
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (1): 3-31.DOI: 10.15541/jim20220218
Special Issue: 【信息功能】敏感陶瓷(202506)
• Topical Section: Anti-epidemic Biomaterials (Contributing Editor: YANG Yong) • Previous Articles Next Articles
LI Yanyan1,2( ), PENG Yusi1,2, LIN Chenglong1,2, LUO Xiaoying3, TENG Zheng4(
), PENG Yusi1,2, LIN Chenglong1,2, LUO Xiaoying3, TENG Zheng4( ), ZHANG Xi4, HUANG Zhengren1,2, YANG Yong1,2(
), ZHANG Xi4, HUANG Zhengren1,2, YANG Yong1,2( )
)
Received:2022-04-12
Revised:2022-05-03
Published:2023-01-20
Online:2022-06-22
Contact:
YANG Yong, professor. E-mail: yangyong@mail.sic.ac.cn;About author:LI Yanyan (1997-), female, PhD candidate. E-mail: liyanyan20@mails.ucas.ac.cn
Supported by:CLC Number:
LI Yanyan, PENG Yusi, LIN Chenglong, LUO Xiaoying, TENG Zheng, ZHANG Xi, HUANG Zhengren, YANG Yong. Nanomaterials and Biosensing Technology for the SARS-CoV-2 Detection[J]. Journal of Inorganic Materials, 2023, 38(1): 3-31.
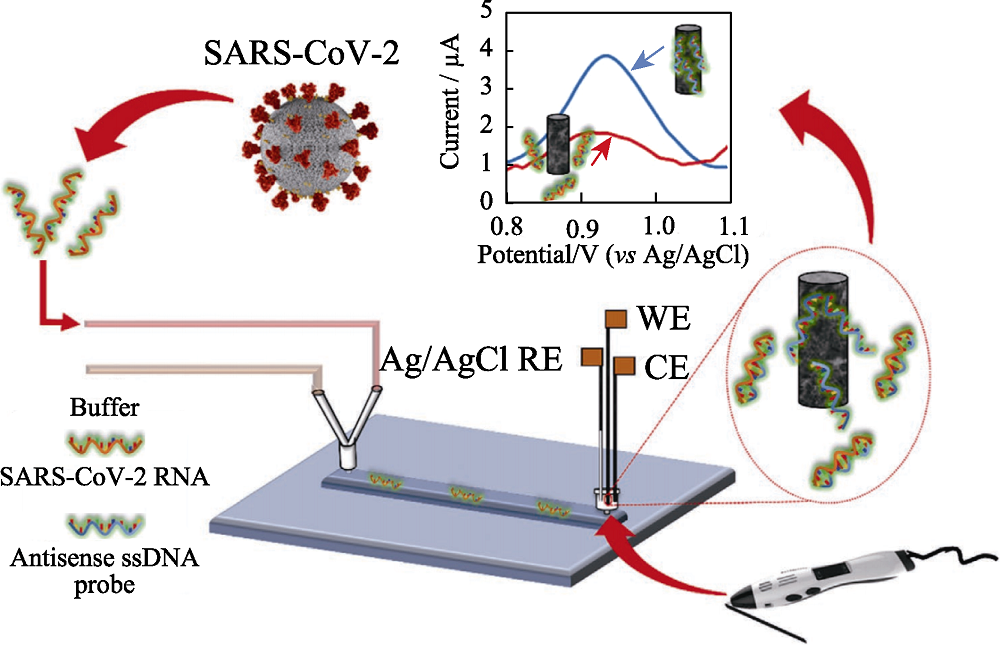
Fig. 3 Scheme of the lab-on-a-chip genosensor for SARS- CoV-2 virus detection[35] WE: working electrode; CE: counter electrode; RE: reference electrode; ssDNA: single strand DNA The color figure can be obtained from online edition
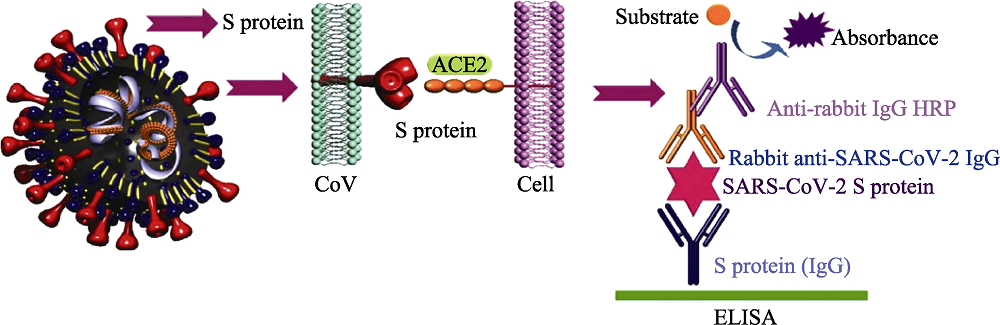
Fig. 7 ELISA method for detection of SARS-CoV-2[16] ACE2: angiotensin converting enzyme 2; HRP: horse radish peroxidase; ELISA: enzyme linked immunosorbent assay The color figure can be obtained from online edition
| Object sample | Characteristic | Detection technology | Advantage | Disadvantage |
|---|---|---|---|---|
| RNA | 1. Target: the gene sequences of SARS-CoV-2 2. Great producibility 3. Long detection period 4. Possibility of being contaminated and false positive result | Whole genome sequencing | 1. High accuracy and sensitivity 2. Reflecting genetic information of pathogen comprehensively | 1. Expensive special instruments 2. Relying on professionals 3. Difficulty in detection on a large scale |
| RT-qPCR | 1. High sensitivity and specificity 2. Low cost | 1. Long amplification time 2. High requirements of equipment 3. Complex operation | ||
| LAMP | 1. Isothermal reaction 2. High efficiency and speed 3. High sensitivity and visualization | 1. Complex design of primer 2. Low specificity | ||
| Microfluidic chip | 1. Multiple detection of pathogens 2. Integration of sample preparation and detection 3. Ability in automate analysis | Difficulty in chip design, material selection, processing, packaging, and storage | ||
| ddPCR | 1. High sensitivity and lowest limitation of detection 2. Facilitation and high degree of automation 3. Quantitative detection | 1. Small reaction volume 2. Expensive equipment and reagents | ||
| CRISPR | 1. High speed and low cost 2. High sensitivity 3. Strong system stability 4. On-site detection | The accuracy of detection needs to be verified | ||
| Antibodies | 1. Target: human antibodies stimulated by SARS-CoV-2 2. Easy sample collection and low detection threshold 3. Simple operation and high throughput 4. Limitation of timeframe 5. Lower sensitivity and specificity than those of nucleic acid detection | ELISA | 1. Low difficulty of standardization of carrier 2. High sensitivity and specificity 3. Simple equipment | 1. Long detection time and cumbersome steps 2. Limited single detection throughout |
| LFIA (Colloidal gold method) | 1. On-site detection caused by easy operation 2. High sensitivity and speed 3. Low cost 4. Mass production | 1. Only qualitative analysis 2. Different reproducibility of different batches of products | ||
| CLIA | 1. High sensitivity and specificity 2. High throughput detection and high degree of automation | 1. Special instrument 2. High detection cost | ||
| Antigen | 1. Target: SARS-CoV-2 antigen 2. Simple and fast operation | LFIA (Colloidal gold method) | 1. Fast and facile operation 2. Visualization 3. On-site detection and large-scale population screening | Low sensitivity |
