无机材料学报 ›› 2025, Vol. 40 ›› Issue (7): 747-753.DOI: 10.15541/jim20240486 CSTR: 32189.14.jim20240486
所属专题: 【能源环境】金属有机框架材料MOF(202510)
江宗玉1( ), 黄红花2, 清江3, 王红宁1(
), 黄红花2, 清江3, 王红宁1( ), 姚超1, 陈若愚1
), 姚超1, 陈若愚1
收稿日期:2024-11-15
修回日期:2025-02-19
出版日期:2025-07-20
网络出版日期:2025-02-25
通讯作者:
王红宁, 副教授. E-mail: hnwang@cczu.edu.cn作者简介:江宗玉(2000-), 女, 硕士研究生. E-mail: 17855227241@163.com
基金资助:
JIANG Zongyu1( ), HUANG Honghua2, QING Jiang3, WANG Hongning1(
), HUANG Honghua2, QING Jiang3, WANG Hongning1( ), YAO Chao1, CHEN Ruoyu1
), YAO Chao1, CHEN Ruoyu1
Received:2024-11-15
Revised:2025-02-19
Published:2025-07-20
Online:2025-02-25
Contact:
WANG Hongning, associate professor. E-mail: hnwang@cczu.edu.cnAbout author:JIANG Zongyu (2000-), female, Master candidate. E-mail: 17855227241@163.com
