无机材料学报 ›› 2024, Vol. 39 ›› Issue (7): 835-844.DOI: 10.15541/jim20230542 CSTR: 32189.14.10.15541/jim20230542
所属专题: 【能源环境】氢能材料(202506)
李家琪1( ), 李小松1, 李煊赫1, 朱晓兵1,2(
), 李小松1, 李煊赫1, 朱晓兵1,2( ), 朱爱民1
), 朱爱民1
收稿日期:2023-11-24
修回日期:2024-02-06
出版日期:2024-07-20
网络出版日期:2024-02-26
通讯作者:
朱晓兵, 副教授. E-mail: xzhu@dlut.edu.cn作者简介:李家琪(1999-), 女, 硕士研究生. E-mail: lijiaqi621@mail.dlut.edu.cn
基金资助:
LI Jiaqi1( ), LI Xiaosong1, LI Xuanhe1, ZHU Xiaobing1,2(
), LI Xiaosong1, LI Xuanhe1, ZHU Xiaobing1,2( ), ZHU Aimin1
), ZHU Aimin1
Received:2023-11-24
Revised:2024-02-06
Published:2024-07-20
Online:2024-02-26
Contact:
ZHU Xiaobing, associate professor. E-mail: xzhu@dlut.edu.cnAbout author:LI Jiaqi (1999-), female, Master candidate. E-mail: lijiaqi621@mail.dlut.edu.cn
Supported by:摘要:
可再生能源发电与质子交换膜水电解结合产生“绿色氢”对能源安全具有战略意义, 其速控步骤是析氧反应。从稳定性、活性和成本角度考虑, 本研究采用滑动弧暖等离子体一步合成了氧化锰(MnOx)及过渡金属掺杂(Fe-MnOx, Co-MnOx, Ni-MnOx)的析氧电催化剂, 并对其晶体结构、形貌尺寸、元素组成和表面价态进行了表征。氧化锰主要由晶相Mn2O3和无定形Mn3O4组成。与之相比, 掺杂的氧化锰尽管晶相组成基本不变, 但其粒径明显变小、比表面积增大; 掺杂Co促使氧化锰的表面电子增多。氧化锰基催化剂在酸性电解液的循环伏安测试中表现出独特的电流阶跃现象(低电势Ⅰ-Ⅱ区: 1.4~1.8~2.4 V; 高电势Ⅲ区: 2.4~2.7 V)。该电流阶跃过程与Bulter-Volmer简化方程的电极动力学参照曲线相吻合, 属于多价态锰参与的电催化反应。低电势区Fe-MnOx的电化学活性最优, 而高电势区Co-MnOx表现最优。Co-MnOx的起始电位比MnOx低160 mV, 且在恒电位电解中其末端电流密度是MnOx的3倍。与其活性趋势一致, Fe-MnOx、Co-MnOx分别在低电势区、高电势区更具稳定性。本研究通过掺杂过渡金属优化氧化锰的颗粒尺寸、比表面积和电子结构, 显著提高了催化剂析氧反应活性及稳定性。
中图分类号:
李家琪, 李小松, 李煊赫, 朱晓兵, 朱爱民. 暖等离子体合成过渡金属掺杂氧化锰析氧电催化剂[J]. 无机材料学报, 2024, 39(7): 835-844.
LI Jiaqi, LI Xiaosong, LI Xuanhe, ZHU Xiaobing, ZHU Aimin. Transition Metal-doped Manganese Oxide: Synthesis by Warm Plasma and Electrocatalytic Performance for Oxygen Evolution Reaction[J]. Journal of Inorganic Materials, 2024, 39(7): 835-844.
