无机材料学报 ›› 2021, Vol. 36 ›› Issue (10): 1047-1052.DOI: 10.15541/jim20210078 CSTR: 32189.14.10.15541/jim20210078
收稿日期:2021-02-05
修回日期:2021-03-02
出版日期:2021-10-20
网络出版日期:2021-03-15
通讯作者:
宋二红, 副研究员. E-mail: ehsong@mail.sic.ac.cn; 王连军, 教授. E-mail: wanglj@dhu.edu.cn
作者简介:何俊龙(1996-), 男, 硕士研究生. E-mail: woaichenzy@outlook.com
基金资助:
HE Junlong1( ), SONG Erhong2(
), SONG Erhong2( ), WANG Lianjun1(
), WANG Lianjun1( ), JIANG Wan1
), JIANG Wan1
Received:2021-02-05
Revised:2021-03-02
Published:2021-10-20
Online:2021-03-15
Contact:
SONG Erhong, associate professor. E-mail: ehsong@mail.sic.ac.cn; WANG Lianjun, professor. E-mail: wanglj@dhu.edu.cn
About author:HE Junlong(1996-), Master candidate. E-mail: woaichenzy@outlook.com
Supported by:摘要:
石墨烯具有较高的比表面积, 其电导率会因吸附微量气体分子而发生显著变化, 有望用作超高灵敏度的气体传感器。本研究基于密度泛函理论(DFT)的计算方法, 探讨了NO在石墨烯和Cr掺杂石墨烯上的吸附行为, 通过对比吸附前后的各自体系的电子结构变化, 发现Cr掺杂石墨烯有助于增强对NO气体分子的吸附能力, 吸附能增大到-1.58 eV, 基底转移到吸附物的电荷数增大了一个数量级, 达到0.143 e, 显著提升了气体探测灵敏度。本研究为工业、环境和军事监测领域中开发新型NO气体传感器提供了新的设计思路。
中图分类号:
何俊龙, 宋二红, 王连军, 江莞. DFT方法研究一氧化氮在铬掺杂石墨烯上的吸附行为[J]. 无机材料学报, 2021, 36(10): 1047-1052.