Supported by:摘要:
挥发性有机化合物(VOCs)危害环境质量和人体健康。为了提高吸附剂对VOCs的吸附性能, 本研究基于金属有机框架(MOFs)中金属位点可以取代的策略, 采用一步溶剂热法在MIL-101(Cr)合成体系中掺杂丰富、廉价且环保的金属离子Al3+, 以增加MIL-101(Cr)表面的不饱和金属位点。通过调节Al3+掺杂量, 得到一系列Al-MIL-101(Cr)样品。通过不同手段表征Al-MIL-101(Cr)样品的形貌和结构, 并研究其对甲苯、正己烷、油气和对二甲苯的吸附性能。MIL-101(Cr)的甲苯、正己烷、油气和对二甲苯静态吸附容量分别为0.676、0.621、0.451和0.812 g·g-1。随着Al3+掺杂量增多, Al-MIL-101(Cr)样品的吸附容量先增加后减小; 当Al3+掺杂量达到0.75 mmol时, Al-0.75- MIL-101(Cr)具有最大的吸附容量(0.911 g·g-1甲苯, 0.755 g·g-1正己烷, 0.713 g·g-1油气, 0.875 g·g-1对二甲苯)。通过单一组分甲苯穿透曲线评估Al-MIL-101(Cr)样品的动态吸附行为, 结果表明Al-MIL-101(Cr)具有良好的VOCs去除能力, 这与其较大的比表面积以及不饱和金属位点增多有关。
中图分类号:
江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753.
JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance[J]. Journal of Inorganic Materials, 2025, 40(7): 747-753.

