
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (11): 1205-1211.DOI: 10.15541/jim20160109
• Orginal Article • Previous Articles Next Articles
LI Jun1, PAN Lei1, WANG Ji-Tong1, LONG Dong-Hui1, QIAO Wen-Ming1,2, LING Li-Cheng1
Received:2016-03-01
Revised:2016-04-14
Published:2016-11-10
Online:2016-10-25
About author:LI Jun. E-mail: lijun8540870@163.com
Supported by:CLC Number:
LI Jun, PAN Lei, WANG Ji-Tong, LONG Dong-Hui, QIAO Wen-Ming, LING Li-Cheng. Low-temperature Removal of NO by Spherical Activated Carbon Loaded with MnOx-CeO2 and Melamine[J]. Journal of Inorganic Materials, 2016, 31(11): 1205-1211.
| Sample | SBET/(m2·g-1) | Smic/(m2·g-1) | Vt/(cm3·g-1) | Vmic/(cm3·g-1) |
|---|---|---|---|---|
| SAC | 1411 | 1295 | 0.62 | 0.52 |
| 400-8(Mn-Ce)/SAC | 710 | 648 | 0.32 | 0.26 |
| 400-8(Mn-Ce)/SAC-5 | 661 | 603 | 0.29 | 0.25 |
| 400-8(Mn-Ce)/SAC-10 | 578 | 528 | 0.26 | 0.22 |
| 400-8(Mn-Ce)/SAC-15 | 476 | 428 | 0.22 | 0.17 |
| 400-8(Mn-Ce)/SAC-20 | 392 | 355 | 0.18 | 0.14 |
Table 1 Porosity parameters of SAC and 400-8(Mn-Ce)/SAC with different melamine loadings
| Sample | SBET/(m2·g-1) | Smic/(m2·g-1) | Vt/(cm3·g-1) | Vmic/(cm3·g-1) |
|---|---|---|---|---|
| SAC | 1411 | 1295 | 0.62 | 0.52 |
| 400-8(Mn-Ce)/SAC | 710 | 648 | 0.32 | 0.26 |
| 400-8(Mn-Ce)/SAC-5 | 661 | 603 | 0.29 | 0.25 |
| 400-8(Mn-Ce)/SAC-10 | 578 | 528 | 0.26 | 0.22 |
| 400-8(Mn-Ce)/SAC-15 | 476 | 428 | 0.22 | 0.17 |
| 400-8(Mn-Ce)/SAC-20 | 392 | 355 | 0.18 | 0.14 |
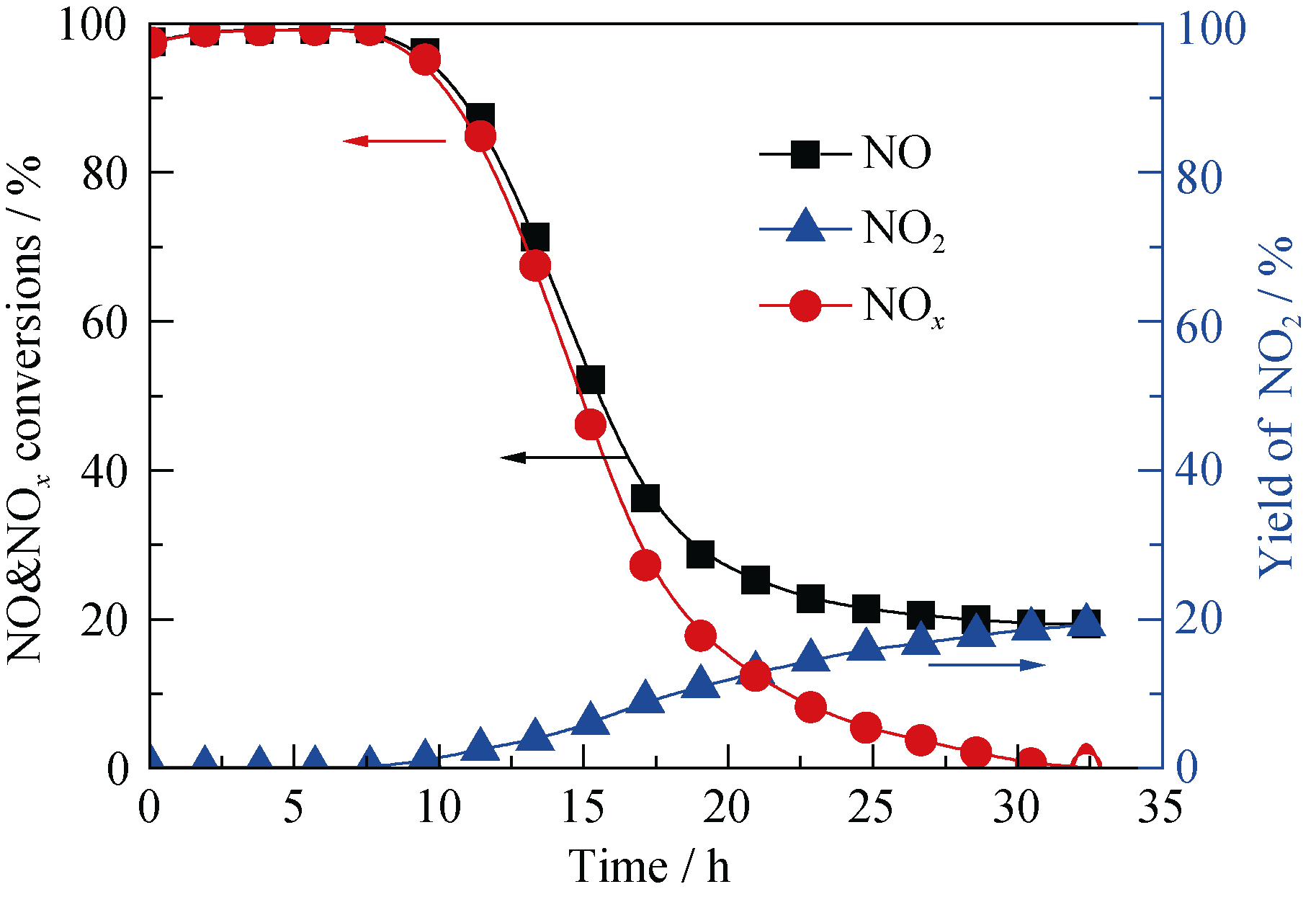
