
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (8): 845-853.DOI: 10.15541/jim20170516
Special Issue: 电催化研究
• Orginal Article • Previous Articles Next Articles
YANG Zhi-Bin1, YUE Tong-Lian1, YU Xiang-Nan1, WU Miao-Miao2
Received:2017-10-28
Revised:2017-12-14
Published:2018-08-28
Online:2018-07-17
CLC Number:
YANG Zhi-Bin, YUE Tong-Lian, YU Xiang-Nan, WU Miao-Miao. Electrocatalytic Activity of Cobalt Doped Ceria Nanoparticles[J]. Journal of Inorganic Materials, 2018, 33(8): 845-853.
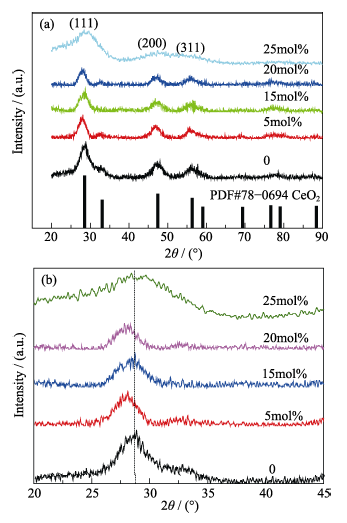
Fig. 1 XRD patterns of ceria nanoparticles with different doping ratio of cobalt(a) Whole range patterns; (b) Magnified patterns of the highest peak (111)
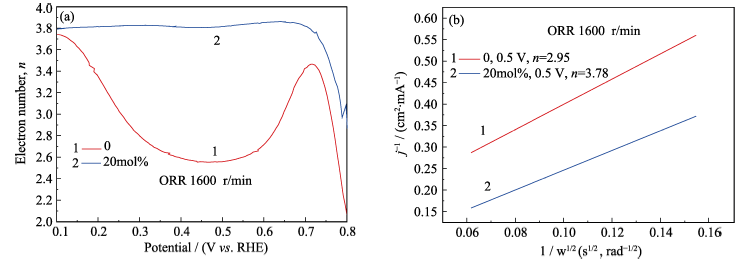
Fig. 5 (a) Corresponding transfer electron number of ceria nanoparticles with different doping amount in RRDE model, and (b) corresponding K-L plots of ceria nanoparticles with different doping ratio at 0.5 V
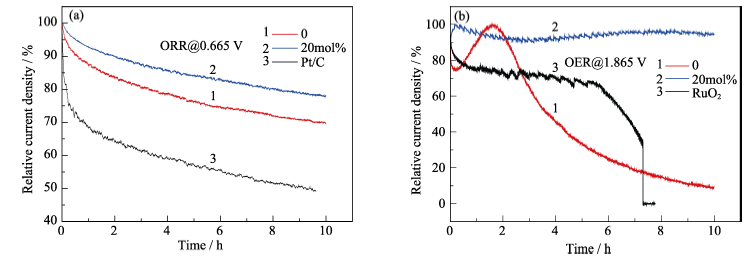
Fig. 6 Current-time(I-t) chronoamperometric responses for ceria nanoparticles (a) at two different doping ratio and Pt/C, and (b) at two different doping ratio and RuO2
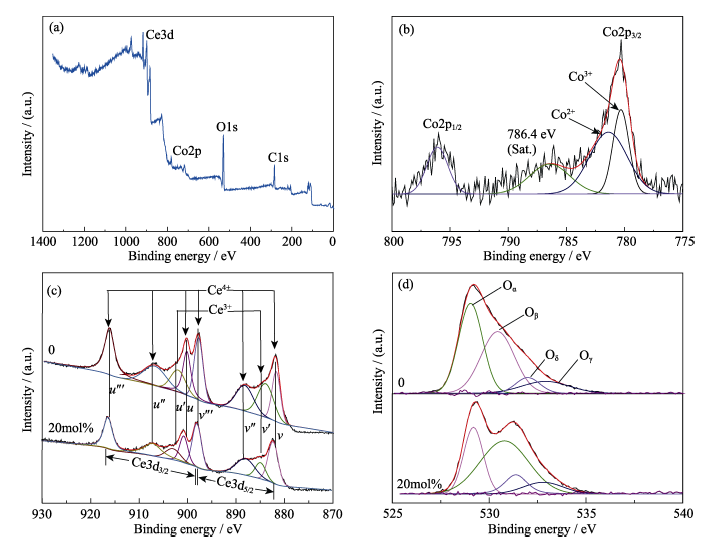
Fig. 10 (a) XPS survey spectra and (b) high-resolution XPS spectra of Co2p of CeO2 doping with 20mol% Co; High-resolution XPS spectra of Ce3d (c) and O1s (d) of CeO2 with different doping ratio
| Sample | Content | |||||
|---|---|---|---|---|---|---|
| Oα/at% | Oβ/at% | Oδ/at% | Oγ/at% | Oβ/Oα | [Ce3+/(Ce4++Ce3+)]/% | |
| 0 | 48.5 | 29.2 | 10.4 | 11.8 | 0.60 | 20.1 |
| 20mol% | 27.2 | 53.1 | 10.5 | 9.2 | 1.95 | 24.1 |
Table 1 Corresponding XPS results of different doping ratio
| Sample | Content | |||||
|---|---|---|---|---|---|---|
| Oα/at% | Oβ/at% | Oδ/at% | Oγ/at% | Oβ/Oα | [Ce3+/(Ce4++Ce3+)]/% | |
| 0 | 48.5 | 29.2 | 10.4 | 11.8 | 0.60 | 20.1 |
| 20mol% | 27.2 | 53.1 | 10.5 | 9.2 | 1.95 | 24.1 |
| [1] | JIE S, KEVIN J M, HUBERT A G,et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles.Science, 2011, 334(6061): 1383-1385. |
| [2] | JIN S, HUBERT A G, YABUUCHI N,et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries.Nature Chemistry, 2011, 3(8): 647. |
| [3] | WU G, KARREN L M, JOHNSTON C M,et al. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt.Science, 2011, 332(6028): 443-447. |
| [4] | LEFÈVRE M, PROIETTI E, JAOUEN F,et al. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells.Science, 2009, 324(5923): 71-74. |
| [5] | CAO Z X, DING Y M, WANG Z C,et al. Porous calcium manganese oxide: preparation and electrocatalytic activity of oxygen reduction reaction.Journal of Inorganic Materials, 2017, 32(5): 535-542. |
| [6] | HOU F, LI H, YANG Y,et al. Preparation and catalytic oxidation of CO with specific morphology and porous nano CeO2.Chemical Industry and Engineering Progress, 2017, 36(7): 2481-2487. |
| [7] | KE J, XIAO J W, ZHU W,et al. Dopant-induced modification of active site structure and surface bonding mode for high-performance nanocatalysts: CO oxidation on capping-free(110)-oriented CeO2: Ln (Ln=La-Lu) nanowires.Journal of the American Chemical Society, 2013, 135(40): 15191-15200. |