HE Junlong, SONG Erhong, WANG Lianjun, JIANG Wan. DFT Calculation of NO Adsorption on Cr Doped Graphene[J]. Journal of Inorganic Materials, 2021, 36(10): 1047-1052.
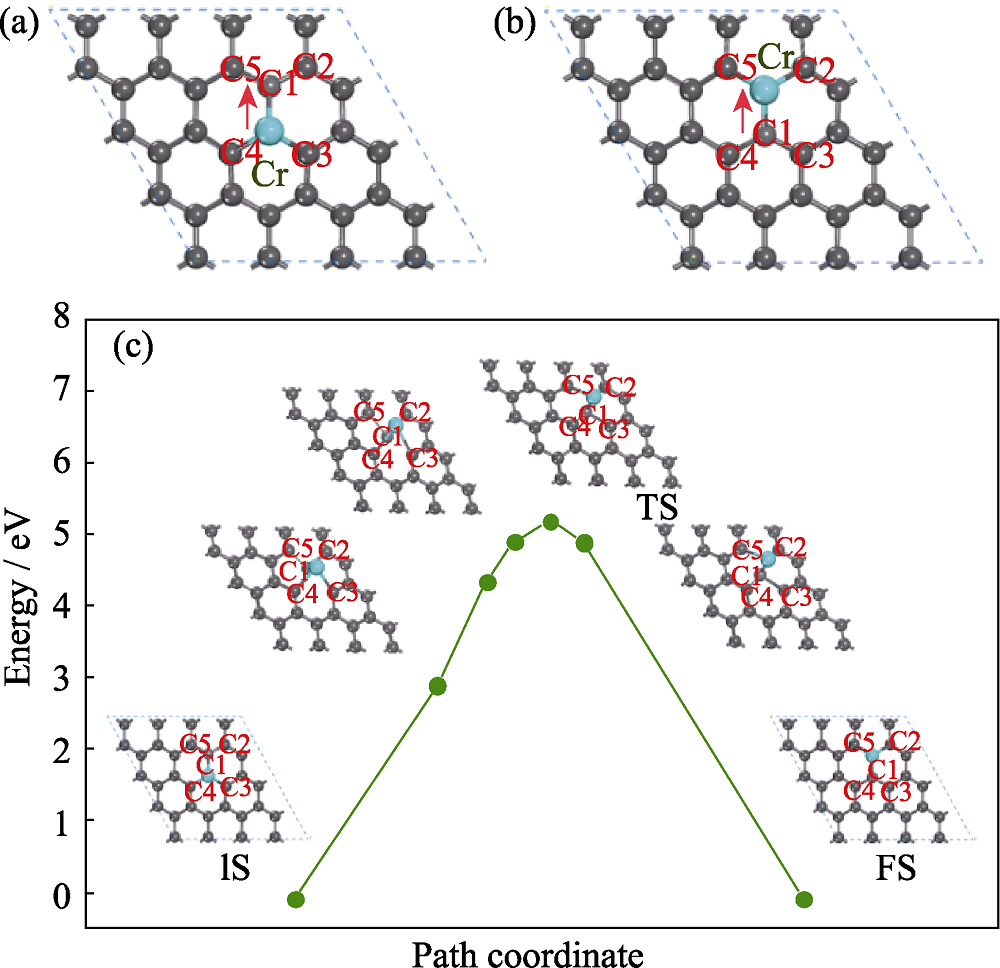
图1 Cr原子掺杂石墨烯扩散过程中优化的原子结构图
Fig. 1 Optimized structure of Cr-doped graphene (a) Initial state (IS); (b) Relaxed configuration of final state (FS); (c) Diffusion period of Cr atom on graphene; Gray and cyan spheres denote C and Cr atoms, respectively

图2 NO吸附在石墨烯和Cr掺杂石墨烯的原子结构示意图
Fig. 2 Atomic configuration of NO adsorption on graphene and Cr-doped graphene Graphene with N-end model (a) and O-end model (b), and Cr-doped graphene with N-end (c) and O-end model (d); Gray, cyan, blue, and red spheres denote C, Cr, N, and O atoms, respectively
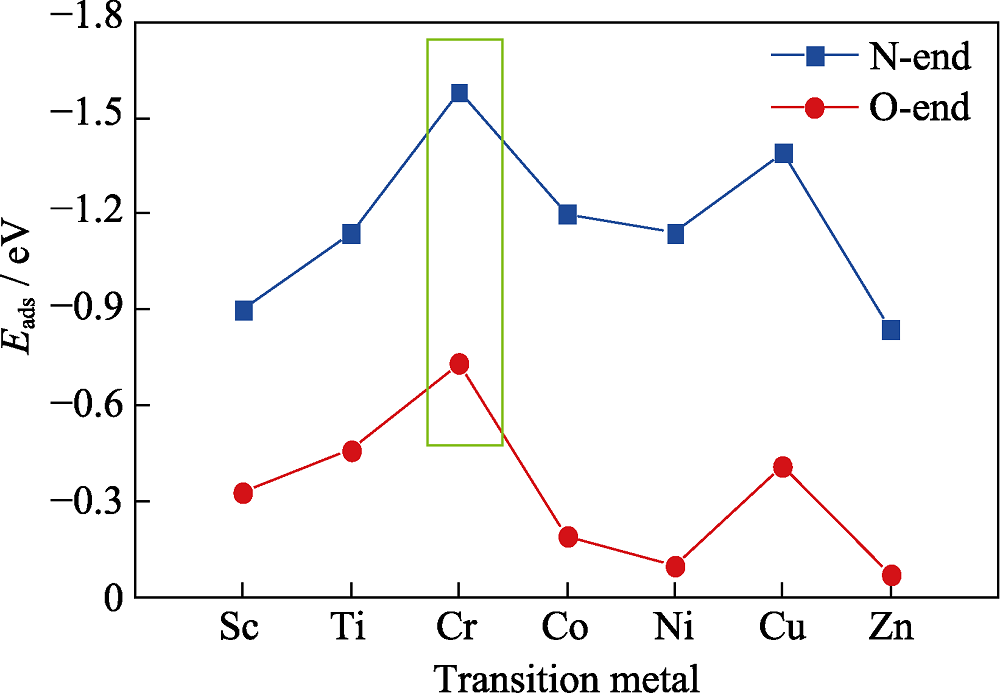
图3 NO以N-end和O-end模式在3d过渡金属掺杂石墨烯上的吸附能
Fig. 3 Adsorption energy of NO adsorbed on 3d transition metal doped graphene via N-end and O-end model, respectively
| System | NO-O-end | NO-N-end |
|---|---|---|
| Graphene | -0.012 e | -0.009 e |