Table 1 Comparison of conventional detection methods for SARS-CoV-2
| Object sample | Characteristic | Detection technology | Advantage | Disadvantage |
|---|---|---|---|---|
| RNA | 1. Target: the gene sequences of SARS-CoV-2 2. Great producibility 3. Long detection period 4. Possibility of being contaminated and false positive result | Whole genome sequencing | 1. High accuracy and sensitivity 2. Reflecting genetic information of pathogen comprehensively | 1. Expensive special instruments 2. Relying on professionals 3. Difficulty in detection on a large scale |
| RT-qPCR | 1. High sensitivity and specificity 2. Low cost | 1. Long amplification time 2. High requirements of equipment 3. Complex operation | ||
| LAMP | 1. Isothermal reaction 2. High efficiency and speed 3. High sensitivity and visualization | 1. Complex design of primer 2. Low specificity | ||
| Microfluidic chip | 1. Multiple detection of pathogens 2. Integration of sample preparation and detection 3. Ability in automate analysis | Difficulty in chip design, material selection, processing, packaging, and storage | ||
| ddPCR | 1. High sensitivity and lowest limitation of detection 2. Facilitation and high degree of automation 3. Quantitative detection | 1. Small reaction volume 2. Expensive equipment and reagents | ||
| CRISPR | 1. High speed and low cost 2. High sensitivity 3. Strong system stability 4. On-site detection | The accuracy of detection needs to be verified | ||
| Antibodies | 1. Target: human antibodies stimulated by SARS-CoV-2 2. Easy sample collection and low detection threshold 3. Simple operation and high throughput 4. Limitation of timeframe 5. Lower sensitivity and specificity than those of nucleic acid detection | ELISA | 1. Low difficulty of standardization of carrier 2. High sensitivity and specificity 3. Simple equipment | 1. Long detection time and cumbersome steps 2. Limited single detection throughout |
| LFIA (Colloidal gold method) | 1. On-site detection caused by easy operation 2. High sensitivity and speed 3. Low cost 4. Mass production | 1. Only qualitative analysis 2. Different reproducibility of different batches of products | ||
| CLIA | 1. High sensitivity and specificity 2. High throughput detection and high degree of automation | 1. Special instrument 2. High detection cost | ||
| Antigen | 1. Target: SARS-CoV-2 antigen 2. Simple and fast operation | LFIA (Colloidal gold method) | 1. Fast and facile operation 2. Visualization 3. On-site detection and large-scale population screening | Low sensitivity |
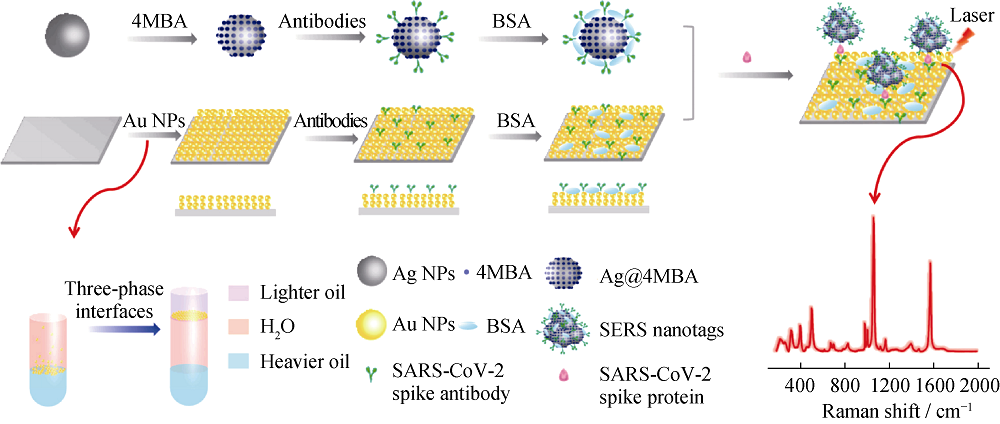
Fig. 8 Schematic illustration of the SERS-based immunoassay[77] MBA: thiosalicylic acid; BSA: bovine serum albumin; NPs: nanoparticles The color figure can be obtained from online edition
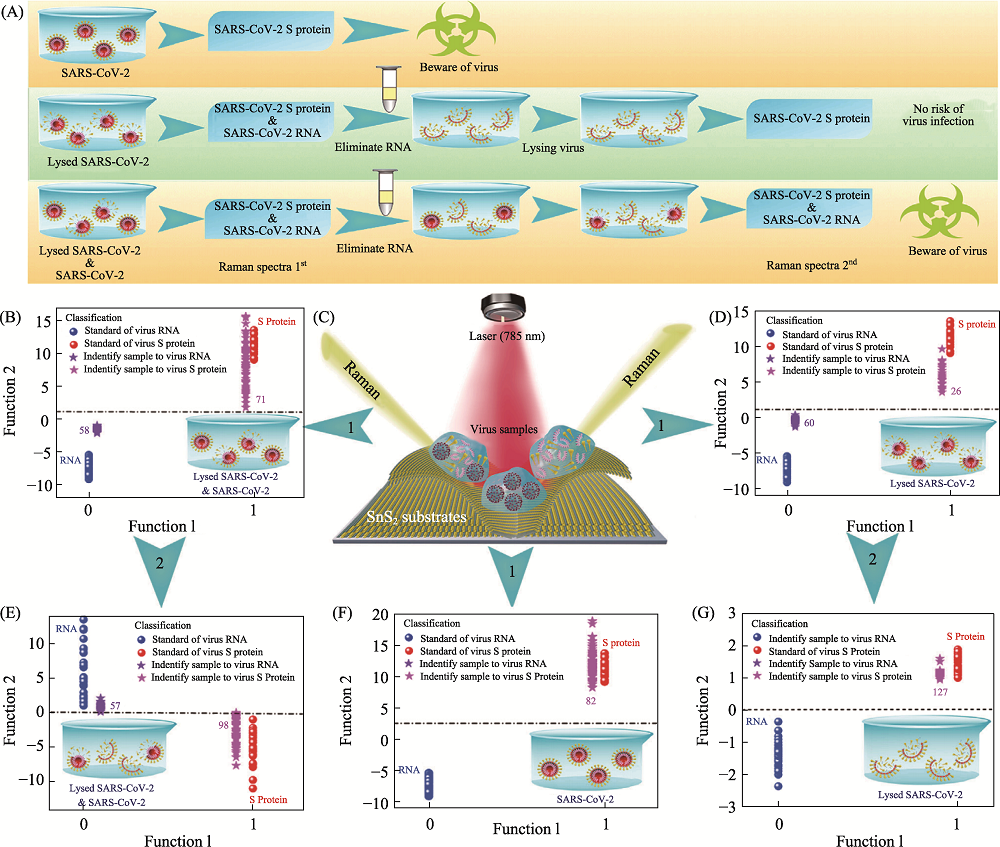
Fig. 10 Application of SnS2 microspheres for diagnosing the infectiousness of SARS-CoV-2[80] (A) Experimental procedure for diagnosing the infectiousness of SARS-CoV-2; (B) SVM analysis results to identify the mixture of the SARS-CoV-2 with complete viral structure and the lysed SARS-CoV-2; (C) Raman scattering diagram of three contamination situations of the novel coronavirus based on SnS2 substrates; (D) SVM analysis results to identify the lysed SARS-CoV-2; (E) SVM analysis results to identify the mixture of the SARS-CoV-2 with complete viral structure and the lysed SARS-CoV-2 after eliminating RNA and relysing virus samples; (F) SVM analysis results to identify the SARS-CoV-2 with complete viral structure; (G) SVM analysis results to identify the lysed SARS-CoV-2 after eliminating RNA and relysing virus samples. SVM: support vector machine The color figure can be obtained from online edition
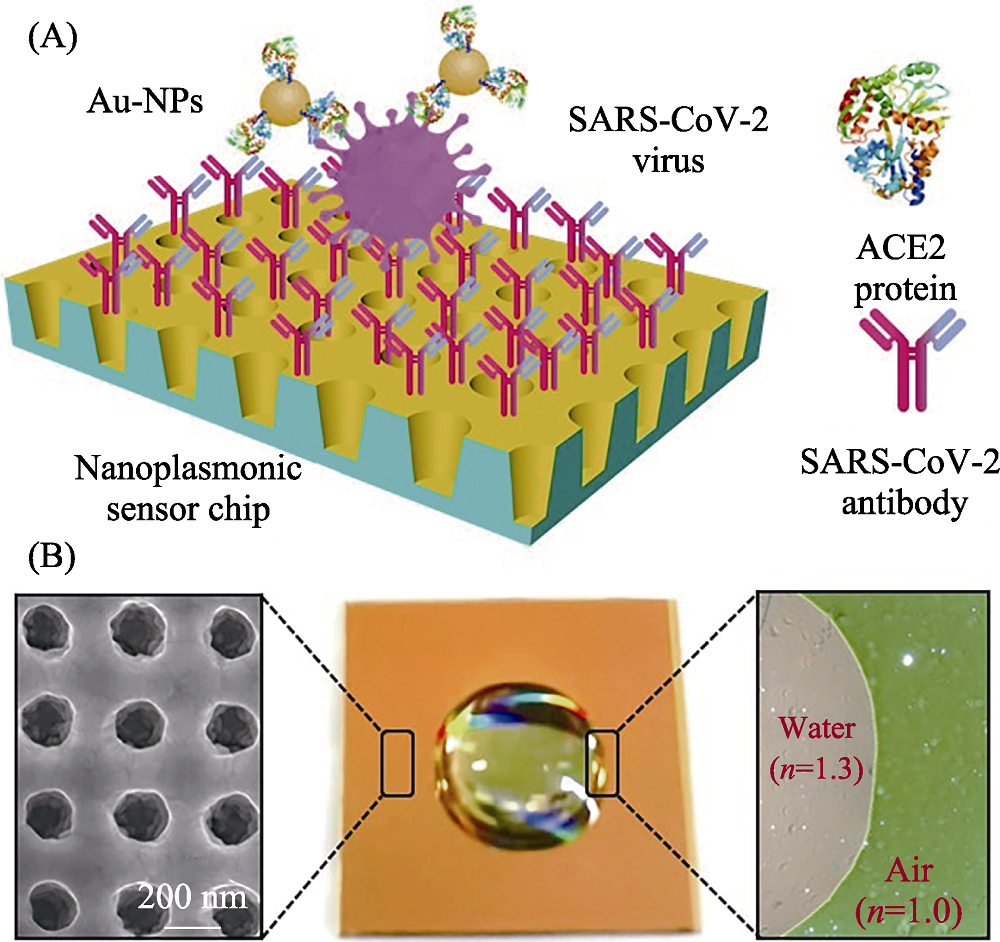