图3 MIL-101(Cr) (a)、Al-0.25-MIL-101(Cr) (b)、Al-0.50- MIL-101(Cr) (c)、Al-0.75-MIL-101(Cr) (d)和Al-1.0-MIL- 101(Cr) (e)的SEM照片
Fig. 3 SEM images of MIL-101(Cr) (a), Al-0.25-MIL-101(Cr) (b), Al-0.50-MIL-101(Cr) (c), Al-0.75-MIL-101(Cr) (d), and Al-1.0-MIL-101(Cr) (e)
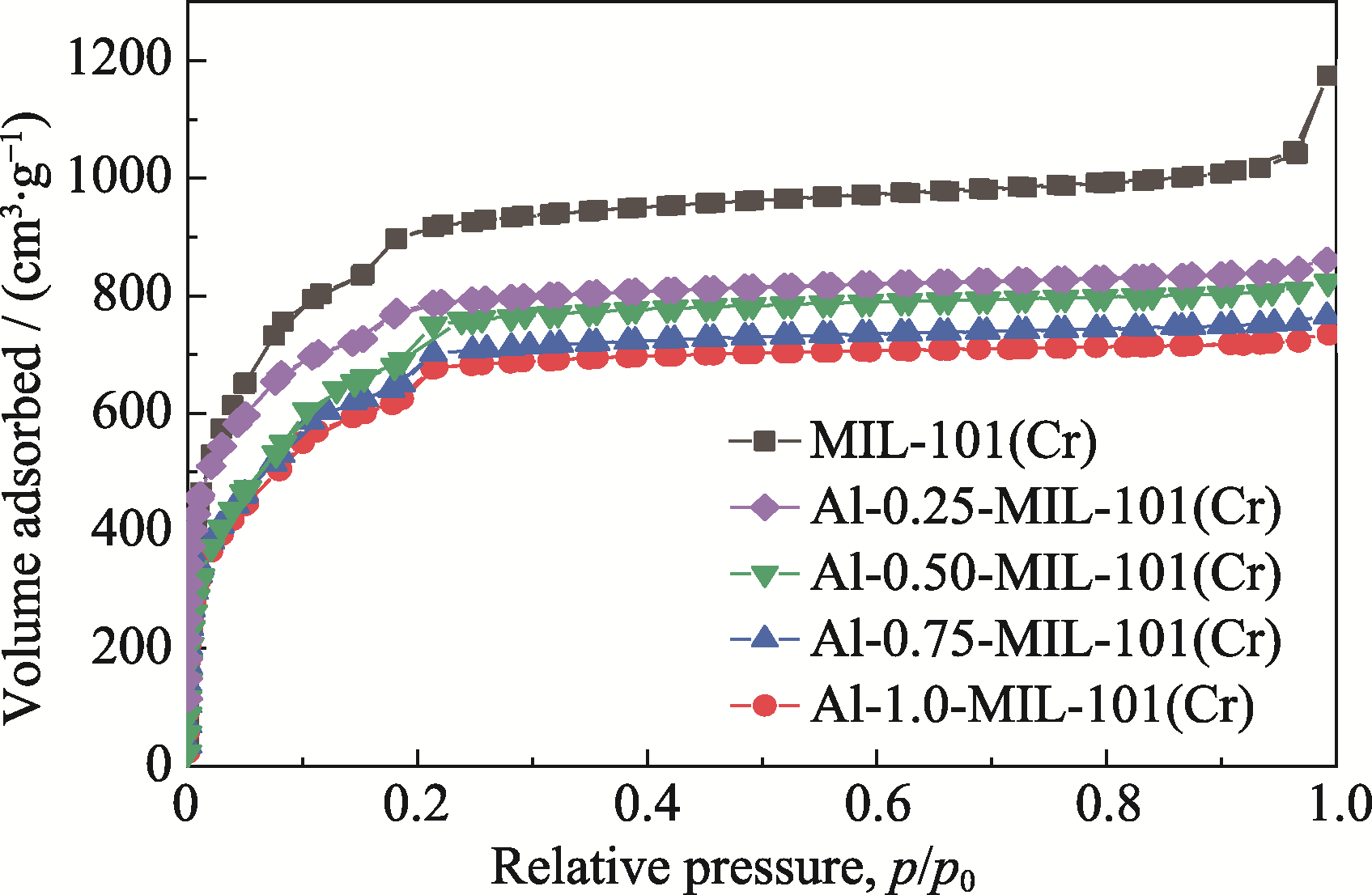
图5 MIL-101(Cr)和Al-MIL-101(Cr)的氮气吸附-脱附等温线
Fig. 5 Nitrogen adsorption-desorption isotherms of MIL-101(Cr) and Al-MIL-101(Cr) Value of the vertical axis of Al-0.25-MIL-101(Cr) is shifted upwards by 80 units while that of Al-0.50-MIL-101(Cr) is shifted downwards by 80 units
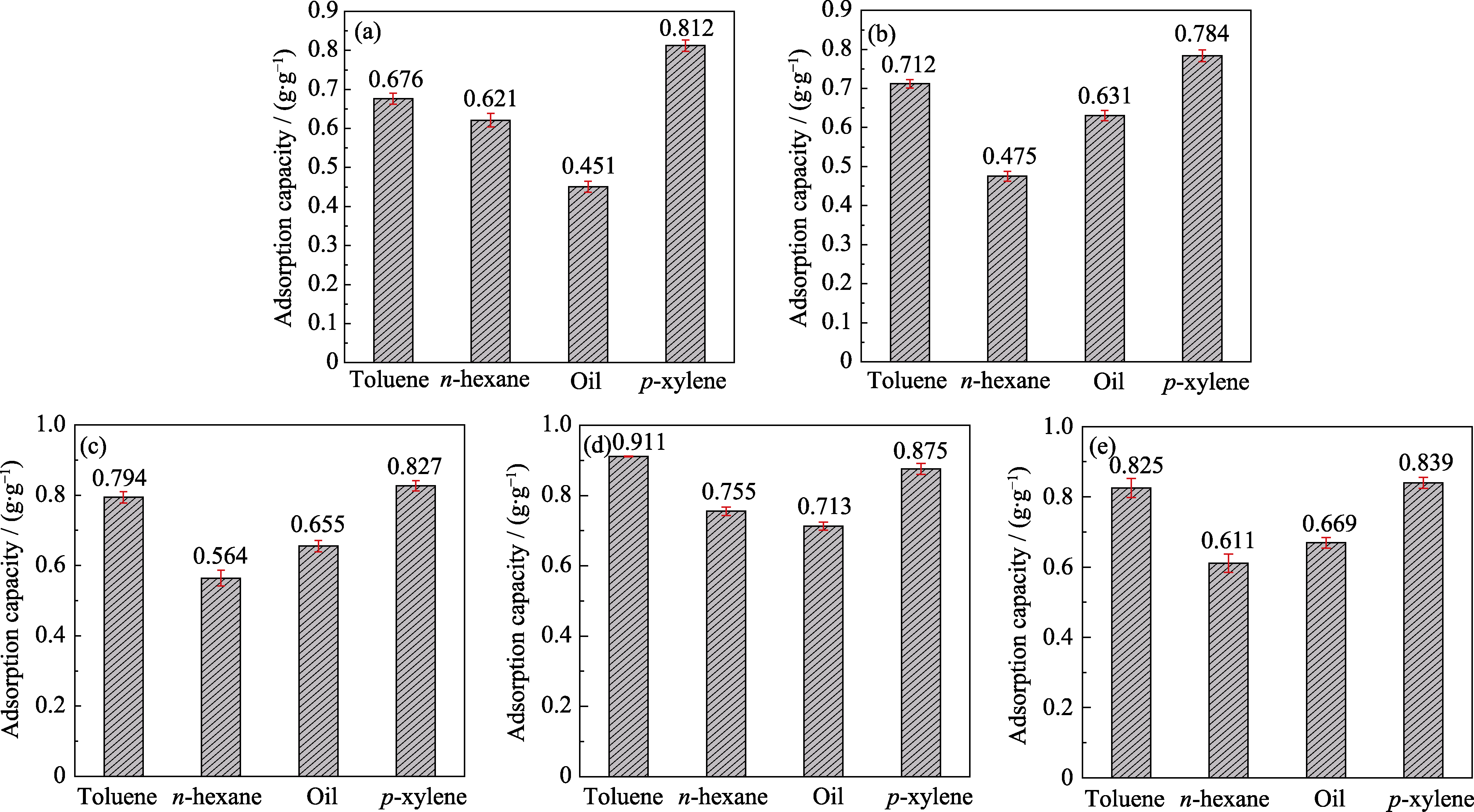
图6 MIL-101(Cr) (a)、Al-0.25-MIL-101(Cr) (b)、Al-0.50-MIL-101(Cr) (c)、Al-0.75-MIL-101(Cr) (d) 和Al-1.0-MIL-101(Cr) (e)的静态吸附容量