| Cr doped graphene | -0.119 e | -0.143 e |
表1 石墨烯和Cr掺杂石墨烯吸附NO分子后的电荷改变量∆Q
Table 1 Charge change (∆Q) of graphene and Cr doped graphene after NO adsorption
| System | NO-O-end | NO-N-end |
|---|---|---|
| Graphene | -0.012 e | -0.009 e |
| Cr doped graphene | -0.119 e | -0.143 e |
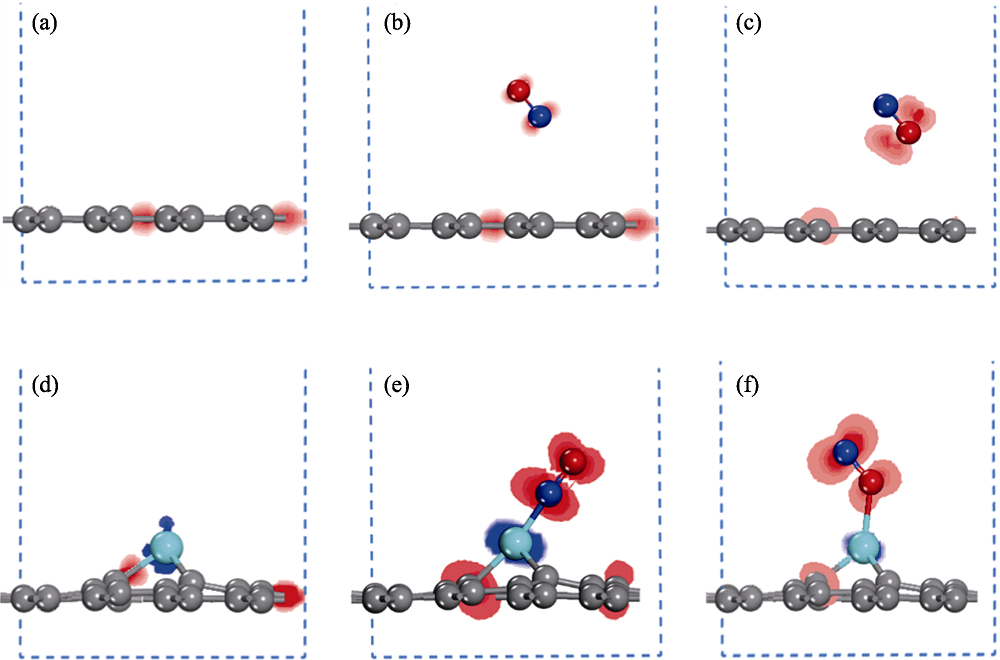
图4 NO吸附在石墨烯和Cr掺杂石墨烯前后的电子密度差示意图
Fig. 4 Charge density difference of graphene and Cr-doped graphene before and after NO adsorption (a) Graphene; NO adsorption on graphene via (b) N-end and (c) O-end model; (d) Cr-doped graphene; NO adsorption on Cr doped graphene via (e) N-end and (f) O-end model; Red and blue regions represent accumulation and loss of electrons, respectively

图5 石墨烯(a, b)与Cr掺杂石墨烯(c, d)吸附NO气体分子前(a, c)后(b, d)的态密度(DOS)图
Fig. 5 Density of states (DOS) of intrinsic graphene (a, b) and Cr-doped graphene (c, d) before (a, c) and after (b, d) NO adsorption
| [1] |
JOHNSON C, HENSHAW J, MCLNNES G. Impact of aircraft and surface emissions of nitrogen oxides ontropospheric ozone and global warming. Nature, 1992, 355(6355):69-71.
