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chemical Society Reviews, 2012, 41(7): 2849.
DOI PMID |
| [46] |
TRIPATHY S, SINGH S G. Label-free electrochemical detection of DNA hybridization: a method for COVID-19 diagnosis. Transactions of the Indian National Academy of Engineering, 2020, 5(2): 205.
DOI URL |
| [47] |
KASHEFI-KHEYRABADI L, NGUYEN H V, GO A, et al. Rapid, multiplexed, and nucleic acid amplification-free detection of SARS-CoV-2 RNA using an electrochemical biosensor. Biosensors & Bioelectronics, 2021, 195: 113649.
DOI URL |
| [48] |
LORENZEN A L, DOS SANTOS A M, DOS SANTOS L P, et al. PEDOT-AuNPs-based impedimetric immunosensor for the detection of SARS-CoV-2 antibodies. Electrochimica Acta, 2022, 404: 139757.
DOI URL |
| [49] |
VADLAMANI B S, UPPAL T, VERMA S C, et al. Functionalized TiO2 nanotube-based electrochemical biosensor for rapid detection of SARS-CoV-2. Sensors, 2020, 20(20): 5871.
DOI URL |
| [50] |
ARDUINI F, CINTI S, MAZZARACCHIO V, et al. Carbon black as an outstanding and affordable nanomaterial for electrochemical (bio) sensor design. Biosensors and Bioelectronics, 2020, 156: 112033.
DOI URL |
| [51] |
EISSA S, ZOUROB M. Development of a low-cost cotton-tipped electrochemical immunosensor for the detection of SARS-CoV-2. Analytical Chemistry, 93(3): 1826.
DOI URL |
| [52] |
TORRENTE-RODRÍGUEZ R, LUKAS H, TU J, et al. SARS-CoV-2 rapidplex: a graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter, 2020, 3: 1981.
DOI URL |
| [53] |
LIV L, OBAN G, NAKIBOLU N, et al. A rapid, ultrasensitive voltammetric biosensor for determining SARS-CoV-2 spike protein in real samples. Biosensors & Bioelectronics, 2021, 192: 113497.
DOI URL |
| [54] |
HASHEMI S A, BEHBAHAN N, BAHRANI S, et al. Ultra-sensitive viral glycoprotein detection nanosystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosensors & Bioelectronics, 2021, 171: 112731.
DOI URL |
| [55] | ALI MA, HU C, JAHAN S, et al. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Advanced Materials, 2021, 33(7): 2006647. |
| [56] | WITT S, ROGIEN A, WERNER D, et al. Boron doped diamond thin films for the electrochemical detection of SARS-CoV-2 S1 protein. Diamond and Related Materials, 2021, 4: 108542. |
| [57] |
MEHMANDOUST M, GUMUS Z P, SOYLAK M, et al. Electrochemical immunosensor for rapid and highly sensitive detection of SARS-CoV-2 antigen in the nasal sample. Talanta, 2022, 240: 123211.
DOI URL |
| [58] |
TIAN J, LIANG Z, HU O, et al. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochimica Acta, 2021, 387: 138533.
DOI URL |
| [59] |
YAKOH A, PIMPITAK U, RENGPIPAT S, et al. Paper-based electrochemical biosensor for diagnosing COVID-19: detection of SARS-CoV-2 antibodies and antigen. Biosensors & Bioelectronics, 2020, 176(14): 112912.
DOI URL |
| [60] |
RAZIQ A, KIDAKOVA A, BOROZNJAK R, et al. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosensors & Bioelectronics, 2021, 178: 113029.
DOI URL |
| [61] |
TORRENTE R, LUKAS H, Tu J, et al. SARS-CoV-2 rapidPlex: a graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter, 2020, 3: 1981.
DOI URL |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | CHEN Xi, YUAN Yuan, TAN Yeqiang, LIU Changsheng. Strategic Study on the Development of Inorganic Non-metallic Biomaterials [J]. Journal of Inorganic Materials, 2025, 40(5): 449-456. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [10] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [11] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [12] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [13] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [14] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [15] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||