Fig. 6 Static adsorption capacities of MIL-101(Cr) (a), Al-0.25-MIL-101(Cr) (b), Al-0.50-MIL-101(Cr) (c), Al-0.75-MIL-101(Cr) (d), and Al-1.0-MIL-101(Cr) (e)
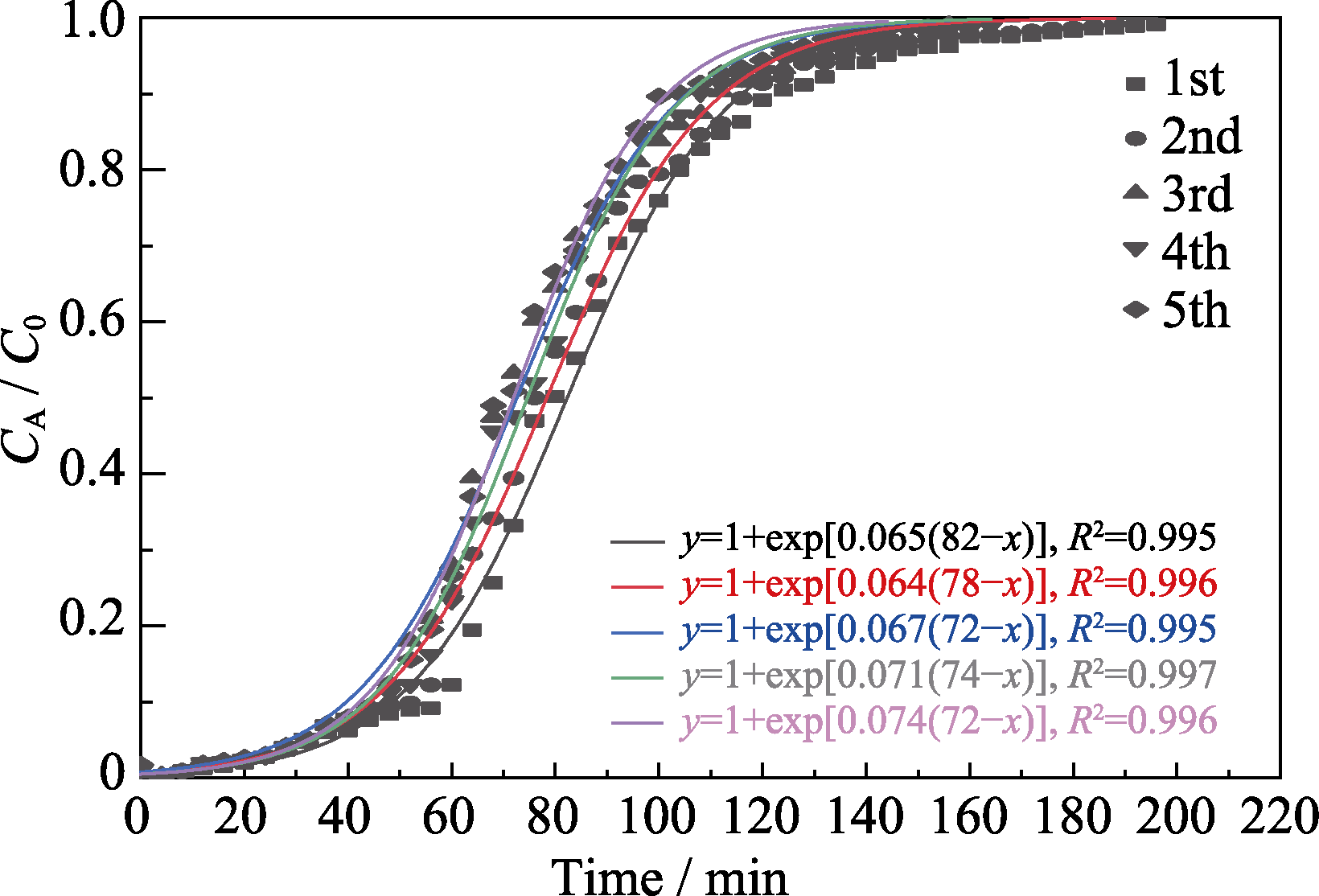
图8 Al-0.75-MIL-101(Cr)的甲苯吸附穿透拟合曲线
Fig. 8 Toluene adsorption breakthrough fitting curves of Al-0.75-MIL-101(Cr) Colorful figure is available on website
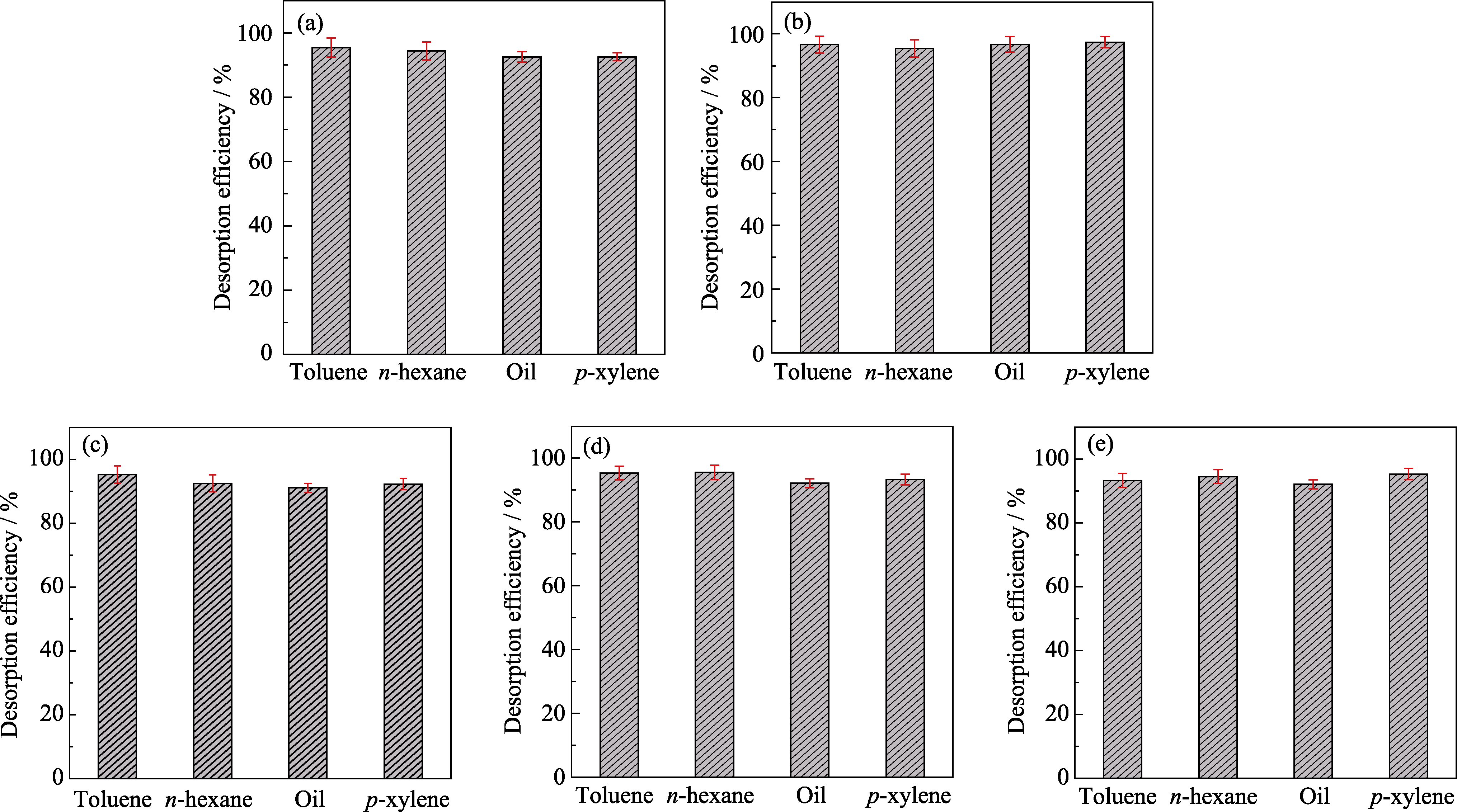
图S3 MIL-101(Cr) (a)、Al-0.25-MIL-101(Cr) (b)、Al-0.50-MIL-101(Cr) (c)、Al-0.75-MIL-101(Cr) (d)和Al-1.0-MIL-101(Cr) (e)对甲苯、正己烷、油气和对二甲苯的解吸效率图
Fig. S3 Desorption efficiency diagrams of toluene, n-hexane, oil and p-xylene of MIL-101(Cr) (a), Al-0.25-MIL-101(Cr) (b), Al-0.50-MIL-101(Cr) (c), Al-0.75-MIL-101(Cr) (d), andAl-1.0-MIL-101(Cr) (e)
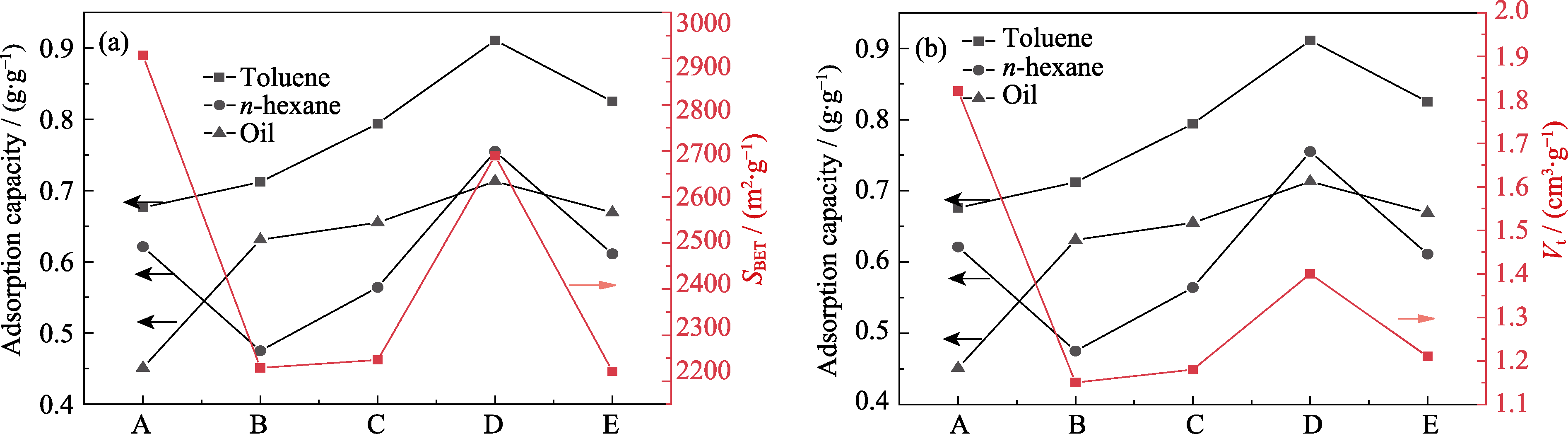