Fig. 4 Reactivity of NO with melamine on 400-8(Mn-Ce)/ SAC-10Reaction conditions: 0.1% NO, 8% O2, balance N2, reaction temperature = 180℃, space velocity = 6000 h-1
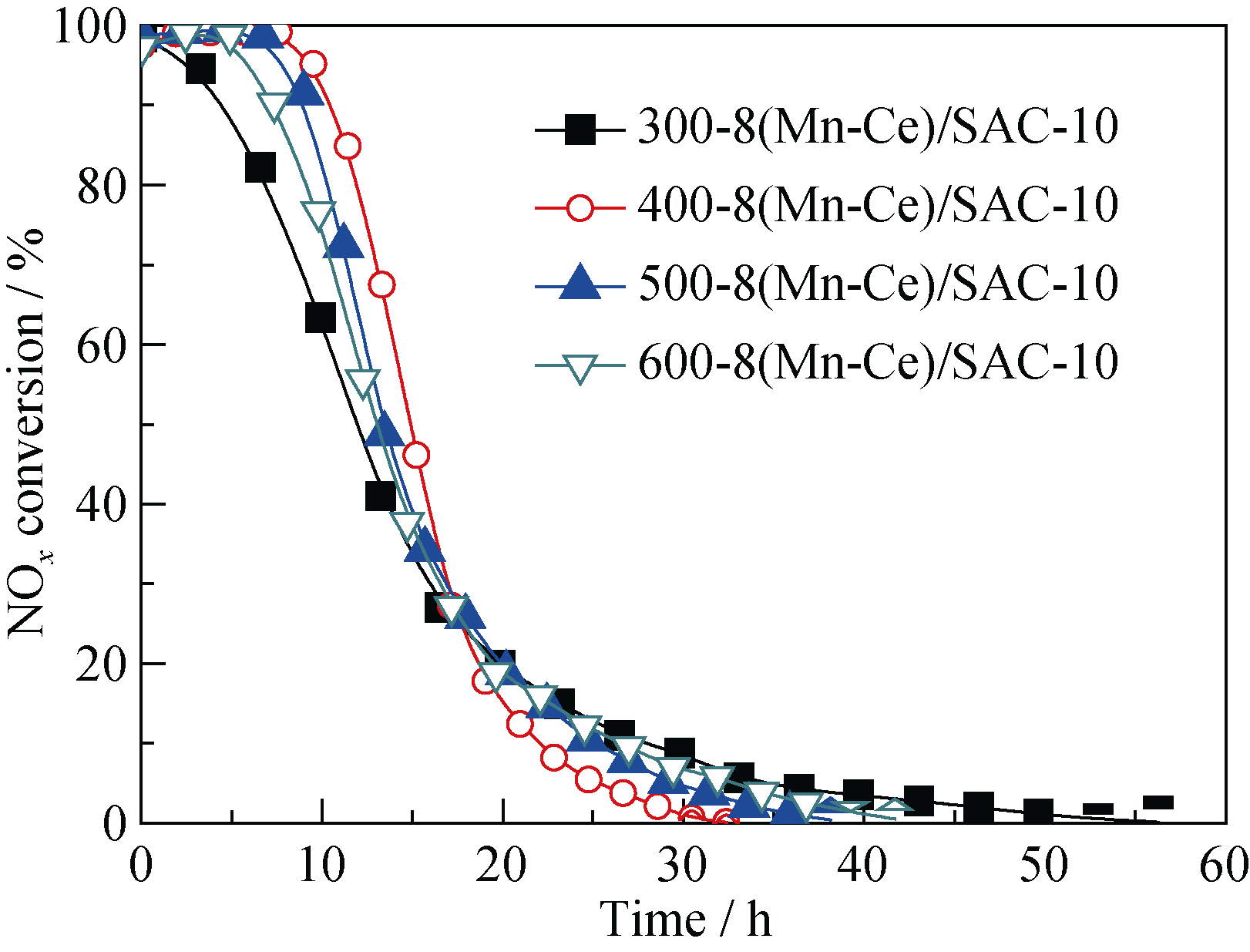
Fig. 5 Effect of calcination temperature on NOx conversion over melamine-supported catalystsReaction conditions: 0.1% NO, 8% O2, balance N2, reaction temperature = 180℃, space velocity= 6000 h-1
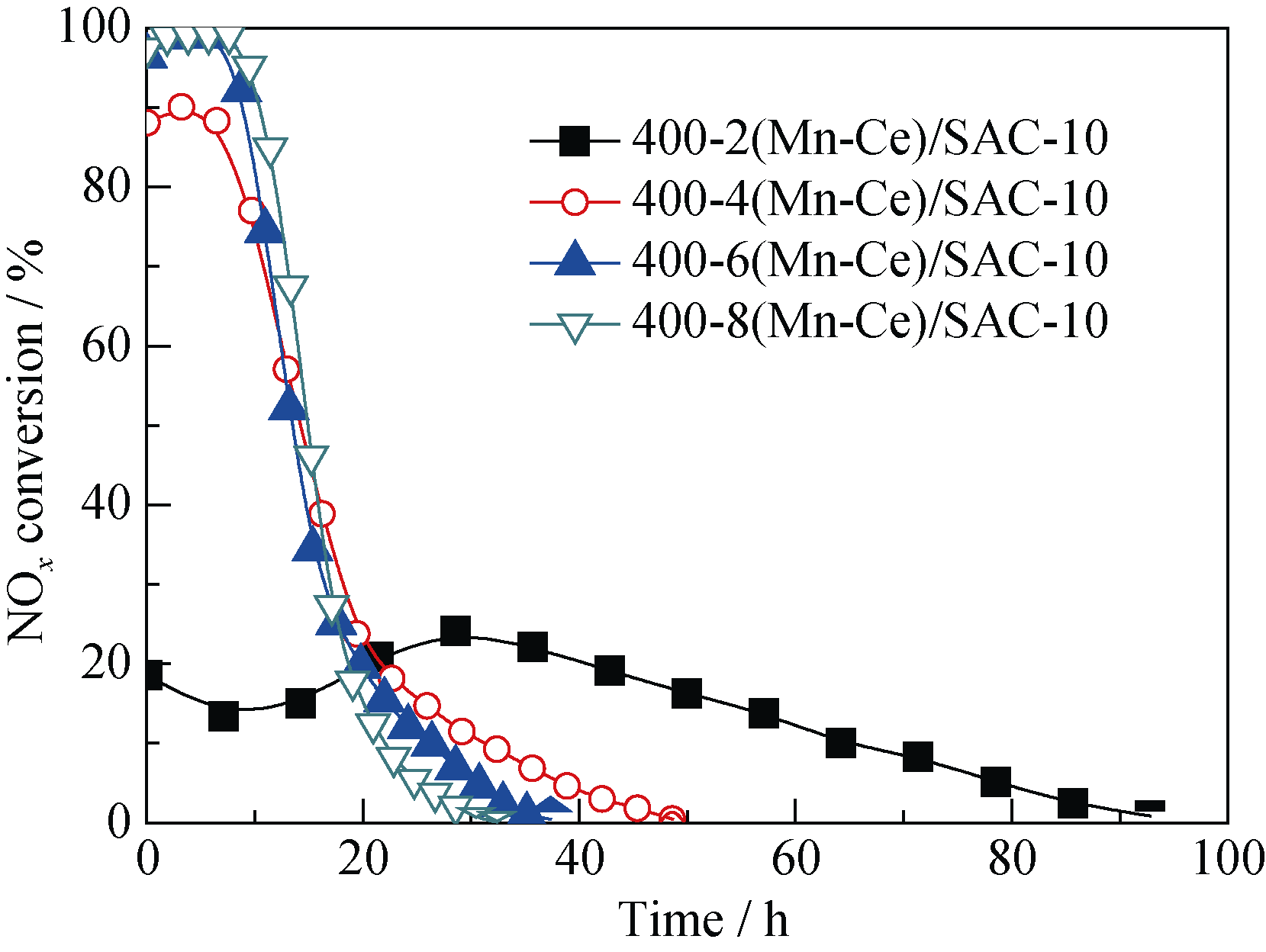
Fig. 6 Effect of metal oxides loading on NOx conversion over melamine-supported catalystsReaction conditions: 0.1% NO, 8% O2, balance N2, reaction temperature = 180℃, space velocity = 6000 h-1
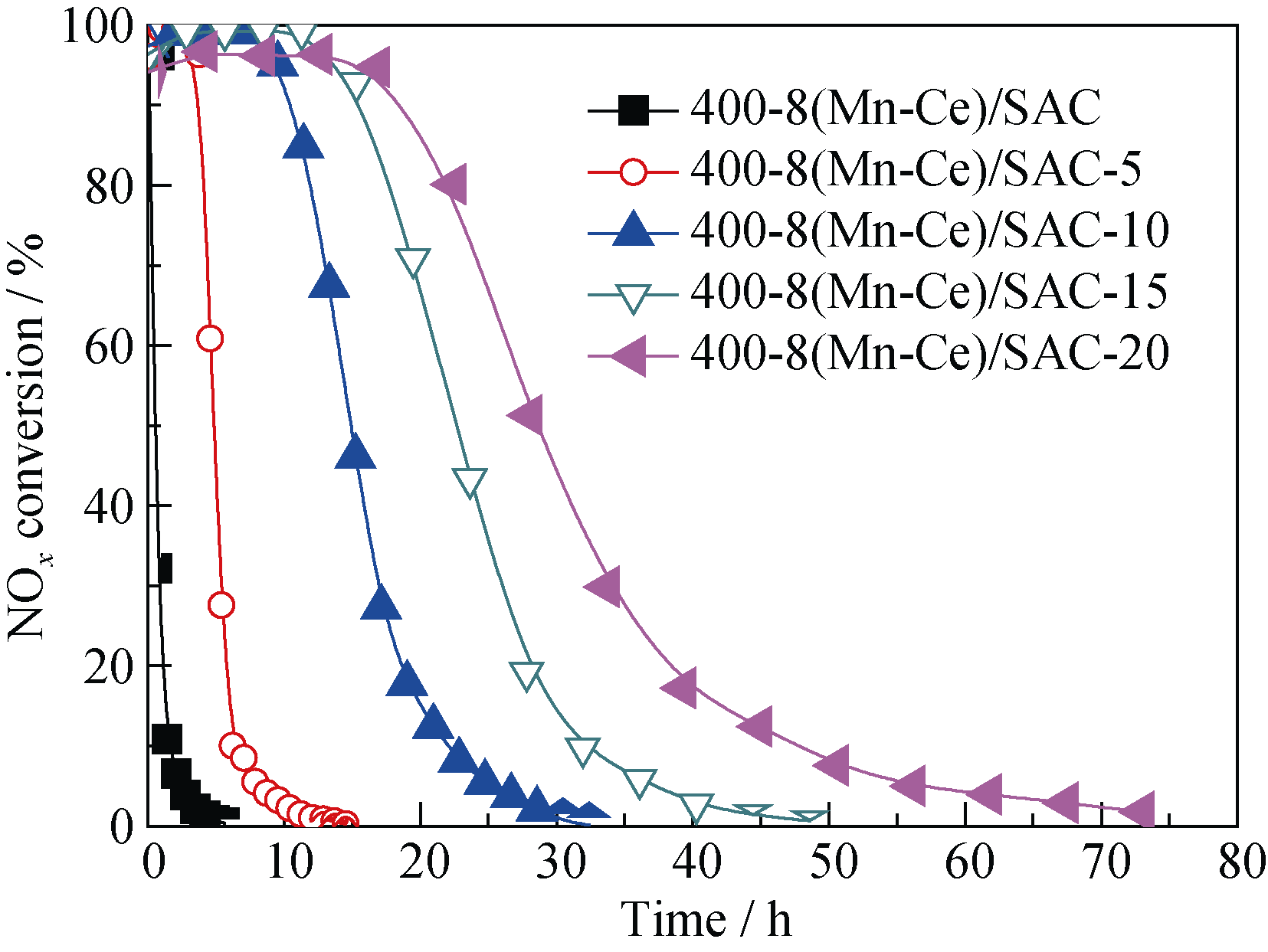
Fig. 7 Effect of melamine loading on NOx conversion over melamine-supported 400-8(Mn-Ce)/SACReaction conditions: 0.1% NO, 8% O2, balance N2, reaction temperature = 180℃, space velocity = 6000 h-1
| Material balance | 400-8(Mn-Ce)/SAC-5 | 400-8(Mn-Ce)/SAC-10 | 400-8(Mn-Ce)/SAC-15 | 400-8(Mn-Ce)/SAC-20 |
|---|---|---|---|---|
| Amount of melamine on sample /×10-4 mol | 2.02 | 4.04 | 6.06 | 8.08 |
| Breakthrough time(BTT) /h | 4.20 | 8.80 | 13.80 | 18.50 |
| Total amount of supplied net NO until BTT /×10-4 mol | 11.30 | 23.70 | 37.10 | 49.80 |
| Ratio of reacted NO to supplied net NO /% | 99.50 | 99.60 | 99.40 | 96.80 |
| Amount of removed NO /× 10-4 mol | 11.20 | 23.60 | 36.90 | 48.20 |
| Mole ratio of reacted NO to melamine on the catalyst | 5.50 | 5.80 | 6.10 | 6.00 |