DOI URL |
| [2] |
LELIEVELD J, KLING MÜ, LLER K, et al. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proceedings of the National Academy of Sciences, 2019, 116(15):7192-7197.
DOI URL |
| [3] |
POTYRAILO R A, GO S, SEXTON D, et al. Extraordinary performance of semiconducting metal oxide gas sensors using dielectric excitation. Nature Electronics, 2020, 3(5):280-289.
DOI URL |
| [4] |
TSAI Y T, CHANG S J, JI L W, et al. High sensitivity of NO gas sensors based on novel Ag-doped ZnO nanoflowers enhanced with a UV light-emitting diode. ACS Omega, 2018, 3(10):13798-13807.
DOI URL |
| [5] |
LI QIANG, SHI WANYAN, ZHANG CHEN, et al. SO2 non- equilibrium gas sensor based on Na3Zr2Si2PO12 solid electrolyte. Journal of Inorganic Materials, 2018, 33(2):229-236
DOI URL |
| [6] |
JIN W, HO H L, CAO Y C, et al. Gas detection with micro- and nano-engineered optical fibers. Optical Fiber Technology, 2013, 19(6 Part B):741-759.
DOI URL |
| [7] | LI J, YAN H, DANG H, et al. Structure design and application of hollow core microstructured optical fiber gas sensor: a review. Optics & Laser Technology, 2021, 135:106658. |
| [8] |
AKSHYA S, JULIET A V. A computational study of a chemical gas sensor utilizing Pd-rGO composite on SnO2 thin film for the detection of NOx. Scientific Reports, 2021, 11(1):970.
DOI URL |
| [9] | HANG T, WU J, XIAO S, et al. Anti-biofouling NH3 gas sensor based on reentrant thorny ZnO/graphene hybrid nanowalls. Microsystems & Nanoengineering, 2020, 6:41. |
| [10] |
CHU Y X, LIU H R, YAN S. Preparation and gas sensing properties of SnO2/NiO composite semiconductor nanofibers. Journal of Inorganic Materials, 2021, 36(9):950-958.
DOI URL |
| [11] |
KRENO L E, LEONG K, FARHA O K, et al. Metal-organic framework materials as chemical sensors. Chemical Reviews, 2012, 112(2):1105-1125.
DOI URL |
| [12] |
LEE J S, KWON O S, PARK S J, et al. Fabrication of ultrafine metal-oxide-decorated carbon nanofibers for DMMP sensor application. ACS Nano, 2011, 5(10):7992-8001.
DOI URL |
| [13] | GUO X, WANG X, YANG R, et al. EDTA assistant preparation and gas sensing properties of Co3O4 nanomaterials. Journal of Inorganic Materials, 2020, 35:1215-1221. |
| [14] |
LIANG JIRAN, ZHANG YE, YANG RAN,et al Room- temperature NH3 gas sensing property of VO2(B)/ZnO hierarchical heterogeneous composite with nanorod structure. Journal of Inorganic Materials, 2018, 33(12):1323-1329.
DOI URL |
| [15] |
XU SHUANG, YANG YING, WU HONGYUAN, et al. Preparation of one-dimensional Pt/SnO2 nanofibers and NOx gas-sensing properties. Journal of Inorganic Materials, 2013, 28(6):584-588.