图S4 MIL-101(Cr)和Al-MIL-101(Cr)的正己烷、甲苯以及油气的静态吸附容量与SBET(a)和Vt(b)的关系图
Fig. S4 Relationships between static adsorption capacities of MIL-101(Cr) and Al-MIL-101(Cr) for n-hexane, toluene, and oil and SBET (a) and Vt (b) A: MIL-101(Cr); B: Al-0.25-MIL-101(Cr); C: Al-0.50-MIL-101(Cr); D: Al-0.75-MIL-101(Cr); E: Al-1.0-MIL-101(Cr)
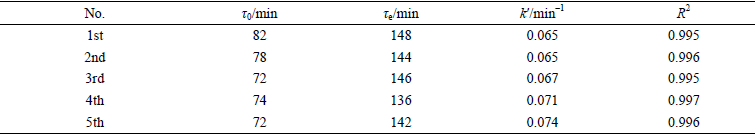 |
表S2 Al-0.75-MIL-101(Cr)的5次动态甲苯吸附Yoon-Nelson模型的拟合参数
Table S2 Yoon-Nelson model fitting parameters of 5 times dynamic toluene adsorption for Al-0.75-MIL-101(Cr)
 |
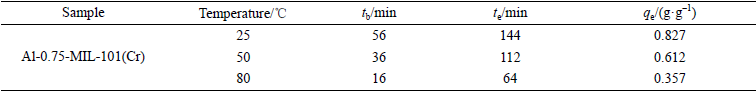 |
表S3 Al-0.75-MIL-101(Cr)在不同温度下的动态甲苯吸附参数比较
Table S3 Comparison of dynamic toluene adsorption parameters of Al-0.75-MIL-101(Cr) at different temperatures
 |
| [1] | YE Q Q, CHEN Y Y, LI Y Z, et al. Management of typical VOCs in air with adsorbents: status and challenges. Dalton Transactions, 2023, 52(35): 12169. |
| [2] | FETISOV V, GONOPOLSKY A M, DAVARDOOST H, et al. Regulation and impact of VOC and CO2 emissions on low-carbon energy systems resilient to climate change: a case study on an environmental issue in the oil and gas industry. Energy Science & Engineering, 2023, 11(4): 1516. |
| [3] | HE T, KONG X J, BIAN Z X, et al. Trace removal of benzene vapour using double-walled metal-dipyrazolate frameworks. Nature Materials, 2022, 21(6): 689. |
| [4] |
GAN G Q, FAN S Y, LI X Y, et al. Adsorption and membrane separation for removal and recovery of volatile organic compounds. Journal of Environmental Sciences, 2023, 123: 96.