Fig. 11 Schematic diagram of nano-plasma optic sensor for detection of SARS-CoV-2[89] (A) Schematic diagram of the nanoplasmonic resonance sensor for determination of SARS-CoV-2 pseudovirus concentration; (B) Photograph (middle) of one piece of Au nanocup array chip with a drop of water on top The color figure can be obtained from online edition
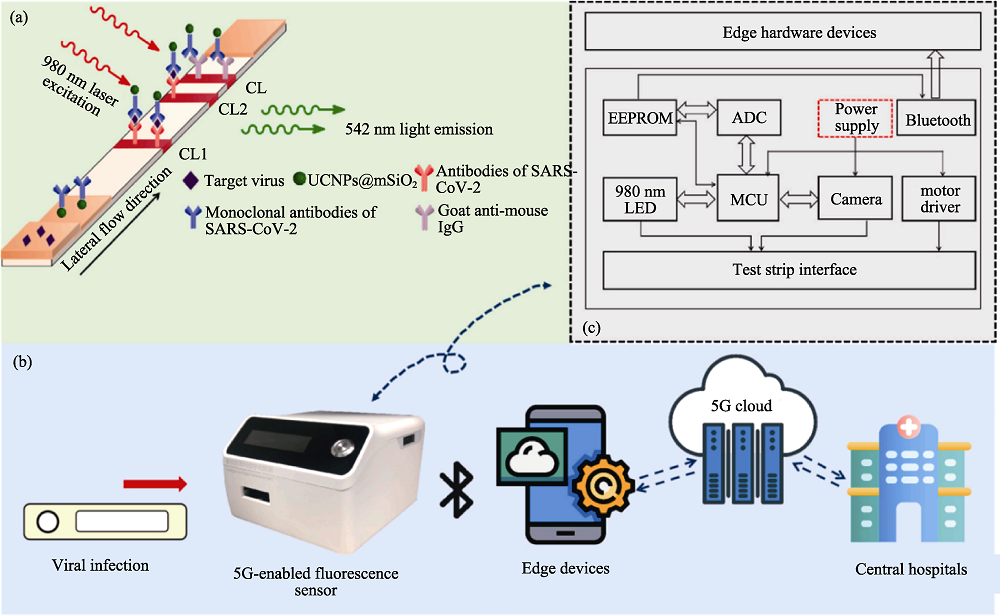
Fig. 12 SARS-CoV-2 detection based on 5G-enabled fluorescence biosensor[100] (a) The principle of the UCNPs based lateral flow assay in detection of SARS-CoV-2; (b) The working process of the proposed 5G-enabled fluorescence sensor; (c) The circuit configuration and hardware composition of the fluorescence sensor; CL: control line; TL1: test line 1; TL2: test line 2; UCNPs: up-conversion nanoparticles; EEPROM: electrically erasable programmable read only memory; ADC: application data center; MCU: motor control unit The color figure can be obtained from online edition
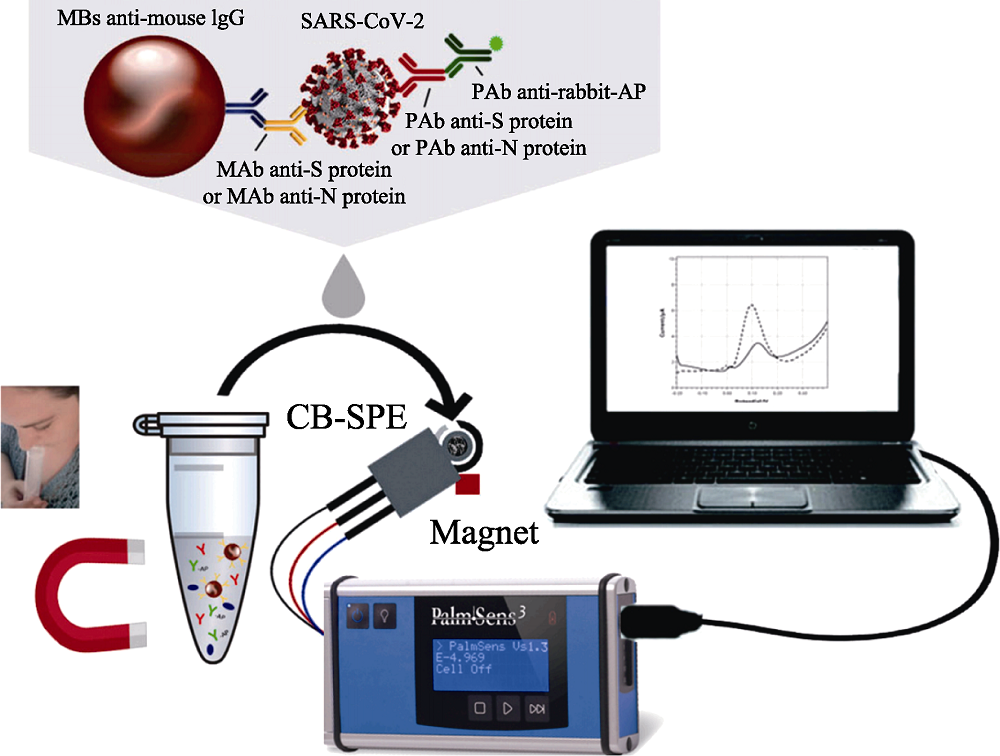
Fig. 13 Magnetic beads-based electrochemical assay for SARS-CoV-2 detection in untreated saliva[126] MBs: magnetic beads; MAb: monoclonal antibody; PAb: polyclonal antibody; AP: alkaline phosphatase; CB-SPE: carbon-based screen-printed electrodes The color figure can be obtained from online edition
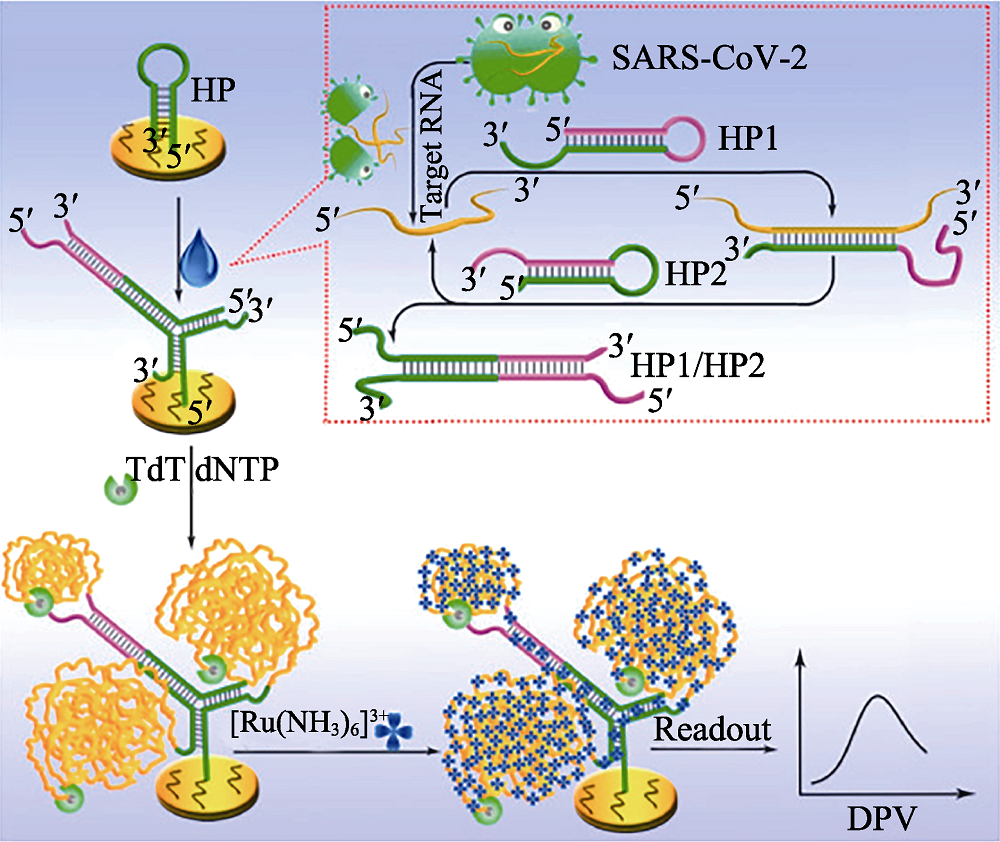
Fig. 14 Principle of the proposed electrochemical biosensor for sensitive analysis of SARS-CoV-2 RNA[136] HP: hairpin; TdT: terminal deoxynucleotidyl transferase; dNTP: deoxyribonucleotides; DPV: differential pulse voltammetry The color figure can be obtained from online edition
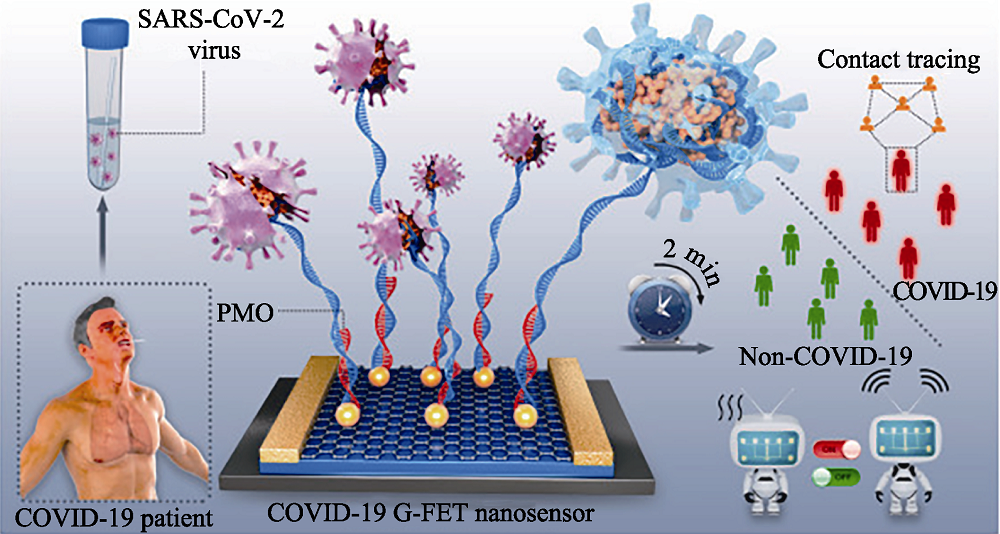
Fig. 15 Schematic diagram of rapid direct identification of SARS-CoV-2 using PMO-functionalized G-FET nano-sensors[148] G-FET: graphene field-effect transistor; PMO: phosphorodiamidate morpholino oligos The color figure can be obtained from online edition
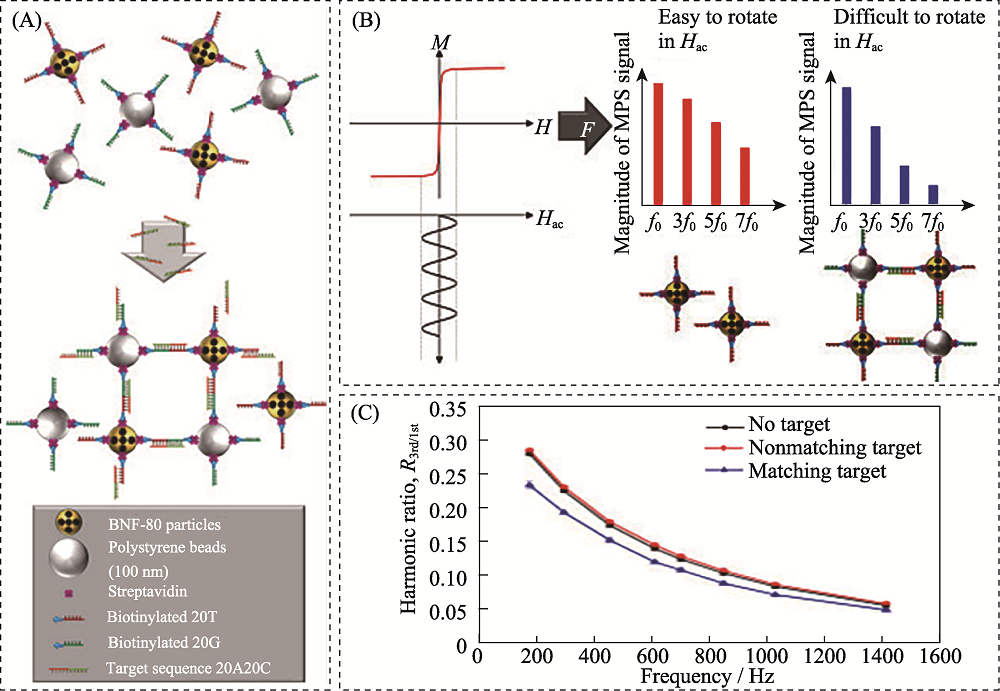
Fig. 16 Schematic diagram of detection of SARS-CoV-2 RNA based on magnetic particle spectroscopy biosensors[166] (A) Magnetic nanoparticles (gold) and polystyrene beads (silver) with streptavidin (purple)-modified surface are equipped with single stranded DNA strands (red and green, respectively) with a specific sequence via biotin-streptavidin-binding; (B) Applying a sinusoidal magnetic field (black) to a solution of nanoparticles results in reorientation of the nanoparticles which can be readout by measuring the magnetic response M of the nanoparticles; (C) Exemplary spectrum of the ratio of received harmonics as a function of excitation frequencies for 80 nm BNF magnetic particles The color figure can be obtained from online edition
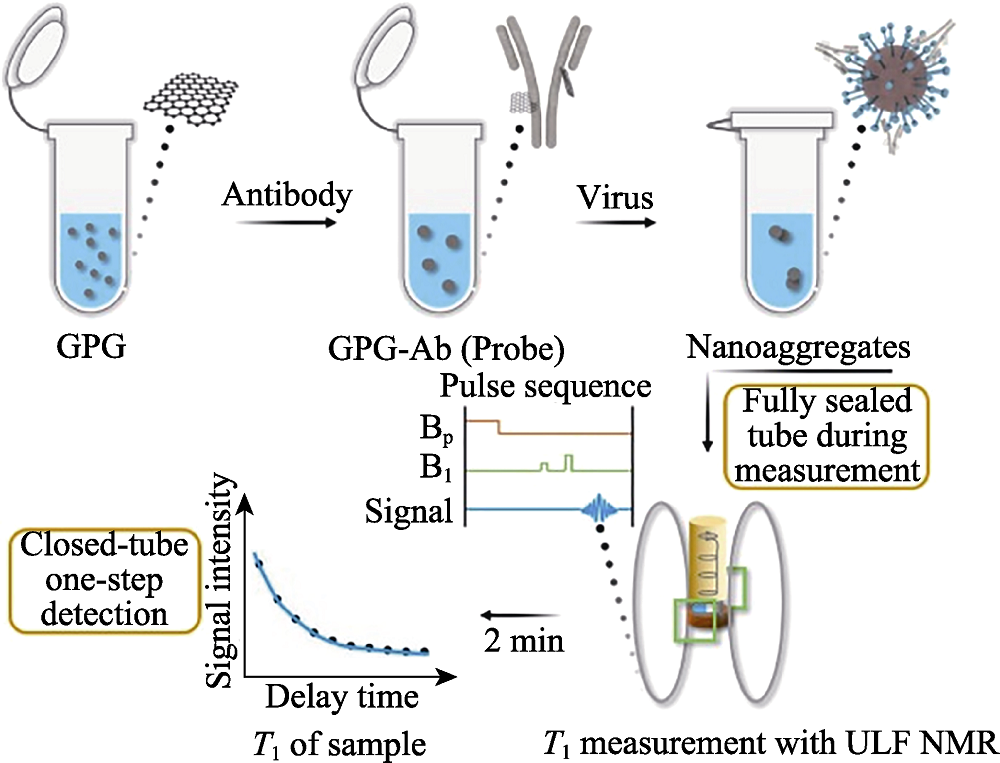
Fig. 17 Detection process of SARS-CoV-2 of the magnetic relaxation switches assay with ULF NMR[169] ULF: ultra-low field; GPG: Gd3+ loaded PEG modified GQDs; GQDs: graphene quantum dots The color figure can be obtained from online edition
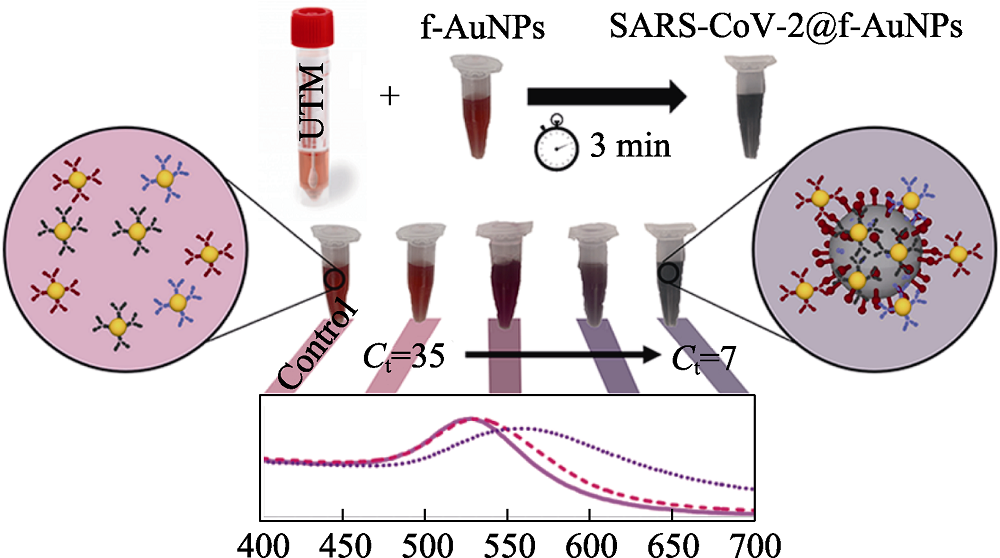
Fig. 18 Schematic diagram of the detection of SARS-CoV-2 based on colorimetric biosensors[187] UTM: universal transport medium The color figure can be obtained from online edition
| Detection technology | Detection method | Object | Sample | Related material | Detection time | Lower detection limit | Ref. |
|---|---|---|---|---|---|---|---|
| SERS-based biosensors | Labelled-SERS | S protein | Lysis solution | Macro/nanostructure Au substrate | 15 min | 10 PFU/mL | [ |
| Label-free SERS | Virus particles | Nasal/throat solution | Macro/nanostructure Au substrate, Au nanoparticles | 15 min | 60 copies/mL | [ | |
| SPR-based biosensors | Combining SPR and LSPR | Pseudovirus particles | N/A | Macro/nanostructure Au substrate, Au nanoparticles | 15 min | 370 vp/mL | [ |