DOI URL |
| [16] |
HAN SHUANGSHUANG, LIU LIYUE, SHAN YONGKUI, et al. Research of graphene/antireflection nanostructure composite transparent conducting films. Journal of Inorganic Materials, 2017, 32(2):197-202.
DOI URL |
| [17] |
NAN HUI, WANG WENLI, HAN JIANHUA, et al. Low-cost preparation of graphene papers from chemical reduction with FeI2/Ni2+ for conductivity and catalytic propert. Journal of Inorganic Materials, 2017, 32(9):997-1003.
DOI URL |
| [18] |
YANG G, LEE C, KIM J, et al. Flexible graphene-based chemical sensors on paper substrates. Physical Chemistry Chemical Physics, 2013, 15(6):1798-1801.
DOI URL |
| [19] |
CHOI J H, LEE J, BYEON M, et al. Graphene-based gas sensors with high sensitivity and minimal sensor-to-sensor variation. ACS Applied Nano Materials, 2020, 3(3):2257-2265.
DOI URL |
| [20] | YUAN W, SHI G. Graphene-based gas sensors. Journal of Materials Chemistry A, 2013, 1(35):10078-10091. |
| [21] |
XING WEIWEI, ZHANG CHENXIAO, FAN SHANGCHUN, et al. Research progress on resonant characteristics of graphene. Journal of Inorganic Materials, 2016, 31(7):673-680.
DOI URL |
| [22] |
PUMERA M, AMBROSI A, BONANNI A, et al. Graphene for electrochemical sensing and biosensing. TrAC Trends in Analytical Chemistry, 2010, 29(9):954-965.
DOI URL |
| [23] |
GOMEZ DE ARCO L, ZHANG Y, SCHLENKER C W, et al. Continuous, highly flexible, and transparent graphene films by chemical vapor deposition for organic photovoltaics. ACS Nano, 2010, 4(5):2865-2873.
DOI URL |
| [24] |
CASTRO NETO A H, GUINEA F, PERES N M R, et al. The electronic properties of graphene. Reviews of Modern Physics, 2009, 81(1):109-162.
DOI URL |
| [25] |
RATINAC K R, YANG W, RINGER S P, et al. Toward ubiquitous environmental gas sensors-capitalizing on the promise of graphene. Environmental Science & Technology, 2010, 44(4):1167-1176.
DOI URL |
| [26] |
NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films. Science, 2004, 306(5696):666-669.
DOI URL |
| [27] |
MEYER J C, GEIM A K, KATSNELSON M I, et al. The structure of suspended graphene sheets. Nature, 2007, 446(7131):60-63.
DOI URL |
| [28] |
LEE C, WEI X, KYSAR J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science, 2008, 321(5887):385-388.
DOI URL |
| [29] |
WANG LIN, TIAN LINHAI, WEI GUODONG, et al. Epitaxial growth of graphene and their applications in devices. Journal of Inorganic Materials, 2011, 26(10):1009-1019.
DOI URL |
| [30] |
WANG X, BAI H, SHI G. Size fractionation of graphene oxide sheets by pH-assisted selective sedimentation. Journal of the American Chemical Society, 2011, 133(16):6338-6342.
DOI URL |
| [31] |
XU Y, SHI G. Assembly of chemically modified graphene: methods and applications. Journal of Materials Chemistry, 2011, 21(10):3311-3323.
DOI URL |
| [32] |
WANG GUIXIN, PEI ZHIBIN, YE CHANGHUI. Inkjet- printing and performance investigation of self-powered flexible graphene oxide humidity sensors. Journal of Inorganic Materials, 2019, 34(1):114-120.
DOI URL |
| [33] |
SCHEDIN F, GEIM A K, MOROZOV S V, et al. Detection of individual gas molecules adsorbed on graphene. Nature Materials, 2007, 6(9):652-655.