DOI PMID |
| [5] | WANG H C, GUO H, ZHAO Y X, et al. Thermodynamic analysis of a petroleum volatile organic compounds (VOCs) condensation recovery system combined with mixed-refrigerant refrigeration. International Journal of Refrigeration, 2020, 116: 23. |
| [6] | WU X M, ZHANG Q G, SOYEKWO F, et al. Pervaporation removal of volatile organic compounds from aqueous solutions using the highly permeable PIM-1 membrane. AIChE Journal, 2016, 62(3): 842. |
| [7] | YANG Y, ZHAO S H, CUI L F, et al. Recent advancement and future challenges of photothermal catalysis for VOCs elimination: from catalyst design to applications. Green Energy & Environment, 2023, 8(3): 654. |
| [8] | QIAN X F, YUE D T, TIAN Z Y, et al. Carbon quantum dots decorated Bi2WO6 nanocomposite with enhanced photocatalytic oxidation activity for VOCs. Applied Catalysis B: Environmental, 2016, 193: 16. |
| [9] | QIN L B, XU Z, LIU L, et al. In-situ biodegradation of volatile organic compounds in landfill by sewage sludge modified waste-char. Waste Management, 2020, 105: 317. |
| [10] | SUGIMOTO I, NAGAOKA T, SEYAMA M, et al. Classification and characterization of atmospheric VOCs based on sorption/ desorption behaviors of plasma polymer films. Sensors and Actuators B: Chemical, 2007, 124(1): 53. |
| [11] | YANG K, XUE F, SUN Q, et al. Adsorption of volatile organic compounds by metal-organic frameworks MOF-177. Journal of Environmental Chemical Engineering, 2013, 1(4): 713. |
| [12] | WANG Y, XIE J, WU Y C, et al. A magnetic metal-organic framework as a new sorbent for solid-phase extraction of copper(II), and its determination by electrothermal AAS. Microchimica Acta, 2014, 181(9): 949. |
| [13] | XIE L H, LIU X M, HE T, et al. Metal-organic frameworks for the capture of trace aromatic volatile organic compounds. Chem, 2018, 4(8): 1911. |
| [14] | YE Z C, YAO J C, ZHENG W, et al. The preparation and adsorption performance of Co-doped MIL-101(Cr) for low-concentration C3F8. Chemical Engineering Science, 2023, 282: 119302. |
| [15] | LLEWELLYN P L, BOURRELLY S, SERRE C, et al. High uptakes of CO2 and CH4 in mesoporous metal-organic frameworks MIL-100 and MIL-101. Langmuir, 2008, 24(14): 7245. |
| [16] | CAO J W, LI Y X, MA X B, et al. Constructing binuclear sites to modulate the charge distribution of MIL-101 for enhanced toluene adsorption performance: experimental and theoretical studies. Separation and Purification Technology, 2025, 354: 129400. |
| [17] | SHAO P, KUANG X Y, DING L P, et al. Can CO2 molecule adsorb effectively on Al-doped boron nitride single walled nanotube? Applied Surface Science, 2013, 285: 350. |
| [18] | XIA X N, XU Y Z, CHEN Y, et al. Fabrication of MIL-101(Cr/Al) with flower-like morphology and its catalytic performance. Applied Catalysis A: General, 2018, 559: 138. |
| [19] | JAVANBAKHT V, RAFIEE Z. Fibrous polyester sponge modified with carboxymethyl cellulose and zeolitic imidazolate frameworks for methylene blue dye removal in batch and continuous adsorption processes. Journal of Molecular Structure, 2022, 1249: 131552. |
| [20] | AWADALLAH-F A, ELKHATAT A M, AL-MUHTASEB S A. Impact of synthesis conditions on meso- and macropore structures of resorcinol-formaldehyde xerogels. Journal of Materials Science, 2011, 46(24): 7760. |
| [21] | WICKENHEISSER M, HERBST A, TANNERT R, et al. Hierarchical MOF-xerogel monolith composites from embedding MIL-100(Fe, Cr) and MIL-101(Cr) in resorcinol-formaldehyde xerogels for water adsorption applications. Microporous and Mesoporous Materials, 2015, 215: 143. |
| [22] | FENG L J, LI J L, OU Y Y, et al. Study on the Pb(II) adsorption mechanism on acid-modified ceramsite made from sewage sludge and industrial solid wastes. International Journal of Applied Ceramic Technology, 2024, 21(5): 3389. |
| [23] |
TIWARI S, SALEEM M, MISHRA A, et al. Structural, optical, and dielectric studies on Sr-doped biferroic YCrO3. Journal of Superconductivity and Novel Magnetism, 2019, 32(8): 2521.