| Fluorescence biosensors | “signal on” mode | RNA | Lysis solution | N/A | 15 samples/ 45 min | 600 copies/mL | [ |
| Electrochemical biosensors | Voltammetric/ amperometric biosensors | RNA | Nasal/throat solution | Au nanoparticles | 5 min | 6900 copies/mL | [ |
| Impedimetric biosensors | Antibodies | Serum | Au nanoparticles | 30 min | N/A | [ | |
| Potentiometric biosensors | Cholinesterase | Blood | Graphene and copper | ~7 s (only detection time) | 7.9 × 10-8 mol/L | [ | |
| FET-based biosensors | RNA | Nasal/throat solution | Graphene | 1 min (only detection time) | 10-20 copies/mL | [ | |
| Magnetic biosensors | Magnetoresistance | Antibodies | Blood | Magnetic nanoparticles | 10 min | 5-10 ng/mL | [ |
| Magnetic particle spectroscopy platforms | S protein and N protein | PBS | Magnetic nanoparticles | N/A | 1.56 nmol/L | [ | |
| Nuclear magnetic resonance | Antibodies | Blood | Magnetic graphene quantum dot | 2 min | 248 vp/mL | [ | |
| Colorimetric biosensors | Agglomeration of nanoparticles | RNA | N/A | Au nanoparticles | >45 min | 160 fmol/L | [ |
Table 2 Comparison of novel biosensors for SARS-CoV-2 detection
| Detection technology | Detection method | Object | Sample | Related material | Detection time | Lower detection limit | Ref. |
|---|---|---|---|---|---|---|---|
| SERS-based biosensors | Labelled-SERS | S protein | Lysis solution | Macro/nanostructure Au substrate | 15 min | 10 PFU/mL | [ |
| Label-free SERS | Virus particles | Nasal/throat solution | Macro/nanostructure Au substrate, Au nanoparticles | 15 min | 60 copies/mL | [ | |
| SPR-based biosensors | Combining SPR and LSPR | Pseudovirus particles | N/A | Macro/nanostructure Au substrate, Au nanoparticles | 15 min | 370 vp/mL | [ |
| Fluorescence biosensors | “signal on” mode | RNA | Lysis solution | N/A | 15 samples/ 45 min | 600 copies/mL | [ |
| Electrochemical biosensors | Voltammetric/ amperometric biosensors | RNA | Nasal/throat solution | Au nanoparticles | 5 min | 6900 copies/mL | [ |
| Impedimetric biosensors | Antibodies | Serum | Au nanoparticles | 30 min | N/A | [ | |
| Potentiometric biosensors | Cholinesterase | Blood | Graphene and copper | ~7 s (only detection time) | 7.9 × 10-8 mol/L | [ | |
| FET-based biosensors | RNA | Nasal/throat solution | Graphene | 1 min (only detection time) | 10-20 copies/mL | [ | |
| Magnetic biosensors | Magnetoresistance | Antibodies | Blood | Magnetic nanoparticles | 10 min | 5-10 ng/mL | [ |
| Magnetic particle spectroscopy platforms | S protein and N protein | PBS | Magnetic nanoparticles | N/A | 1.56 nmol/L | [ | |
| Nuclear magnetic resonance | Antibodies | Blood | Magnetic graphene quantum dot | 2 min | 248 vp/mL | [ | |
| Colorimetric biosensors | Agglomeration of nanoparticles | RNA | N/A | Au nanoparticles | >45 min | 160 fmol/L | [ |
| [1] |
YUCE M, FILIZTEKIN E, OZKAYA K G. COVID-19 diagnosis-a review of current methods. Biosensors and Bioelectronics, 2021, 172: 112752.
DOI URL |
| [2] |
JI T X, LIU Z W, WANG G Q, et al. Detection of COVID-19: a review of the current literature and future perspectives. Biosensors and Bioelectronics, 2020, 166: 112455.
DOI URL |
| [3] |
XUE X, BALL J K, ALEXANDER C, et al. All surfaces are not equal in contact transmission of SARS-CoV-2. Matter, 2020, 3(5): 1433.
DOI PMID |
| [4] |
CHEN W, CAI B, GENG Z, et al. Reducing false negatives in COVID-19 testing by using microneedle-based oropharyngeal swabs. Matter, 2020, 3(5): 1589.
DOI PMID |
| [5] |
CUI F Y, ZHOU H S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosensors and Bioelectronics, 2020, 165: 112349.
DOI URL |
| [6] | LIN D C, LIU L, ZHANG M X, et al. Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. European Journal of Clinical Microbiology & Infectious Diseases, 2020, 39(12): 2271. |
| [7] |
SEO G, LEE G, KIM M J, et al. Correction to rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano, 2020, 14(9): 12257.