DOI URL |
| [34] | HWANG E H, ADAM S, DAS SARMA S. Transport in chemically doped graphene in the presence of adsorbed molecules. Physical Review B, 2007, 76(19):195421. |
| [35] | ROMERO H E, JOSHI P, GUPTA A K, et al. Adsorption of ammonia on graphene. Nanotechnology, 2009, 20(24):245501. |
| [36] | CHEN C W, HUNG S C, YANG M D, et al. Oxygen sensors made by monolayer graphene under room temperature. Applied Physics Letters, 2011, 99(24):243502. |
| [37] |
YU K, WANG P, LU G, et al. Patterning vertically oriented graphene sheets for nanodevice applications. The Journal of Physical Chemistry Letters, 2011, 2(6):537-542.
DOI URL |
| [38] |
RUMYANTSEV S, LIU G, SHUR M S, et al. Selective gas sensing with a single pristine graphene transistor. Nano Letters, 2012, 12(5):2294-2298.
DOI URL |
| [39] |
AO Z M, YANG J, LI S, et al. Enhancement of CO detection in Al doped graphene. Chemical Physics Letters, 2008, 461(4):276-279.
DOI URL |
| [40] |
ZHENG Z, WANG H. Different elements doped graphene sensor for CO2 greenhouse gases detection: the DFT study. Chemical Physics Letters, 2019, 721:33-37.
DOI URL |
| [41] | KAUSHAL S, KAUR M, KAUR N, et al. Heteroatom-doped graphene as sensing materials: a mini review. RSC Advances, 2020, 10(48):28608-28629. |
| [42] |
SRIVASTAVA S, JAIN S K, GUPTA G, et al. Boron-doped few- layer graphene nanosheet gas sensor for enhanced ammonia sensing at room temperature. RSC Advances, 2020, 10(2):1007-1014.
DOI URL |
| [43] | ZHANG X, LU Z, TANG Y, et al. A density function theory study on the NO reduction on nitrogen doped graphene. Phys.Chem.Chem.Phys., 2014, 16(38): 20561-1-9. |
| [44] | PRAMANIK A, KANG H S. Density functional theory study of O2 and NO adsorption on heteroatom-doped graphenes including the van der Waals interaction. The Journal of Physical Chemistry C, 2011, 115(22):10971-10978. |
| [45] |
LIAO Y, PENG R, PENG S, et al. The adsorption of H2 and C2H2 on Ge-doped and Cr-doped graphene structures: a DFT study. Nanomaterials, 2021, 11(1):231.
DOI URL |
| [46] |
DELLEY B. An all-electron numerical method for solving the local density functional for polyatomic molecules. The Journal of Chemical Physics, 1990, 92(1):508-517.
DOI URL |
| [47] |
DELLEY B. From molecules to solids with the DMol3 approach. The Journal of Chemical Physics, 2000, 113(18):7756-7764.
DOI URL |
| [48] |
PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple. Physical Review Letters, 1996, 77(18):3865-3868.
DOI URL |
| [49] | DELLEY B. Hardness conserving semilocal pseudopotentials. Physical Review B, 2002, 66(15):155125. |
| [50] |
HIRSHFELD F L. Bonded-atom fragments for describing molecular charge densities. Theoretica Chimica Acta, 1977, 44(2):129-138.
DOI URL |
| [51] |
HENKELMAN G, JÓNSSON H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. The Journal of Chemical Physics, 2000, 113(22):9978-9985.
DOI URL |
| [52] |
GEIM A K, NOVOSELOV K S. The rise of graphene. Nature Materials, 2007, 6(3):183-191.
DOI URL |
| [53] |
SONG E H, WEN Z, JIANG Q. CO catalytic oxidation on copper-embedded graphene. The Journal of Physical Chemistry C, 2011, 115(9):3678-3683.