| [8] | XU B, ZHANG Q, YUAN S,et al. Synthesis and photocatalytic performance of yttrium-doped CeO2 with a porous broom-like hierarchical structure.Applied Catalysis B: Environmental, 2016, 183: 361-370. |
| [9] | HAO L, YU J, XU X,et al. Nitrogen-doped MoS2/carbon as highly oxygen-permeable and stable catalysts for oxygen reduction reaction in microbial fuel cells.Journal of Power Sources, 2017, 339: 68-79. |
| [10] | CHEN P, ZHOU T, XING L,et al. Atomically dispersed iron-nitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions.Angewandte Chemie International Edition, 2017, 56(2): 610-614. |
| [11] | ZHU Q L, XIA W, AKITA T,et al. Metal-organic framework-derived honeycomb-like open porous nanostructures as precious-metal-free catalysts for highly efficient oxygen electroreduction.Advanced Materials, 2016, 28(30): 6391-6398. |
| [12] | FENG J, LIANG Y G, WANG H L,et al. Engineering manganese oxide/nanocarbon hybrid materials for oxygen reduction electrocatalysis.Nano Research, 2012, 5(10): 718-725. |
| [13] | TAN Y M, XU C F, CHEN G X,et al. Facile synthesis of manganese-oxide-containing mesoporous nitrogen-doped carbon for efficient oxygen reduction.Advanced Functional Materials, 2012, 22(21): 4584-4591. |
| [14] | DONG C, LIU Z W, LIU Y J, ,et al.Modest oxygen-defective amorphous manganese-based nanoparticle mullite with superior overall electrocatalytic performance for oxygen reduction reaction. Small, 2017,13(16): 1603903-1-9. |
| [15] | LIANG Y Y, WANG H L, ZHOU J G,et al. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts.Journal of the American Chemical Society, 2012, 134(7): 3517-3523. |
| [16] | PENDASHTEH A, PALAMA J, ANDERSON M,et al. NiCoMnO4 nanoparticles on N-doped graphene: highly efficient bifunctional electrocatalyst for oxygen reduction/evolution reactions.Applied Catalysis B: Environmental, 2017, 201: 241-252. |
| [17] | HARDIN W G, MEFFORD J T, SLANAC D A,et al. Tuning the electrocatalytic activity of perovskites through active site variation and support interactions.Chemistry of Materials, 2014, 26(11): 3368-3376. |
| [18] | HARDIN W G, SLANAC D A, WANG X,et al. Highly active, nonprecious metal perovskite electrocatalysts for bifunctional metal-air battery electrodes.The Journal of Physical Chemistry Letters, 2013, 4(8): 1254-1259. |
| [19] | HARTMANN P, BREZESINSKI T, SANN J,et al. Defect chemistry of oxide nanomaterials with high surface area: ordered mesoporous thin films of the oxygen storage catalyst CeO2-ZrO2.ACS Nano. 2013, 7(4): 2999-3013. |
| [20] | XU X M, SU C, ZHOU W, ,et al.3 Co-doping strategy for developing perovskite oxides as highly efficient electrocatalysts for oxygen evolution reaction.Advanced Science., 2016, 3(2): 1500187-1-6. |
| [21] | ZHU Y L, ZHOU W, SUNARSO J,et al. Phosphorus-doped perovskite oxide as highly efficient water oxidation electrocatalyst in alkaline solution.Advanced Functional Materials, 2016, 26(32): 5862-5872. |