DOI PMID |
| [8] |
YANG Y, PENG Y S, LIN C L, et al. Human ACE2-functionalized gold “virus-trap” nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano-Micro Letters, 2021, 13: 109.
DOI URL |
| [9] |
YAO H P, SONG Y T, CHEN Y, et al. Molecular architecture of the SARS-CoV-2 virus. Cell, 2020, 183(3): 730.
DOI PMID |
| [10] |
ZHOU P, YANG X L, WANG X G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 2020, 579(7798): 270.
DOI URL |
| [11] |
LI W H, MOORE J M, VASILIEVA N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 2003, 426: 450.
DOI URL |
| [12] |
HOFMANN H, PYRC K, HOEK V D L, et al. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(22): 7988.
DOI PMID |
| [13] |
LU G W, HU Y W, WANG Q H, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature, 2013, 500(7461): 227.
DOI URL |
| [14] |
RAJ V S, MOU H, SMITS S L, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature, 2013, 495 (7440): 251.
DOI URL |
| [15] |
MENACHERY V D, YOUNT B L JR, DEBBINK K, et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nature Medicine, 2015, 21(12): 1508.
DOI PMID |
| [16] |
OROOJI Y, SOHRABI H, HEMMAT N, et al. An overview on SARS-CoV-2 (COVID-19) and other human coronaviruses and their detection capability via amplification assay, chemical sensing, biosensing, immunosensing, and clinical assays. Nano-Micro Letters, 2021, 13: 18.
DOI URL |
| [17] |
TO K K W, TSANG O T Y, LEUNG W S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. The Lancet Infectious Diseases, 2020, 20(5): 565.
DOI URL |
| [18] |
DONG X, CAO Y Y, LU X X, et al. Eleven faces of coronavirus disease 2019. Allergy, 2020, 75(7): 1699.
DOI PMID |
| [19] | CORMAN V M, LANDT O, KAISER M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillnace, 2020, 25(3): 23. |
| [20] |
LU R J, ZHAO X, LI J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet, 2020, 395(10224): 565.
DOI URL |
| [21] |
REN L L, WANG Y M, WU Z Q, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chinese Medical Journal, 2020, 133(9): 1015.
DOI URL |
| [22] |
PEIRIS S M J, PHIL D, YUEN Y K, et al. The severe acute respiratory syndrome. The New England Journal of Medicine, 2003, 349: 2431.
DOI PMID |
| [23] | CHEN L J, LIU W Y, ZHANG Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerging Microbes & Infections, 2020, 9(1): 313. |
| [24] |
SHI M, ZHANG Y Z, HOLMES E C. Meta-transcriptomics and the evolutionary biology of RNA viruses. Virus Research, 2018, 243: 83.
DOI PMID |
| [25] |
SAKAMOTO Y, SEREEWATTANAWOOT S, SUZUKI A. A new era of long-read sequencing for cancer genomics. Journal of Human Genetics, 2020, 65(1): 3.
DOI PMID |
| [26] |
WANG M, FU A S, HU B, et al. Nanopore targeted sequencing for the accurate and comprehensive detection of SARS-CoV-2 and other respiratory viruses. Small, 2020, 16: 2002169.
DOI URL |
| [27] |
NOTOMI T, OKAYAMA H, MASUBUCHI H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Research, 2000, 28: 63.
PMID |
| [28] |
ZHU X, WANG X X, HAN L M, et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosensors and Bioelectronics, 2020, 166: 112437.
DOI URL |
| [29] |
ZHUANG J J, YIN J X, LV S W, et al. Advanced "lab-on-a-chip" to detect viruses-current challenges and future perspectives. Biosensors and Bioelectronics, 2020, 163: 112291.
DOI URL |
| [30] |
WANG L J, PUMERA M. Recent advances of 3D printing in analytical chemistry: focus on microfluidic, separation, and extraction devices. TRAC Trends in Analytical Chemistry, 2021, 135: 116151.
DOI URL |
| [31] |
WANG G H, TAN J, TANG M H, et al. Binary centrifugal microfluidics enabling novel, digital addressable functions for valving and routing. Lab on a Chip, 2018, 18(8): 1141.
DOI URL |
| [32] |
CHEN J J, KANG Z W, WANG G H, et al. Optofluidic guiding, valving, switching and mixing based on plasmonic heating in a random gold nanoisland substrate. Lab on a Chip, 2015, 15(11): 2504.
DOI PMID |
| [33] |
TANG M H, LOO J F, WANG Y Y, et al. Motor-assisted chip-in-a-tube (MACT): a new 2- and 3-dimensional centrifugal microfluidic platform for biomedical applications. Lab on a Chip, 2017, 17(3): 474.
DOI PMID |
| [34] | TANG M H, WANG G H, KONG S K, et al. A review of biomedical centrifugal microfluidic platforms. Micromachines (Basel), 2016, 7: 26. |
| [35] |
CREVILLEN A G, MAYORGA-MARTINEZ C C, VAGHASIYA J V, et al. 3D-Printed SARS-CoV-2 RNA genosensing microfluidic system. Advanced Materials Technologies, 2022, 7(6): 2101121.
DOI URL |
| [36] |
WIENCKE J K, BRACCI P M, HSUANG G, et al. A comparison of DNA methylation specific droplet digital PCR (ddPCR) and real time qPCR with flow cytometry in characterizing human T cells in peripheral blood. Epigenetics, 2014, 9(10): 1360.
DOI PMID |
| [37] |
CHEN B, JIANG Y F, CAO X H, et al. Droplet digital PCR as an emerging tool in detecting pathogens nucleic acids in infectious diseases. Clinica Chimica Acta, 2021, 517: 156.
DOI PMID |
| [38] |
SEDLAK R H, JEROME K R. Viral diagnostics in the era of digital polymerase chain reaction. Diagnostic Microbiology and Infectious Disease, 2013, 75(1): 1.
DOI PMID |
| [39] |
DONG L H, ZHOU J B, NIU C Y, et al. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta, 2021, 224: 121726.
DOI URL |
| [40] | SUO T, LIU X J, GUO M, et al. ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerging Microbes & Infections, 2020, 9: 1259. |
| [41] | HOU T Y, ZENG W Q, YANG M L, et al. Development and evaluation of a rapid CRISPR-based diagnostic for COVID-19. PLoS Pathogens, 2020, 16(8): e1008705. |
| [42] |
JOUNG J, LADHA A, SAITO M, et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. MedRxiv, 2020, 10.1101/2020.05.04.20091231
DOI |
| [43] | 上海交大人投身一线众志成城科技战“疫”, 上海交通大学·新闻学术网, 2020-02-05, https://news.sjtu.edu.cn/jdyw/20200204/119621.html. |
| [44] |
CHEN J S, MA E, HARRINGTON L B, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science, 2018, 360(6387): 436.
DOI PMID |
| [45] |
BROUGHTON J P, DENG X D, YU G X, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nature Biotechnology, 2020, 38(7): 870.
DOI PMID |
| [46] | HOU H Y, WANG T, ZHANG B, et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clinical Translational Immunology, 2020, 9(5): 01136. |
| [47] | LIU W B, LIU L, KOU G M, et al. Evaluation of nucleocapsid and spike protein-based enzymeLinked immunosorbent assays for detecting antibodies against SARS-CoV-2. Journal of Clinical Microbiology, 2020, 58(6): 00461. |
| [48] |
AMANAT F, STADLBAUER D, STROHMEIER S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nature Medicine, 2020, 26(7): 1033.
DOI PMID |
| [49] | 葛均波院士团队联合研发 SARS-CoV-2 IgM 抗体快检试剂盒通过注册检验,新民晚报, 2020-2-18, https://baijiahao.baidu.com/s?id=1658849335805344872&wfr=spider&for=pc. |
| [50] |
DUAN D M, FAN K L, ZHANG D X, et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosensors and Bioelectronics, 2015, 74: 134.
DOI PMID |
| [51] | WANG H H, LI X M, LI T, et al. The genetic sequence, origin, and diagnosis of SARS-CoV-2. European Journal of Clinical Microbiology & Infectious Diseases, 2020, 39(9): 1629. |
| [52] | ALBERT E, TORRES I, BUENO F, et al. Field evaluation of a rapid antigen test (Panbio COVID-19 Ag rapid test device) for COVID-19 diagnosis in primary healthcare centres. Clinical Microbiology and Infection, 2021, 27(3): 472. |
| [53] |
BARO B, RODO P, OUCHI D, et al. Performance characteristics of five antigen-detecting rapid diagnostic test (Ag-RDT) for SARS-CoV-2 asymptomatic infection: a head-to-head benchmark comparison. Journal of Infection, 2021, 82(6): 269.
DOI PMID |
| [54] |
SEREBRENNIKOVA K V, BYZOVA N A, ZHERDEV A V, et al. Lateral flow immunoassay of SARS-CoV-2 antigen with SERS-based registration: development and comparison with traditional immunoassays. Biosensors, 2021, 11(12): 510.
DOI URL |
| [55] | 国家药监局应急审批2款SARS-CoV-2抗原检测产品广东企业上榜,潇湘晨报, 2020-11-24, https://baijiahao.baidu.com/s?id=1684237376273195168&wfr=spider&for=pc. |
| [56] |
PENG Y S, LIN C L, LONG L, et al. Charge-transfer resonance and electromagnetic enhancement synergistically enabling MXenes with excellent SERS sensitivity for SARS-CoV-2 S protein detection. Nano-Micro Letters, 2021, 13: 52.
DOI PMID |
| [57] |
MORAIS L M C, PARASKEVAIDI M, CUI L, et al. Standardization of complex biologically derived spectrochemical datasets. Nature Protocols, 2019, 14: 1546.
DOI PMID |
| [58] |
YANG L L, YANG Y, MA Y F, et al. Fabrication of semiconductor ZnO nanostructures for versatile SERS application. Nanomaterials (Basel), 2017, 7: 398.
DOI URL |
| [59] |
SHAN Y F, YANG Y, CAO Y Q, et al. Synthesis of wheatear-like ZnO nanoarrays decorated with Ag nanoparticles and its improved SERS performance through hydrogenation. Nanotechnology, 2016, 27(14): 145502.