| Catalyst | Doping element content/% (in atomic) | SBET/ (m2∙g-1) | DXRD / nm | DTEM / nm |
|---|---|---|---|---|
| MnOx | - | 35.7 | 32.7 | 37.9 |
| Ni-MnOx | 1.23 | 57.5 | 27.4 | 37.5 |
| Co-MnOx | 1.41 | 51.1 | 26.9 | 20.1 |
| Fe-MnOx | 1.22 | 54.8 | 28.1 | 19.1 |
表1 采用ICP-OES, BET, XRD, TEM表征MnOx, Ni-MnOx, Co-MnOx和Fe-MnOx催化剂的物理化学参数
Table 1 Physicochemical parameters of MnOx, Ni-MnOx, Co-MnOx, and Fe-MnOx catalysts by ICP-OES, BET, XRD, and TEM
| Catalyst | Doping element content/% (in atomic) | SBET/ (m2∙g-1) | DXRD / nm | DTEM / nm |
|---|---|---|---|---|
| MnOx | - | 35.7 | 32.7 | 37.9 |
| Ni-MnOx | 1.23 | 57.5 | 27.4 | 37.5 |
| Co-MnOx | 1.41 | 51.1 | 26.9 | 20.1 |
| Fe-MnOx | 1.22 | 54.8 | 28.1 | 19.1 |

图1 MnOx, Ni-MnOx, Co-MnOx和Fe-MnOx催化剂的XRD谱图