DOI |
| [24] | WANG Y Q, FU X, PAN T T, et al. Stable effect on MIL-101(Cr) with Cu2+ for the toluene adsorption. Journal of Solid State Chemistry, 2022, 316: 123633. |
| [25] | ZHENG Y, CHU F C, ZHANG B, et al. Ultrahigh adsorption capacities of carbon tetrachloride on MIL-101 and MIL-101/graphene oxide composites. Microporous and Mesoporous Materials, 2018, 263: 71. |
| [26] | ZHANG F F, SHANG H, WANG L, et al. Substituent-induced electron-transfer strategy for selective adsorption of N2 in MIL-101(Cr)-X metal-organic frameworks. ACS Applied Materials & Interfaces, 2022, 14(1): 2146. |
| [27] | CAO E P, ZHENG Y H, ZHANG H, et al. In-situ regenerable Cu/Zeolite adsorbent with excellent H2S adsorption capacity for blast furnace gas. Separation and Purification Technology, 2024, 336: 126305. |
| [28] | WANG H Y, WANG B D, LI J H, et al. Adsorption equilibrium and thermodynamics of acetaldehyde/acetone on activated carbon. Separation and Purification Technology, 2019, 209: 535. |
| [29] |
KONGGIDINATA M I, CHAO B, LIAN Q Y, et al. Equilibrium, kinetic and thermodynamic studies for adsorption of BTEX onto ordered mesoporous carbon (OMC). Journal of Hazardous Materials, 2017, 336: 249.
DOI PMID |
| [1] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [2] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [3] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [4] | 瞿牡静, 张淑兰, 朱梦梦, 丁浩杰, 段嘉欣, 代恒龙, 周国红, 李会利. CsPbBr3@MIL-53纳米复合荧光粉的合成、性能及其白光LEDs应用[J]. 无机材料学报, 2024, 39(9): 1035-1043. |
| [5] | 潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919. |
| [6] | 苗鑫, 闫世强, 韦金豆, 吴超, 樊文浩, 陈少平. Te基热电器件反常界面层生长行为及界面稳定性研究[J]. 无机材料学报, 2024, 39(8): 903-910. |
| [7] | 陈甜, 罗媛, 朱刘, 郭学益, 杨英. 有机-无机共添加增强柔性钙钛矿太阳能电池机械弯曲及环境稳定性能[J]. 无机材料学报, 2024, 39(5): 477-484. |
| [8] | 杨博, 吕功煊, 马建泰. 镍铁氢氧化物-磷化钴复合电极电催化分解水研究[J]. 无机材料学报, 2024, 39(4): 374-382. |
| [9] | 张宇晨, 陆知遥, 赫晓东, 宋广平, 朱春城, 郑永挺, 柏跃磊. 硫族MAX相硼化物的物相稳定性和性能预测[J]. 无机材料学报, 2024, 39(2): 225-232. |
| [10] | 王煜, 熊浩, 黄孝坤, 江琳沁, 吴波, 黎健生, 杨爱军. 低剂量异辛酸亚锡调控两步法制备Sn-Pb混合钙钛矿太阳能电池[J]. 无机材料学报, 2024, 39(12): 1339-1347. |
| [11] | 周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158. |
| [12] | 方万丽, 沈黎丽, 李海艳, 陈薪羽, 陈宗琦, 寿春晖, 赵斌, 杨松旺. NiOx介孔层的成膜过程对碳电极钙钛矿太阳能电池性能的影响[J]. 无机材料学报, 2023, 38(9): 1103-1109. |
| [13] | 陈雨, 林埔安, 蔡冰, 张文华. 钙钛矿太阳能电池无机空穴传输材料的研究进展[J]. 无机材料学报, 2023, 38(9): 991-1004. |
| [14] | 胡忠良, 傅赟天, 蒋蒙, 王连军, 江莞. Nb/Mg3SbBi界面层热稳定性研究[J]. 无机材料学报, 2023, 38(8): 931-937. |
| [15] | 刘建, 王凌坤, 许保亮, 赵倩, 王耀萱, 丁艺, 张胜泰, 段涛. 熔盐法低温合成掺钕ZrSiO4陶瓷的物相演变和化学稳定性[J]. 无机材料学报, 2023, 38(8): 910-916. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||