Table 2 Mole ratio of reacted NO to melamine supported on 400-8(Mn-Ce)/SAC
| Material balance | 400-8(Mn-Ce)/SAC-5 | 400-8(Mn-Ce)/SAC-10 | 400-8(Mn-Ce)/SAC-15 | 400-8(Mn-Ce)/SAC-20 |
|---|---|---|---|---|
| Amount of melamine on sample /×10-4 mol | 2.02 | 4.04 | 6.06 | 8.08 |
| Breakthrough time(BTT) /h | 4.20 | 8.80 | 13.80 | 18.50 |
| Total amount of supplied net NO until BTT /×10-4 mol | 11.30 | 23.70 | 37.10 | 49.80 |
| Ratio of reacted NO to supplied net NO /% | 99.50 | 99.60 | 99.40 | 96.80 |
| Amount of removed NO /× 10-4 mol | 11.20 | 23.60 | 36.90 | 48.20 |
| Mole ratio of reacted NO to melamine on the catalyst | 5.50 | 5.80 | 6.10 | 6.00 |
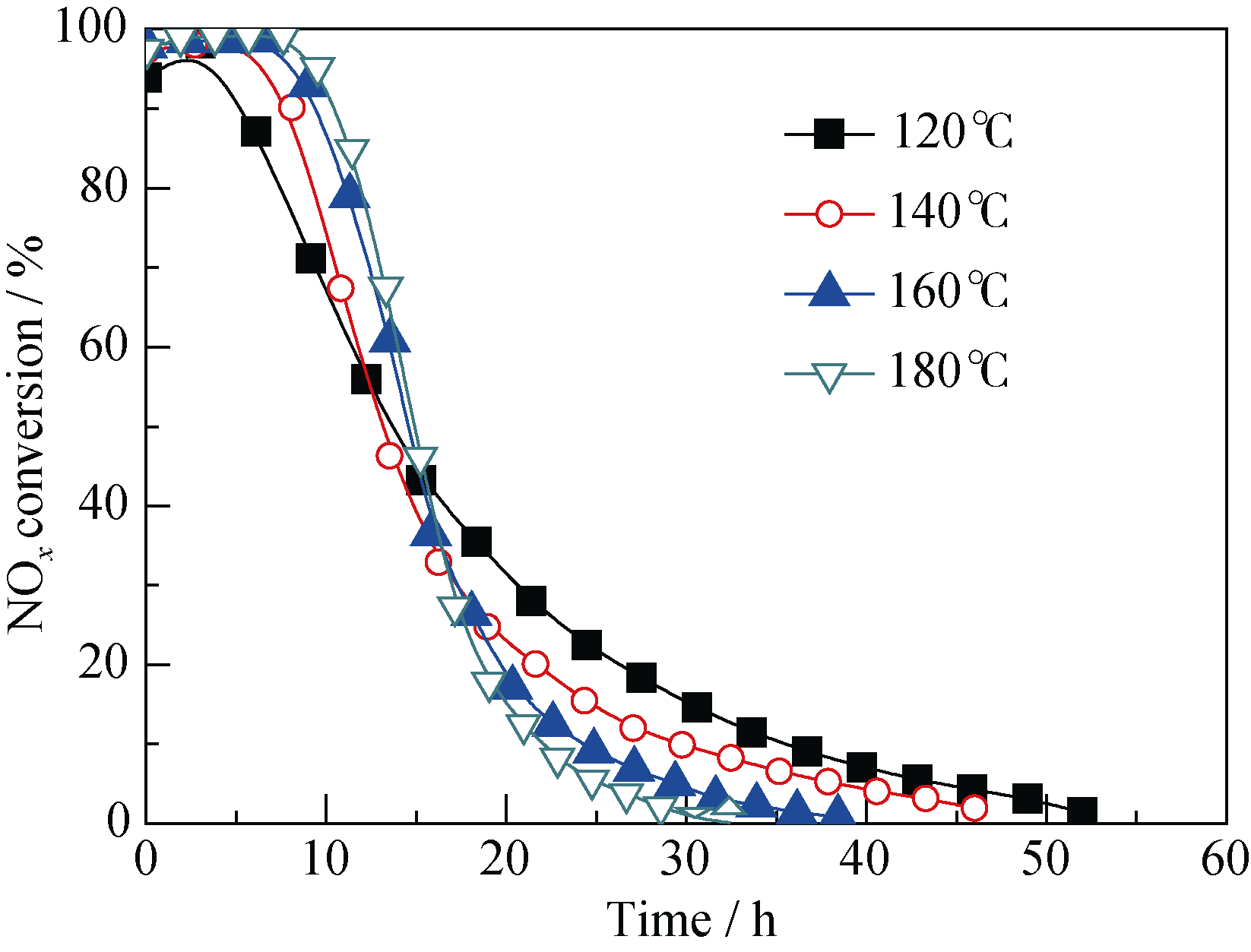
Fig. 8 Effect of reaction temperature on NOx conversion over 400-8(Mn-Ce)/SAC-10Reaction conditions: 0.1% NO, 8% O2, balance N2, space velocity = 6000 h-1

Fig. 9 Effect of (a) NO concentration and (b) O2 concentration on NOx conversion over 400-8(Mn-Ce)/SAC-10Reaction conditions: 0.01%~0.1% NO, 0~8% O2, balance N2, reaction temperature = 180℃, space velocity = 6000 h-1
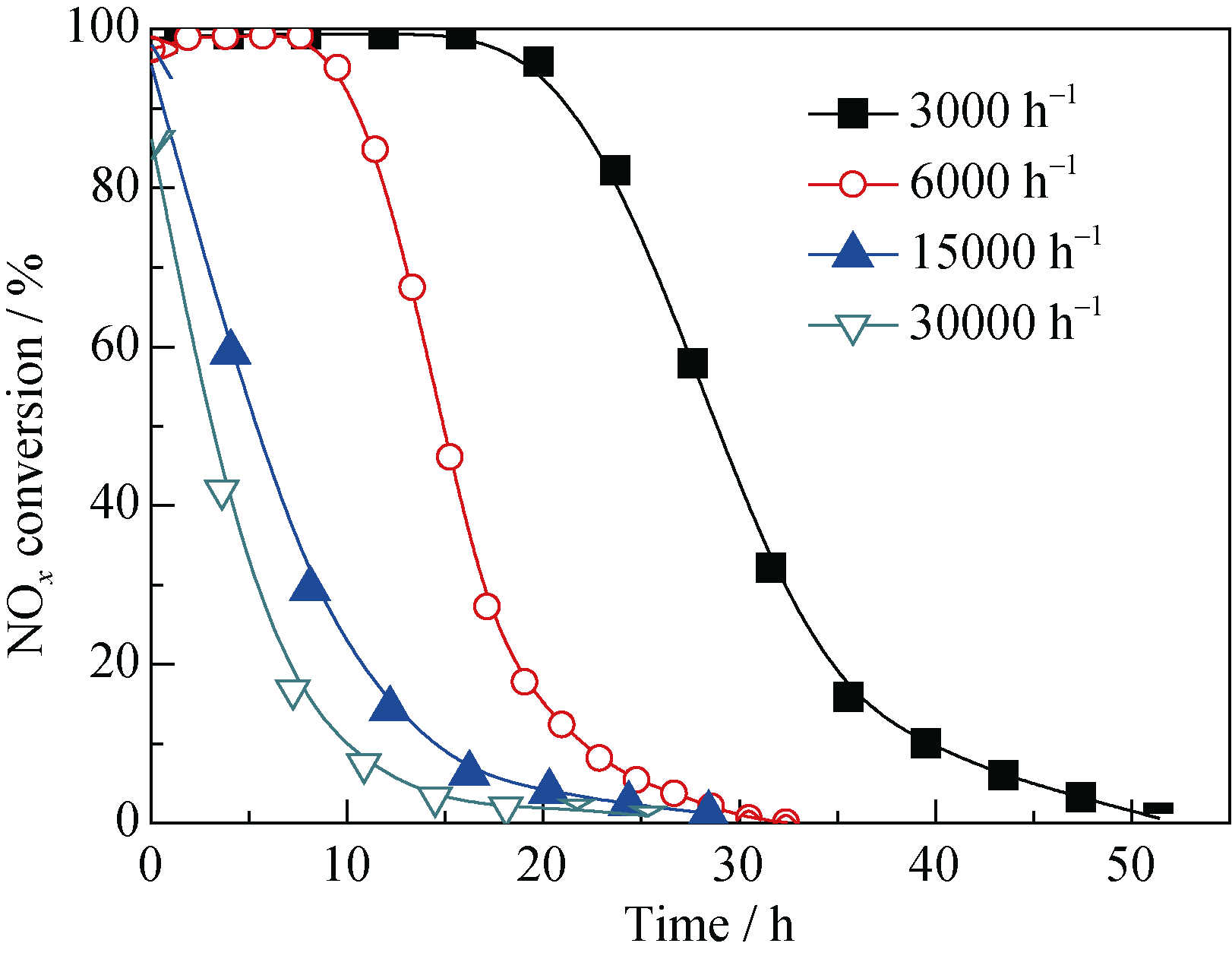
Fig. 10 Effect of space velocity on NOx conversion over 400-8(Mn-Ce)/SAC-10Reaction conditions: 0.1% NO, 8% O2, balance N2, reaction temperature = 180℃
| [1] | BOSCH H, JANSSEN F.Catalytic reduction of nitrogen oxides: A review on the fundamentals and technology. Catalysis Today, 1988, 2(4): 369-532. |
| [2] | YUAN CONG-HUI, LIU HUA-YAN, LU HAN-FENG, et al.Catalytic oxidation-reductive absorption process for NOx removal in humid waste gas.Chinese Journal of Environmental Engineering, 2008, 2(9): 1207-1212. |