| [22] | HE Y, ZHANG J F, HE G W,et al. Ultrathin Co3O4 nanofilm as an efficient bifunctional catalyst for oxygen evolution and reduction reaction in rechargeable zinc-air batteries.Nanoscale, 2017, 9(25): 8623-8630. |
| [23] | MENG Y T, SONG W Q, HUANG H,et al. Structure-property relationship of bifunctional MnO2 nanostructures: highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media.Journal of the American Chemical Society, 2014, 136(32): 11452-11464. |
| [24] | ZHAO X, FU Y, WANG J,et al. Ni-doped CoFe2O4 hollow nanospheres as efficient bi-functional catalysts.Electrochimica Acta, 2016, 201: 172-178. |
| [25] | XU B, ZHANG Q T, YUAN S S,et al. Synthesis and photocatalytic performance of yttrium-doped CeO2 with a hollow sphere structure.Catalysis Today, 2017, 281: 135-143. |
| [26] | LI H, MENG F, GONG J,et al. Structural, morphological and optical properties of shuttle-like CeO2 synthesized by a facile hydrothermal method.Journal of Alloys and Compounds, 2017, 722: 489-498. |
| [27] | DENG W, DAI Q G, LAO Y J,et al. Low temperature catalytic combustion of 1, 2-dichlorobenzene over CeO2-TiO2 mixed oxide catalysts.Applied Catalysis B: Environmental, 2016, 181: 848-861. |
| [28] | ZANG C J, ZHANG X S, HU S Y,et al. The role of exposed facets in the Fenton-like reactivity of CeO2 nanocrystal to the Orange II.Applied Catalysis B: Environmental, 2017, 216: 106-113. |
| [29] | FENG J, YE S H, XU H,et al. Design and synthesis of FeOOH/ CeO2 heterolayered nanotube electrocatalysts for the oxygen evolution reaction.Advanced Materials, 2016, 28(23): 4698-4703. |
| [30] | BECHE E, CHARVIN P, PERARNAU D,et al. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (C.xTiyOz). Surface and Interface Analysis, 2008, 40(3/4): 264-267. |
| [31] | LI L L, ZHANG L, MA K L,et al. Ultra-low loading of copper modified TiO2/CeO2 catalysts for low-temperature selective catalytic reduction of NO by NH3.Applied Catalysis B: Environmental, 2017, 207: 366-375. |
| [32] | LIANG F L, YU Y, ZHOU W,et al. Highly defective CeO2 as a promoter for efficient and stable water oxidation.Journal of Material Chemistry, 2015, 3(2): 634-640. |
| [33] | XU L, JIANG Q Q, XIAO Z H,et al. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction.Angewandte Chemie International Edition, 2016, 55(17): 5277-5281. |
| [34] | ZHUANG L Z, GE L, YANG Y S, ,et al..Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Advanced Materials, 2017, 29(17): 1606793- 1-7. |
| [35] | WANG F, ZHANG L, XU L,et al. Low temperature CO oxidation and CH4 combustion over Co3O4 nanosheets.Fuel, 2017, 203: 419-429. |
| [1] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [2] | ZHOU Yangyang, ZHANG Yanyan, YU Ziyi, FU Zhengqian, XU Fangfang, LIANG Ruihong, ZHOU Zhiyong. Enhancement of Piezoelectric Properties in CaBi4Ti4O15-based Ceramics through Bi3+ Self-doping Strategy [J]. Journal of Inorganic Materials, 2025, 40(6): 719-728. |