DOI URL |
| [60] |
YANG Y, NOGAMI M, SHI J L, et al. Self-assembled semiconductor capped metal composite nanoparticles embedded in BaTiO3 thin films for nonlinear optical applications. Journal of Materials Chemistry, 2003, 13(12): 3026.
DOI URL |
| [61] |
YANG L L, PENG Y S, YANG Y, et al. Green and sensitive flexible semiconductor SERS substrates: hydrogenated black TiO2 nanowires. ACS Applied Nano Materials, 2018, 1(9): 4516.
DOI URL |
| [62] |
KIM H, KANG H, KIM H N, et al. Development of 6E3 antibody-mediated SERS immunoassay for drug-resistant influenza virus. Biosensors and Bioelectronics, 2021, 187: 113324.
DOI URL |
| [63] |
WANG C W, WANG C G, WANG X L, et al. Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses. ACS Applied Materials Interfaces, 2019, 11(21): 19495.
DOI URL |
| [64] |
WANG J F, WU X Z, WANG C W, et al. Facile synthesis of Au-coated magnetic nanoparticles and their application in bacteria detection via a SERS method. ACS Applied Materials Interfaces, 2016, 8(31): 19958.
DOI URL |
| [65] |
WANG C G, LIU M, WANG Z F, et al. Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today, 2021, 37: 101092.
DOI URL |
| [66] |
PRAMANIK A, GAO Y, PATIBANDLA S, et al. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Advances, 2021, 3(6): 1588.
DOI PMID |
| [67] |
ZHANG D Y, ZHANG X L, MA R, et al. Ultra-fast and onsite interrogation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in waters via surface enhanced Raman scattering (SERS). Water Research, 2021, 200: 117243.
DOI URL |
| [68] |
LIN C L, LIANG S S, PENG Y S, et al. Visualized SERS imaging of single molecule by Ag/black phosphorus nanosheets. Nano-Micro Letters, 2022, 14: 75.
DOI PMID |
| [69] |
ZAVYALOVA E, AMBARTSUMYAN O, ZHDANOV G, et al. SERS-based aptasensor for rapid quantitative detection of SARS-CoV-2. Nanomaterials (Basel), 2021, 11: 1394.
DOI URL |
| [70] |
WU Y X, DANG H J, PARK S G, et al. SERS-PCR assays of SARS-CoV-2 target genes using Au nanoparticles-internalized Au nanodimple substrates. Biosensors and Bioelectronics, 2022, 197: 113736.
DOI URL |
| [71] |
SANCHEZ J E, JARAMILLO S A, SETTLES E, et al. Detection of SARS-CoV-2 and its S and N proteins using surface enhanced Raman spectroscopy. RSC Advances, 2021, 11(41): 25788.
DOI PMID |
| [72] |
LI Y Y, LIN C L, PE Y S. High-sensitivity and point-of-care detection of SARS-CoV-2 from throat and nasal swabs by magnetic SERS biosensor. Sensors and Actuators B: Chemical, 2022, 365: 131974.
DOI URL |
| [73] |
CHEN H, PARK S G, CHOI N, et al. Sensitive detection of SARS-CoV-2 using a SERS-based aptasensor. ACS Sensors, 2021, 6(6): 2378.
DOI PMID |
| [74] |
LEONG S X, LEONG Y X, TAN E X, et al. Noninvasive and point-of-care surface-enhanced Raman scattering (SERS)-based breathalyzer for mass screening of coronavirus disease 2019 (COVID-19) under 5 min. ACS Nano, 2022, 16(2): 2629.
DOI PMID |
| [75] |
PARIA D, KWOK K S, RAJ P, et al. Label-free spectroscopic SARS-CoV-2 detection on versatile nanoimprinted substrates. Nano Letters, 2022, 22(9): 3620.
DOI URL |
| [76] |
LI J R, WUETHRICH A, EDWARDRAJA S, et al. Amplification-free SARS-CoV-2 detection using nanoyeast-scFv and ultrasensitive plasmonic nanobox-integrated nanomixing microassay. Analytical Chemistry, 2021, 93(29): 10251.
DOI PMID |
| [77] |
ZHANG M L, LI X D, PAN J L, et al. Ultrasensitive detection of SARS-CoV-2 spike protein in untreated saliva using SERS-based biosensor. Biosensors and Bioelectronics, 2021, 190: 113421.
DOI URL |
| [78] |
DAOUDI K, RAMACHANDRAN K, ALAWADHI H, et al. Ultra-sensitive and fast optical detection of the spike protein of the SARS-CoV-2 using AgNPs/SiNWs nanohybrid based sensors. Surfaces and Interfaces, 2021, 27: 101454.
DOI URL |
| [79] |
YANG Y, TANEMURA M, HUANG Z R, et al. Aligned gold nanoneedle arrays for surface-enhanced Raman scattering. Nanotechnology, 2010, 21: 325701.
DOI URL |
| [80] |
PENG Y S, LIN C L, LI Y Y, et al. Identifying infectiousness of SARS-CoV-2 by ultra-sensitive SnS2 SERS biosensors with capillary effect. Matter, 2022, 5(2): 694.
DOI URL |
| [81] |
MARQUES A C, PINHEIRO T, MORAIS M, et al. Bottom-up microwave-assisted seed-mediated synthesis of gold nanoparticles onto nanocellulose to boost stability and high performance for SERS applications. Applied Surface Science, 2021, 561: 150060.
DOI URL |
| [82] |
SITJAR J, XU H Z, LIU C Y, et al. Synergistic surface-enhanced Raman scattering effect to distinguish live SARS-CoV-2 S pseudovirus. Analytica Chimica Acta, 2022, 1193: 339406.
DOI URL |
| [83] |
CHEN H, PARK S K, JOUNG Y, et al. SERS-based dual-mode DNA aptasensors for rapid classification of SARS-CoV-2 and influenza A/H1N1 infection. Sensors and Actuators: B. Chemical, 2022, 355: 131324.
DOI URL |
| [84] |
CHEN C, WANG J S. Optical biosensors: an exhaustive and comprehensive review. Analyst, 2020, 145(5): 1605.
DOI PMID |
| [85] |
FIRDOUS S, ANWAR S, RAFYA R. Development of surface plasmon resonance (SPR) biosensors for use in the diagnostics of malignant and infectious diseases. Laser Physics Letters, 2018, 15: 065602.
DOI URL |
| [86] |
LYNN N S, DANDY D S. Passive microfluidic pumping using coupled capillary/evaporation effects. Lab on a Chip, 2009, 9(23): 3422.
DOI PMID |
| [87] |
MARCHESINI G R, KOOPAL K, MEULENBERG E, et al. Spreeta-based biosensor assays for endocrine disruptors. Biosensors and Bioelectronics, 2007, 22: 1908.
DOI URL |
| [88] |
YANO T A, KAJISA T, ONO M, et al. Ultrasensitive detection of SARS-CoV-2 nucleocapsid protein using large gold nanoparticle-enhanced surface plasmon resonance. Scientific Reports, 2022, 12: 1060.
DOI URL |
| [89] |
HUANG L P, DING L F, ZHOU J, et al. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point- of-care device. Biosensors and Bioelectronics, 2021, 171: 112685.
DOI URL |
| [90] |
KAJIURA M, NAKANISHI T, IIDA H, et al. Biosensing by optical waveguide spectroscopy based on localized surface plasmon resonance of gold nanoparticles used as a probe or as a label. Journal of Colloid and Interface Science, 2009, 335(1): 140.
DOI PMID |
| [91] |
PASHCHENKO O, SHELBY T, BANERJEE T, et al. A comparison of optical, electrochemical, magnetic, and colorimetric point-of-care biosensors for infectious disease diagnosis. ACS Infectious Diseases, 2018, 4(8): 1162.
DOI PMID |
| [92] | ZHANG R Q, LIU S L, ZHAO W, et al. A simple point-of-care microfluidic immunomagnetic fluorescence assay for pathogens. Analtical Chemistry, 2013, 85(5): 2645. |
| [93] | TAKEMURA K, ADEGOKE O, SUZUKI T, et al. A localized surface plasmon resonance-amplified immunofluorescence biosensor for ultrasensitive and rapid detection of nonstructural protein 1 of Zika virus. PLoS ONE, 2019, 14(1): 0211517. |
| [94] |
GUERREIRO M R, FREITAS D F, ALVES P M, et al. Detection and quantification of label-free infectious adenovirus using a switch-on cell-based fluorescent biosensor. ACS Sensors, 2019, 4(6): 1654.
DOI PMID |
| [95] |
HUANG R R, HE L, LI S, et al. A simple fluorescence aptasensor for gastric cancer exosome detection based on branched rolling circle amplification. Nanoscale, 2020, 12(4): 2445.
DOI PMID |
| [96] |
WANG Y, LI Z H, WANG J, et al. Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends in Biotechnology, 2011, 29(5): 205.
DOI PMID |
| [97] |
SAHA K, AGASTI S S, KIM C, et al. Gold nanoparticles in chemical and biological sensing. Chemical Reviews, 2012, 112(5): 2739.
DOI PMID |
| [98] |
YANG D D, LIU M, XU J, et al. Carbon nanosphere-based fluorescence aptasensor for targeted detection of breast cancer cell MCF-7. Talanta, 2018, 185: 113.
DOI PMID |
| [99] |
LI B M, YU Q L, DUAN Y X. Fluorescent labels in biosensors for pathogen detection. Critical Reviews in Biotechnology, 2015, 35(1): 82.
DOI PMID |
| [100] |
GUO J C, CHEN S Q, TIAN S L, et al. 5G-enabled ultra-sensitive fluorescence sensor for proactive prognosis of COVID-19. Biosensors and Bioelectronics, 2021, 181: 113160.
DOI URL |
| [101] |
ZHOU Y F, CHEN Y, LIU W J, et al. Development of a rapid and sensitive quantum dot nanobead-based double-antigen sandwich lateral flow immunoassay and its clinical performance for the detection of SARS-CoV-2 total antibodies. Sensors and Actuators B: Chemical, 2021, 343: 130139.
DOI URL |
| [102] | CHU Y J, QIU J Y, WANG Y H, et al. Rapid and high-throughput SARS-CoV-2 RNA detection without RNA extraction and amplification by using a microfluidic biochip. Chemistry, 2022, 28: 202104054. |
| [103] |
HAMD-GHADAREH S, HAMAH-AMEEN B A, SALIMI A, et al. Ratiometric enhanced fluorometric determination and imaging of intracellular microRNA-155 by using carbon dots, gold nanoparticles and rhodamine B for signal amplification. Mikrochim Acta, 2019, 186: 469.