| [3] | YAO YAO, ZHANG SHU-LE, ZHONG QIN, et al.Low temperature selective catalytic reduction of NO over manganese supported on TiO2 nanotubes. Journal of Fuel Chemistry and Technology, 2011, 39(9): 694-701. |
| [4] | AN ZHONG-YI, ZHUO YU-QUN, CHEN CHANG-HE.Influence of calcinations temperature on the catalytic activity of Mn/TiO2 for NO oxidation.Journal of Fuel Chemistry and Technology, 2014, 42(3): 370-376. |
| [5] | GUO JING, LI CAI-TING, LU PEI, et al.Research on SCR Denitrification of MnOx/Al2O3 modified by CeO2 and its mechanism at low temperature.Environmental Science, 2011, 32(8): 2240-2246. |
| [6] | LI LI, HUANG HUA-CUN, WEI TENG-YOU, et al.Influence of cerium additive on selective catalytic reduction of NOx with MnOx/ACFN catalyst. Chemical industry and Engineering progress, 2013, 32(11): 2655-2660. |
| [7] | WANG YAN-LI, LI XIAO-XIAO, ZHAN LIANG, et al.Effect of metal additives on the catalytic performance of MnOx-CeO2 supported on activated carbon honeycomb in NO removal at low temperature. Journal of Fuel Chemistry and Technology, 2014, 42(11): 1365-1371. |
| [8] | OZKAN U S, KUMTHEKAR M W, CAI Y P.Selective catalytic reduction of nitric oxide over vanadia/titania catalysts: temperature-programmed desorption and isotopically labeled oxygen-exchange studies.Industrial & Engineering Chemistry Research, 1994, 33(12): 2924-2929. |