DOI URL |
| [54] | SONG E H, YAN J M, LIAN J S, et al. External electric field catalyzed N2O decomposition on Mn-embedded graphene. The Journal of Physical Chemistry C, 2012, 116(38):20342-20348. |
| [55] | MAITARAD P, JUNKAEW A, PROMARAK V, et al. Complete catalytic cycle of NO decomposition on a silicon-doped nitrogen- coordinated graphene: mechanistic insight from a DFT study. Applied Surface Science, 2020, 508:145255. |
| [56] | LÜ Y A, ZHUANG G L, WANG J G, et al. Enhanced role of Al or Ga-doped graphene on the adsorption and dissociation of N2O under electric field. Phys. Chem. Chem. Phys., 2011, 13(27):12472-12477. |
| [1] | 崔宁, 张玉新, 王鲁杰, 李彤阳, 于源, 汤华国, 乔竹辉. (TiVNbMoW)Cx高熵陶瓷的单相形成过程与碳空位调控[J]. 无机材料学报, 2025, 40(5): 511-520. |
| [2] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [3] | 刘磊, 郭瑞华, 王丽, 王艳, 张国芳, 关丽丽. Pt3Co高指数晶面氧还原过程的密度泛函理论研究[J]. 无机材料学报, 2025, 40(1): 39-46. |
| [4] | 李家琪, 李小松, 李煊赫, 朱晓兵, 朱爱民. 暖等离子体合成过渡金属掺杂氧化锰析氧电催化剂[J]. 无机材料学报, 2024, 39(7): 835-844. |
| [5] | 张睿, 张侃, 袁梦雅, 谷鑫磊, 郑伟涛. 氮空位调控晶格畸变度强化(NbMoTaW)Nx薄膜的力学性质和耐磨损性[J]. 无机材料学报, 2024, 39(6): 715-725. |
| [6] | 李红兰, 张俊苗, 宋二红, 杨兴林. Mo/S共掺杂的石墨烯用于合成氨: 密度泛函理论研究[J]. 无机材料学报, 2024, 39(5): 561-568. |
| [7] | 杨恩东, 李宝乐, 张珂, 谭鲁, 娄永兵. ZnCo2O4-ZnO@C@CoS核壳复合材料的制备及其在超级电容器中的应用[J]. 无机材料学报, 2024, 39(5): 485-493. |
| [8] | 吴光宇, 舒松, 张洪伟, 李建军. 接枝内酯基活性炭增强苯乙烯吸附性能研究[J]. 无机材料学报, 2024, 39(4): 390-398. |
| [9] | 谢天, 宋二红. 弹性应变对C、H、O在过渡金属氧化物表面吸附的影响[J]. 无机材料学报, 2024, 39(11): 1292-1300. |
| [10] | 郭凌翔, 唐颖, 黄世伟, 肖博澜, 夏东浩, 孙佳. C/C复合材料高熵氧化物涂层抗烧蚀性能[J]. 无机材料学报, 2024, 39(1): 61-70. |
| [11] | 丁浩明, 李勉, 李友兵, 陈科, 肖昱琨, 周洁, 陶泉争, 尹航, 柏跃磊, 张毕堃, 孙志梅, 王俊杰, 张一鸣, 黄振莺, 张培根, 孙正明, 韩美康, 赵双, 王晨旭, 黄庆. 三元层状材料结构调控及性能研究进展[J]. 无机材料学报, 2023, 38(8): 845-884. |
| [12] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [13] | 孙炼, 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵. 二维过渡金属硫属化合物氧还原反应催化剂的研究进展[J]. 无机材料学报, 2022, 37(7): 697-709. |
| [14] | 王鹏, 靳遵龙, 陈宁光, 刘勇豪. Mo掺杂α-MnO2电催化析氧反应的理论研究[J]. 无机材料学报, 2022, 37(5): 541-546. |
| [15] | 李友兵, 秦彦卿, 陈科, 陈露, 张霄, 丁浩明, 李勉, 张一鸣, 都时禹, 柴之芳, 黄庆. 熔盐法合成纳米层状Sc2SnC MAX相[J]. 无机材料学报, 2021, 36(7): 773-778. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||