| [3] | SUN Yuxuan, WANG Zheng, SHI Xue, SHI Ying, DU Wentong, MAN Zhenyong, ZHENG Liaoying, LI Guorong. Defect Dipole Thermal-stability to the Electro-mechanical Properties of Fe Doped PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(5): 545-551. |
| [4] | AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao. Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance [J]. Journal of Inorganic Materials, 2025, 40(5): 457-465. |
| [5] | PAN Yuzhou, HE Fajian, XU Lulu, DAI Shixun. Broadband 3 μm Mid-infrared Emission in Dy3+/Yb3+ Co-doped Tellurite Glass under 980 nm LD Excitation [J]. Journal of Inorganic Materials, 2025, 40(5): 521-528. |
| [6] | QU Jifa, WANG Xu, ZHANG Weixuan, ZHANG Kangzhe, XIONG Yongheng, TAN Wenyi. Enhanced Sulfur-resistance for Solid Oxide Fuel Cells Anode via Doping Modification of NaYTiO4 [J]. Journal of Inorganic Materials, 2025, 40(5): 489-496. |
| [7] | MU Haojie, ZHANG Yuanjiang, YU Bin, FU Xiumei, ZHOU Shibin, LI Xiaodong. Preparation and Properties of ZrO2 Doped Y2O3-MgO Nanocomposite Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 281-289. |
| [8] | SHEN Hao, CHEN Qianqian, ZHOU Boxiang, TANG Xiaodong, ZHANG Yuanyuan. Preparation and Energy Storage Properties of A-site La/Sr Co-doped PbZrO3 Thin Films [J]. Journal of Inorganic Materials, 2024, 39(9): 1022-1028. |
| [9] | CHENG Jun, ZHANG Jiawei, QIU Pengfei, CHEN Lidong, SHI Xun. Preparation and Thermoelectric Transport Properties of P-doped β-FeSi2 [J]. Journal of Inorganic Materials, 2024, 39(8): 895-902. |
| [10] | ZHAO Zhihan, GUO Peng, WEI Jing, CUI Li, LIU Shanze, ZHANG Wenlong, CHEN Rende, WANG Aiying. Ti Doped Diamond Like Carbon Films: Piezoresistive Properties and Carrier Transport Behavior [J]. Journal of Inorganic Materials, 2024, 39(8): 879-886. |
| [11] | LI Jiaqi, LI Xiaosong, LI Xuanhe, ZHU Xiaobing, ZHU Aimin. Transition Metal-doped Manganese Oxide: Synthesis by Warm Plasma and Electrocatalytic Performance for Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2024, 39(7): 835-844. |
| [12] | TAM YU Puy Mang, XU Yu, GAO Quanhao, ZHOU Haiqiong, ZHANG Zhen, YIN Hao, LI Zhen, LÜ Qitao, CHEN Zhenqiang, MA Fengkai, SU Liangbi. Spectroscopic Properties and Optical Clusters in Erbium-doped CaF2, SrF2 and PbF2 Crystals [J]. Journal of Inorganic Materials, 2024, 39(3): 330-336. |
| [13] | LI Qiushi, YIN Guangming, LÜ Weichao, WANG Huaiyao, LI Jinglin, YANG Hongguang, GUAN Fangfang. Preparation of Na+/g-C3N4 Materials and Their Photocatalytic Degradation Mechanism on Methylene Blue [J]. Journal of Inorganic Materials, 2024, 39(10): 1143-1150. |
| [14] | DAI Le, LIU Yang, GAO Xuan, WANG Shuhao, SONG Yating, TANG Mingmeng, DMITRY V Karpinsky, LIU Lisha, WANG Yaojin. Self-polarization Achieved by Compositionally Gradient Doping in BiFeO3 Thin Films [J]. Journal of Inorganic Materials, 2024, 39(1): 99-106. |
| [15] | LI Guanglan, WANG Tianyu, LIU Yichen, LU Zhongfa. Layered NiFeCo-LDH-Ti6C3.75 Catalyst: Preparation and Performance for Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2023, 38(7): 823-829. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||