| [9] | SHIRAHAMA N, MOCHIDA I, KORAI Y, et al.Reaction of NO2 in air at room temperature with urea supported on pitch based activated carbon fiber.Applied Catalysis B: Environmental, 2004, 52(3): 173-179. |
| [10] | SHIRAHAMA N, MOCHIDA I, KORAI Y, et al.Reaction of NO with urea supported on activated carbons. Applied Catalysis B: Environmental, 2005, 57(4): 237-245. |
| [11] | MIYAWAKI J, SHIMOHARA T, SHIRAHAMA N, et al.Removal of NOx from air through cooperation of the TiO2 photocatalyst and urea on activated carbon fiber at room temperature.Applied Catalysis B: Environmental, 2011, 110: 273-278. |
| [12] | WANG Z, WANG Y L, WANG D J, et al.Low-temperature selective catalytic reduction of NO with urea supported on pitch-based spherical activated carbon.Industrial & Engineering Chemistry Research, 2010, 49(14): 6317-6322. |
| [13] | WANG Z, WANG Y L, LONG D H, et al.Kinetics and mechanism study of low-temperature selective catalytic reduction of NO with urea supported on pitch-based spherical activated carbon.Industrial & Engineering Chemistry Research, 2011, 50(10): 6017-6027. |
| [14] | ZENG Z, LU P, LI C, et al.Selective catalytic reduction (SCR) of NO by urea loaded on activated carbon fiber (ACF) and CeO2/ACF at 30℃: The SCR mechanism.Environmental Technology, 2012, 33(11): 1331-1337. |
| [15] | LU P, ZENG Z, LI C T, et al.Room temperature removal of NO by activated carbon fibres loaded with urea and La2O3. Environmental Technology, 2012, 33(9): 1029-1036. |