DOI URL |
| [104] |
ZHU S J, ZHANG J H, QIAO C Y, et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chemical Communication, 2011, 47(24): 6858.
DOI URL |
| [105] | NEW S Y, LEE S T, SU X D. DNA-templated silver nanocusters: structural correlation and fluorescence modulation. Nanocale, 2016, 8(41): 17729. |
| [106] |
LIAN J Y, LIU Q, JIN Y, et al. Histone-DNA interation: an effective approach to improve the fluorescence intensity and stability of DNA-templated Cu nanoclusters. Chemical Communication, 2017, 53(93): 12568.
DOI URL |
| [107] |
LIU Y Y, JIANG L P, FAN X J, et al. Intracellular fluorometric determination of microRNA-21 by using a switch-on nanoprobe composed of carbon nanotubes and gold nanoclusters. Mikrochim Acta, 2019, 186: 447.
DOI PMID |
| [108] |
WANG Y H, HE L L, HUANG K J, et al. Recent advances in nanomaterial-based electrochemical and optical sensing platforms for microRNA assays. Analyst, 2019, 144(9): 2849.
DOI URL |
| [109] |
ZHU W Y, SHEN X, ZHU C H, et al. Turn-on fluorescent assay based on purification system via magnetic separation for highly sensitive probing of adenosine. Sensors and Actuators B: Chemical, 2018, 259: 855.
DOI URL |
| [110] |
KUMAR N, HU Y, SINGH S, et al. Emerging biosensor platforms for the assessment of water-borne pathogens. Analyst, 2018, 143(2): 359.
DOI PMID |
| [111] |
ZHANG Z Y, TANG Z M, FAROKHZAD N, et al. Sensitive, rapid, low-cost, and multiplexed COVID-19 monitoring by the wireless telemedicine platform. Matter, 2020, 3(6): 1818.
DOI PMID |
| [112] |
TORRENTE-RODRIGUEZ R M, LUKAS H, TU J, et al. SARS-CoV-2 rapid plex: a graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter, 2020, 3(6): 1981.
DOI URL |
| [113] |
AKSHATH U S, SHUBHA L R, BHATT P, et al. Quantum dots as optical labels for ultrasensitive detection of polyphenols. Biosensors and Bioelectronics, 2014, 57: 317.
DOI PMID |
| [114] |
NISHITANI S, SAKATA T. Enhancement of signal-to-noise ratio for serotonin detection with well-designed nanofilter-coated potentiometric electrochemical biosensor. ACS Applied Materials Interfaces, 2020, 12(13): 14761.
DOI URL |
| [115] |
DAI Y F, LIU C C. Recent advances on electrochemical biosensing strategies toward universal point-of-care systems. Angewante Chemie International Edition, 2019, 58(36): 12355.
DOI URL |
| [116] |
GOLICHENARI B, NOSRATI R, FAROKHI-FARD A, et al. Electrochemical-based biosensors for detection of Mycobacterium tuberculosis and tuberculosis biomarkers. Critical Reviews in Biotechnology, 2019, 39(8): 1056.
DOI URL |
| [117] |
CHAND R, RAMALINGAM S, NEETHIRAJAN S. A 2D transition-metal dichalcogenide MoS2 based novel nanocomposite and nanocarrier for multiplex miRNA detection. Nanoscale, 2018, 10(17): 8217.
DOI URL |
| [118] |
REICH P, PREUSS J A, BAHNER N, et al. Impedimetric aptamer-based biosensors: principles and techniques. Advances in Biochemical Engineering-Biotechnology, 2020, 174: 17.
DOI PMID |
| [119] | PREUSS J A, REICH P, BAHNER N, et al. Impedimetric aptamer-based biosensors: applications. Advances in Biochemical Engineering- Biotechnology, 2020, 174: 43. |
| [120] |
LOW S S, CHIA J S, TAN M T, et al. A proof of concept: detection of avian influenza H5 gene by a graphene-enhanced electrochemical genosensor. Journal of Nanoscience and Nanotechnology, 2016, 16(3): 2438.
PMID |
| [121] |
MOUSAVI M P S, AINLA A, TAN E K W, et al. Ion sensing with thread-based potentiometric electrodes. Lab on a Chip, 2018, 18(15): 2279.
DOI PMID |
| [122] |
LABIB M, SARGENT E H, KELLEY S O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chemical Reviews, 2016, 116(16): 9001.
DOI PMID |
| [123] | LI Q, LU N, WANG L H, et al. Advances in nanowire transistor-based biosensors. Small Methods, 2018, 2: 1700263. |
| [124] |
BOLLELLA P, GORTON L. Enzyme based amperometric biosensors. Current Opinion in Electrochemistry, 2018, 10: 157.
DOI URL |
| [125] |
CHEN A, CHATTERJEE S. Nanomaterials based electrochemical sensors for biomedical applications. Chemical Society Reviews, 2013, 42(12): 5425.
DOI PMID |
| [126] |
FABIANI L, SAROGLIA M, GALATA G, et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: a reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosensors and Bioelectronics, 2021, 171: 112686.
DOI URL |
| [127] |
EL-SAID W A, AL-BOGAMI A S, ALSHITARI W, et al. Electrochemical microbiosensor for detecting COVID-19 in a patient sample based on gold microcuboids pattern. BioChip Journal, 2021, 15: 287.
DOI URL |
| [128] |
ALAFEEF M, DIGHE K, MOITRA P, et al. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano, 2020, 14(12): 17028.
DOI PMID |
| [129] |
BROSEL-OLIU S, MERGEL O, URIA N, et al. 3D impedimetric sensors as a tool for monitoring bacterial response to antibiotics. Lab on a Chip, 2019, 19(8): 1436.
DOI URL |
| [130] |
ELSHAFEY R, TLILI C, ABULROB A, et al. Label-free impedimetric immunosensor for ultrasensitive detection of cancer marker murine double minute 2 in brain tissue. Biosensors and Bioelectronics, 2013, 39(1): 220.
DOI PMID |
| [131] |
PERSHINA L V, GRABEKLIS A R, ISANKINA L N, et al. Determination of sodium and potassium ions in patients with SARS-CoV-2 disease by ion-selective electrodes based on polyelectrolyte complexes as a pseudo-liquid contact phase. RSC Advances, 2021, 11(57): 36215.
DOI PMID |
| [132] |
TORRES M D T, DE ARAUJO W R, DE LIMA L F, et al. Low-cost biosensor for rapid detection of SARS-CoV-2 at the point of care. Matter, 2021, 4(7): 2403.
DOI URL |
| [133] |
DANIELS J S, POURMAND N. Label-free impedance biosensors: opportunities and challenges. Electroanalysis, 2007, 19(12): 1239.
DOI PMID |
| [134] | AYDIN E B, AYDIN M, SEZGINTURK M K. New impedimetric sandwich immunosensor for ultrasensitive and highly specific detection of spike receptor binding domain protein of SARS-CoV-2. ACS Biomaterials Science Engneering, 2021, 7(8): 3874. |
| [135] |
LORENZEN A L, DOS SANTOS A M, DOS SANTOS L P, et al. PEDOT-AuNPs-based impedimetric immunosensor for the detection of SARS-CoV-2 antibodies. Electrochimica Acta, 2022, 404: 139757.
DOI URL |
| [136] |
PENG Y, PAN Y H, SUN Z W, et al. An electrochemical biosensor for sensitive analysis of the SARS-CoV-2 RNA. Biosensors and Bioelectronics, 2021, 186: 113309.
DOI URL |
| [137] |
KIMMEL D W, LEBLANC G, MESCHIEVITZ M E, et al. Electrochemical sensors and biosensors. Analytical Chemistry, 2012, 84(2): 685.
DOI PMID |
| [138] |
LUO X L, DAVIS J J. Electrical biosensors and the label free detection of protein disease biomarkers. Chemical Society Reviews, 2013, 42(13): 5944.
DOI PMID |
| [139] |
ELDIN N B, EL-RAHMAN M K A, ZAAZAA H E, et al. Microfabricated potentiometric sensor for personalized methacholine challenge tests during the COVID-19 pandemic. Biosensors and Bioelectronics, 2021, 190: 113439.
DOI URL |
| [140] |
CHAIBUN T, PUENPA J, NGAMDEE T, et al. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nature Communication, 2021, 12: 802.
DOI URL |
| [141] |
LEE M Y, LEE H R, PARK C H, et al. Organic transistor-based chemical sensors for wearable bioelectronics. Accounts Chemical Research, 2018, 51(11): 2829.
DOI URL |
| [142] |
MATSUMOTO A, MIYAHARA Y. Current and emerging challenges of field effect transistor based bio-sensing. Nanoscale, 2013, 5(22): 10702.
DOI PMID |
| [143] |
GUTIERREZ-SANZ O, ANDOY N M, FILIPIAK M S, et al. Direct, label-free, and rapid transistor-based immunodetection in whole serum. ACS Sensors, 2017, 2(9): 1278.
DOI URL |
| [144] |
KANG H, WANG X J, GUO M Q, et al. Ultrasensitive detection of SARS-CoV-2 antibody by graphene field-effect transistors. Nano Letters, 2021, 21(19): 7897.
DOI URL |
| [145] |
WANG Z, YI K Y, LIN Q Y, et al. Free radical sensors based on inner-cutting graphene field-effect transistors. Nature Communications, 2019, 10: 1544.
DOI PMID |
| [146] |
GANGULI A, FARAMARZI V, MOSTAFA A, et al. High sensitivity graphene field effect transistor-based detection of DNA amplification. Advanced Functional Materials, 2020, 30: 2001031.
DOI URL |
| [147] |
PICCA R A, MANOLI K, MACCHIA E, et al. Ultimately sensitive organic bioelectronic transistor sensors by materials and device structure design. Advanced Functional Materials, 2019, 30: 1904513.
DOI URL |
| [148] |
SHAO W T, SHURIN M R, WHEELER S E, et al. Rapid detection of SARS-CoV-2 antigens using high-purity semiconducting single-walled carbon nanotube-based field-effect transistors. ACS Applied Materials Interfaces, 2021, 13(8): 10321.
DOI URL |
| [149] |
LI J H, WU D, YU Y, et al. Rapid and unamplified identification of COVID-19 with morpholino-modified graphene field-effect transistor nanosensor. Biosensors and Bioelectronics, 2021, 183: 113206.