| [16] | CHEN H Y, VOSKOBOINIKOV T, SACHTLER W M H. Reaction intermediates in the selective catalytic reduction of NOx over Fe/ZSM-5. Journal of Catalysis, 1999, 186(1): 91-99. |
| [17] | JOUBERT E, COURTOIS X, MARECOT P, et al.The chemistry of DeNOx reactions over Pt/Al2O3: the oxime route to N2 or N2O.Journal of Catalysis, 2006, 243(2): 252-262. |
| [18] | YANG JUN-BING, KANG FEI-YU.Activated carbon spheres and their applications.Materials Review, 2002, 16(5): 59-61. |
| [19] | MACHIDA M, KUROGI D, KIJIMA T, et al.MnOx-CeO2 binary oxides for catalytic NOx sorption at low temperatures. Selective reduction of sorbed NOx.Chemistry of Materials, 2000, 12(10): 3165-3170. |
| [20] | MACHIDA M, UTO M, KUROGI D, et al.Solid-gas interaction of nitrogen oxide adsorbed on MnOx-CeO2: a DRIFTS study. Journal of Materials Chemistry, 2001, 11: 900-904. |
| [21] | QI G, YANG R T.Characterization and FTIR studies of MnOx-CeO2 catalyst for low-temperature selective catalytic reduction of NO with NH3.The Journal of Physical Chemistry B, 2004, 108(40): 15738-15747. |
| [22] | QI G, YANG R T, CHANG R.MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Applied Catalysis B: Environmental, 2004, 51(2): 93-106. |
| [23] | ETTIREDDY P R, ETTIREDDY N, SMIRNIOTIS P G, et al.Investigation of the selective catalytic reduction of nitric oxide with ammonia over Mn/TiO2 catalysts through transient isotopic labeling and in situ FT-IR studies.Journal of Catalysis, 2012, 292: 53-63. |
| [24] | XIE J L, FANG D, CHEN X L, et al.Performance and mechanism about MnOx species included in MnOx/TiO2 catalysts for SCR at low temperature.Catalysis Communications, 2012, 28: 77-81. |
| [1] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [2] | TANG Xinli, DING Ziyou, CHEN Junrui, ZHAO Gang, HAN Yingchao. In vivo Distribution and Metabolism of Calcium Phosphate Nanomaterials Based on Fluorescent Labeling with Rare Earth Europium Ions [J]. Journal of Inorganic Materials, 2025, 40(7): 754-764. |
| [3] | ZHOU Houlin, SONG Zhiqing, TIAN Guo, GAO Xingsen. Effects of Growth Conditions on the Formation of Self-assembly Grown Topological Domain in BiFeO3 Nanoislands [J]. Journal of Inorganic Materials, 2025, 40(6): 667-674. |
| [4] | AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao. Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance [J]. Journal of Inorganic Materials, 2025, 40(5): 457-465. |
| [5] | QU Jifa, WANG Xu, ZHANG Weixuan, ZHANG Kangzhe, XIONG Yongheng, TAN Wenyi. Enhanced Sulfur-resistance for Solid Oxide Fuel Cells Anode via Doping Modification of NaYTiO4 [J]. Journal of Inorganic Materials, 2025, 40(5): 489-496. |
| [6] | CHEN Xi, YUAN Yuan, TAN Yeqiang, LIU Changsheng. Strategic Study on the Development of Inorganic Non-metallic Biomaterials [J]. Journal of Inorganic Materials, 2025, 40(5): 449-456. |