DOI URL |
| [150] |
WANG L Q, WANG X J, WU Y G, et al. Rapid and ultrasensitive electromechanical detection of ions, biomolecules and SARS-CoV-2 RNA in unamplified samples. Nature Biomedical Engineering, 2022, 6(3): 276.
DOI PMID |
| [151] |
YOU C C, CHOMPOOSOR A, ROTELLO V M. The biomacromolecule- nanoparticle interface. Nano Today, 2007, 2(3): 34.
DOI URL |
| [152] |
DANIEL M C, ASTRUC D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical Reviews, 2004, 104: 293.
DOI URL |
| [153] |
BANKS C E, CROSSLEY A, SALTER C, et al. Carbon nanotubes contain metal impurities which are responsible for the “electrocatalysis” seen at some nanotube-modified electrodes. Angewandte Chemie International Edition, 2006, 45(16): 2533.
DOI URL |
| [154] |
SHEN J F, HU Y Z, LI C, et al. Synthesis of amphiphilic graphene nanoplatelets. Small, 2009, 5(1): 82.
DOI PMID |
| [155] |
AYTUR T, FOLEY J, ANWAR M, et al. A novel magnetic bead bioassay platform using a microchip-based sensor for infectious disease diagnosis. Journal of Immunological Methods, 2006, 314: 21.
PMID |
| [156] |
HASH S, MARTINEZ-VIEDMA M P, FUNG F, et al. Nuclear magnetic resonance biosensor for rapid detection of Vibrio parahaemolyticus. Biomedical Journal, 2019, 42(3): 187.
DOI PMID |
| [157] |
SU D Q, WU K, KRISHNA V D, et al. Detection of influenza a virus in swine nasal swab samples with a wash-free magnetic bioassay and a handheld giant magnetoresistance sensing system. Frontiers in Microbiology, 2019, 10: 1077.
DOI PMID |
| [158] |
PASTUCHA M, FARKA Z, LACINA K, et al. Magnetic nanoparticles for smart electrochemical immunoassays: a review on recent developments. Mikrochimica Acta, 2019, 186: 312.
DOI PMID |
| [159] |
SCHOTTER J, KAMP P B, BECKER A, et al. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosensors and Bioelectronics, 2004, 19(10): 1149.
PMID |
| [160] |
BAIBICH M N, BROTO J M, FERT A, et al. Giant magnetoresistance of (001)Fe/(001)Cr magnetic superlattices. Physical Review Letters, 1988, 61(21): 2472.
PMID |
| [161] |
BINASCH G, GRUNBERG P, SAURENBACH F, et al. Enhanced magnetoresistance in layered magnetic structures with antiferromagnetic interlayer exchange. Physical Review B: Condensed Matter, 1989, 39(7): 4828.
DOI URL |
| [162] |
BAYIN Q, HUANG L, REN C H, et al. Anti-SARS-CoV-2 IgG and IgM detection with a GMR based LFIA system. Talanta, 2021, 227: 122207.
DOI URL |
| [163] |
ZHANG X J, REEVES D B, PERREARD I M, et al. Molecular sensing with magnetic nanoparticles using magnetic spectroscopy of nanoparticle Brownian motion. Biosensors and Bioelectronics, 2013, 50: 441.
DOI PMID |
| [164] |
ZNOYKO S L, ORLOV A V, BRAGINA V A, et al. Nanomagnetic lateral flow assay for high-precision quantification of diagnostically relevant concentrations of serum TSH. Talanta, 2020, 216: 120961.
DOI URL |
| [165] |
WU K, CHUGH V K, D. KRISHNA V, et al. One-step, wash-free, nanoparticle clustering-based magnetic particle spectroscopy bioassay method for detection of SARS-CoV-2 spike and nucleocapsid proteins in the liquid phase. ACS Applied Materials Interfaces, 2021, 13(37): 44136.
DOI URL |
| [166] |
RÖSCH E L, ZHONG J, LAK A, et al. Point-of-need detection of pathogen-specific nucleic acid targets using magnetic particle spectroscopy. Biosensors and Bioelectronics, 2021, 192: 113536.
DOI URL |
| [167] |
ZALESSKIY S S, DANIELI E, BLUMICH B, et al. Miniaturization of NMR systems: desktop spectrometers, microcoil spectroscopy, and “NMR on a chip” for chemistry, biochemistry, and industry. Chemical Reviews, 2014, 114(11): 5641.
DOI URL |
| [168] |
BEMETZ J, WEGEMANN A, SAATCHI K, et al. Microfluidic- based synthesis of magnetic nanoparticles coupled with miniaturized NMR for online relaxation studies. Analytical Chemistry, 2018, 90(16): 9975.
DOI URL |
| [169] |
LI Y Q, MA P X, TAO Q, et al. Magnetic graphene quantum dots facilitate closed-tube one-step detection of SARS-CoV-2 with ultra-low field NMR relaxometry. Sensors and Actuators B: Chemical, 2021, 337: 129786.
DOI URL |
| [170] |
SCHOENLE M V, LI Y, YUAN M, et al. NMR based SARS-CoV-2 antibody screening. Journal of the American Chemical Society, 2021, 143(21): 7930.
DOI URL |
| [171] |
CANTRELLE F X, BOLL E, BRIER L, et al. NMR spectroscopy of the main protease of SARS-CoV-2 and fragment-based screening identify three protein hotspots and an antiviral fragment. Angewandte Chemie International Edition, 2021, 60(48): 25428.
DOI URL |
| [172] |
NOVAKOVIC M, KUPCE E, SCHERF T, et al. Magnetization transfer to enhance NOE cross-peaks among labile protons: applications to imino-imino sequential walks in SARS-CoV-2-derived RNAs. Angewandte Chemie International Edition, 2021, 60(21): 11884.
DOI URL |
| [173] |
WU K, SAHA R, SU D Q, et al. Magnetic-nanosensor-based virus and pathogen detection strategies before and during COVID-19. ACS Applied Nano Materials, 2020, 3(10): 9560.
DOI URL |
| [174] |
CHOI Y, HWANG J H, LEE S Y. Recent trends in nanomaterials-based colorimetric detection of pathogenic bacteria and viruses. Small Methods, 2018, 2(4): 1700351.
DOI URL |
| [175] | CALVERT A E, BIGGERSTAFF B J, TANNER N A, et al. Rapid colorimetric detection of Zika virus from serum and urine specimens by reverse transcription loop-mediated isothermal amplification (RT-LAMP). PLoS ONE, 2017, 12(9): 0185340. |
| [176] |
ROY S, MOHD-NAIM N F, SAFAVIEH M, et al. Colorimetric nucleic acid detection on paper microchip using loop mediated isothermal amplification and crystal violet dye. ACS Sensors, 2017, 2(11): 1713.
DOI PMID |
| [177] |
WU J J, WANG X Y, WANG Q, et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chemical Society Reviews, 2019, 48(4): 1004.
DOI PMID |
| [178] |
WANG Z Q, LI Z S, ZOU Z G. Application of binder-free TiOxN1-x nanogrid film as a high-power supercapacitor electrode. Journal of Power Sources, 2015, 296: 53.
DOI URL |
| [179] |
WANG Z F, YANG X, FENG J, et al. Label-free detection of DNA by combining gated mesoporous silica and catalytic signal amplification of platinum nanoparticles. Analyst, 2014, 139(23): 6088.
DOI PMID |
| [180] | VILELA D, GONZALEZ M C, ESCARPA A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: chemical creativity behind the assay. a review. Chemical Society Reviews, 2012, 751: 24. |
| [181] |
ALHOGAIL S, SUAIFAN G, BIKKER F J, et al. Rapid colorimetric detection of pseudomonas aeruginosa in clinical isolates using a magnetic nanoparticle biosensor. ACS Omega, 2019, 4(26): 21684.
DOI URL |
| [182] |
GUO L H, XU Y, FERHAN A R, et al. Oriented gold nanoparticle aggregation for colorimetric sensors with surprisingly high analytical figures of merit. Journal of the American Chemical Society, 2013, 135(33): 12338.
DOI PMID |
| [183] |
ALDEWACHI H, CHALATI T, WOODROOFE M N, et al. Gold nanoparticle-based colorimetric biosensors. Nanoscale, 2017, 10(1): 18.
DOI PMID |
| [184] |
SUN J S, XIANYU Y L, JIANG X Y. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chemical Society Reviews, 2014, 43(17): 6239.
DOI PMID |
| [185] |
GODAKHINDI V S, KANG P, SERRE M, et al. Tuning the gold nanoparticle colorimetric assay by nanoparticle size, concentration, and size combinations for oligonucleotide detection. ACS Sensors, 2017, 2(11): 1627.
DOI PMID |
| [186] | BÜYÜKSÜNETCI Y T, CITIL B E, TAPAN U, et al. Development and application of a SARS-CoV-2 colorimetric biosensor based on the peroxidase-mimic activity of gamma-Fe2O3 nanoparticles. Mikrochimica Acta, 2021, 188: 335. |
| [187] |
VENTURA B D, CENNAMO M, MINOPOLI A, et al. Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat Swabs. ACS Sensors, 2020, 5(10): 3043.
DOI URL |
| [188] |
GAO Y K, HAN Y K, WANG C, et al. Rapid and sensitive triple-mode detection of causative SARS-CoV-2 virus specific genes through interaction between genes and nanoparticles. Analytica Chimica Acta, 2021, 1154: 338330.
DOI URL |
| [189] |
LEE J K, CHI Y S, S CHOI I. Reactivity of acetylenyl-terminated self-assembled monolayers on gold: triazole formation. Langmuir, 2004, 20: 3844.
PMID |
| [190] |
CHE J, PARK K, GRABOWSKI C A, et al. Preparation of ordered monolayers of polymer grafted nanoparticles: impact of architecture, concentration, and substrate surface energy. Macromolecules, 2016, 49(5): 1834.
DOI URL |
| [191] | KIMLING J, MAIER M, OKENVE B, et al. Turkevich method for gold nanoparticle synthesis revisited. The Journal of Chemical Physics, 2006, 110: 15700. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | CHEN Xi, YUAN Yuan, TAN Yeqiang, LIU Changsheng. Strategic Study on the Development of Inorganic Non-metallic Biomaterials [J]. Journal of Inorganic Materials, 2025, 40(5): 449-456. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [10] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [11] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [12] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [13] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [14] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [15] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||