| [7] | LI Jianjun, CHEN Fangming, ZHANG Lili, WANG Lei, ZHANG Liting, CHEN Huiwen, XUE Changguo, XU Liangji. Peroxymonosulfate Activation by CoFe2O4/MgAl-LDH Catalyst for the Boosted Degradation of Antibiotic [J]. Journal of Inorganic Materials, 2025, 40(4): 440-448. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | JIA Xianghua, ZHANG Huixia, LIU Yanfeng, ZUO Guihong. Cu2O/Cu Hollow Spherical Heterojunction Photocatalysts Prepared by Wet Chemical Approach [J]. Journal of Inorganic Materials, 2025, 40(4): 397-404. |
| [10] | XIN Zhenyu, GUO Ruihua, WUREN Tuoya, WANG Yan, AN Shengli, ZHANG Guofang, GUAN Lili. Pt-Fe/GO Nanocatalysts: Preparation and Electrocatalytic Performance on Ethanol Oxidation [J]. Journal of Inorganic Materials, 2025, 40(4): 379-387. |
| [11] | YUAN Liping, WU Yuanbo, YU Jiajing, ZHANG Shiyan, SUN Yi, HU Yunchu, FAN Youhua. CNFs Aerogel Composite with Phosphomolybdic Acid Intercalated Hydrotalcite: Preparation and Thermal Insulation Performance [J]. Journal of Inorganic Materials, 2025, 40(4): 415-424. |
| [12] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [13] | MU Haojie, ZHANG Yuanjiang, YU Bin, FU Xiumei, ZHOU Shibin, LI Xiaodong. Preparation and Properties of ZrO2 Doped Y2O3-MgO Nanocomposite Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 281-289. |
| [14] | YE Junhao, ZHOU Zhenzhen, HU Chen, WANG Yanbin, JING Yanqiu, LI Tingsong, CHENG Ziqiu, WU Junlin, IVANOV Maxim, HRENIAK Dariusz, LI Jiang. Yb:Sc2O3 Transparent Ceramics Fabricated from Co-precipitated Nano-powders: Microstructure and Optical Property [J]. Journal of Inorganic Materials, 2025, 40(2): 215-224. |
| [15] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||