Fig. 1 XRD patterns of MnOx, Ni-MnOx, Co-MnOx, and Fe-MnOx catalysts Colorful figure is available on website
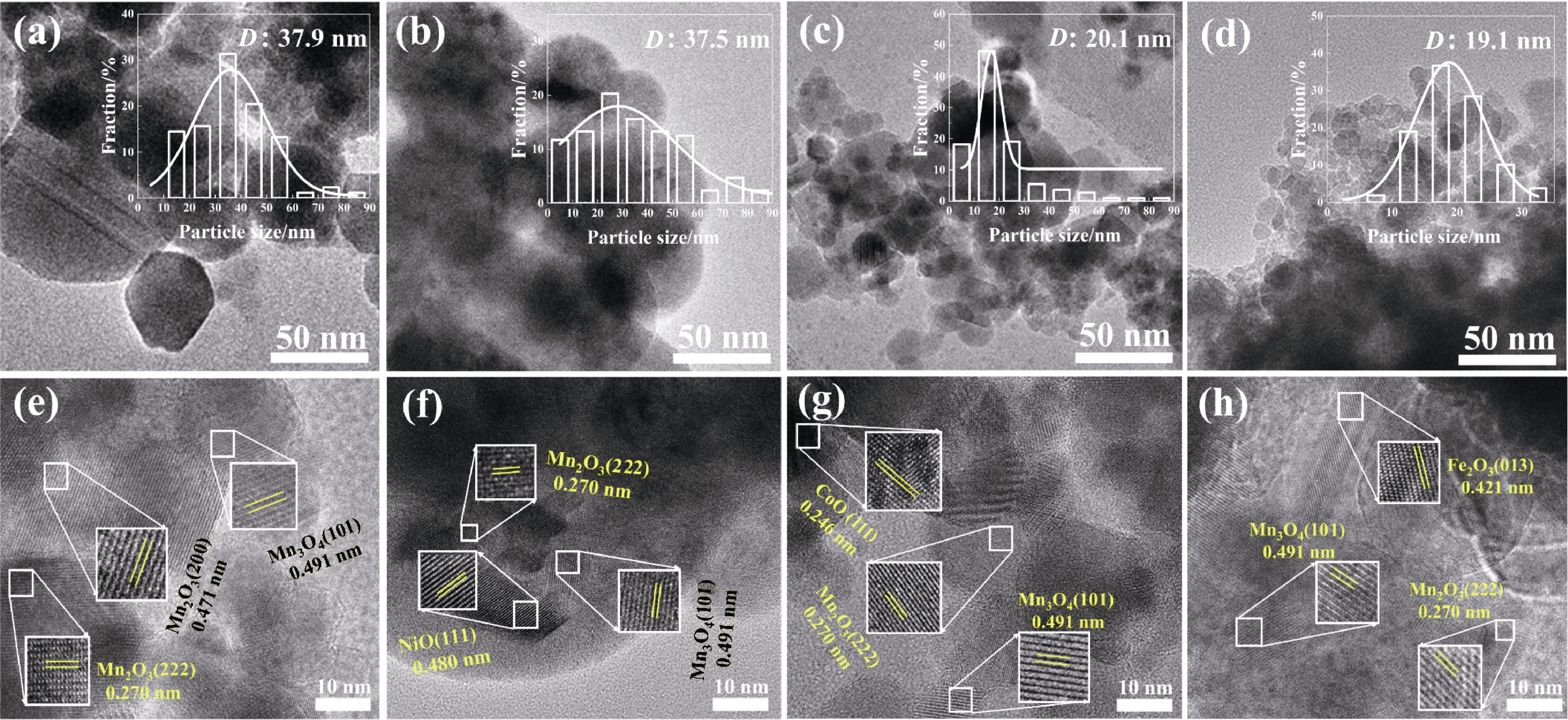
图2 (a, e)MnOx, (b, f)Ni-MnOx, (c, g)Co-MnOx和(d, h)Fe-MnOx催化剂的(a~d)TEM和(e~h)HRTEM照片((a~d)中的插图为相应的颗粒粒径分布直方图)
Fig. 2 (a-d) TEM and (e-h) HRTEM images of (a, e) MnOx, (b, f) Ni-MnOx, (c, g) Co-MnOx, and (d, h) Fe-MnOx catalysts with insets in (a-d) showing corresponding histograms of particle size distributions

图S3 Ni-MnOx催化剂的(a)Ni2p, Co-MnOx催化剂的(b)Co2p和Fe-MnOx催化剂的(c)Fe2p XPS谱图
Fig. S3 XPS spectra of (a) Ni2p for Ni-MnOx, (b) Co2p for Co-MnOx and (c) Fe2p for Fe-MnOx catalysts
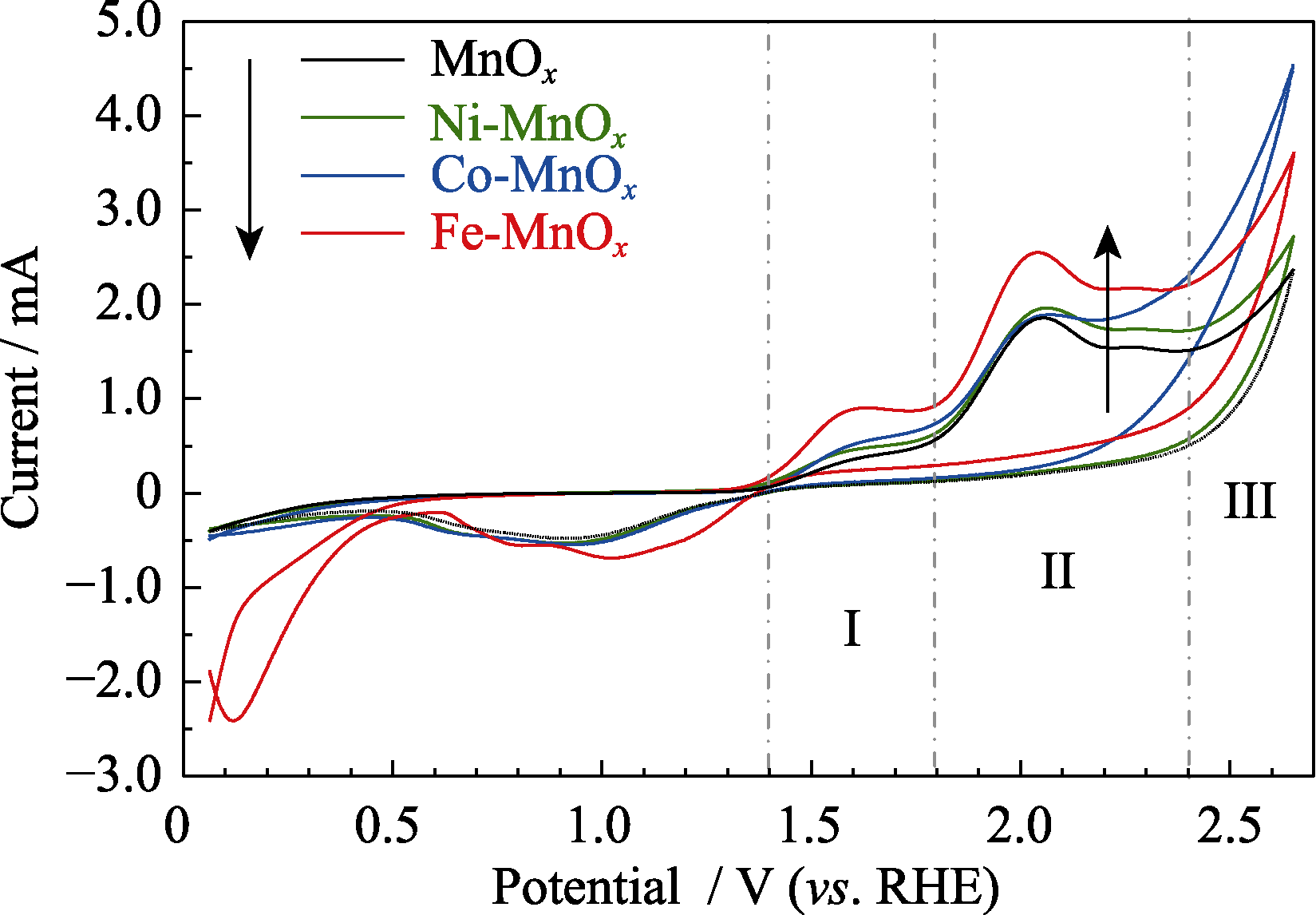
图4 MnOx, Ni-MnOx, Co-MnOx和Fe-MnOx催化剂的循环伏安曲线
Fig. 4 Cyclic voltammetric curves of MnOx, Ni-MnOx, Co-MnOx, and Fe-MnOx catalysts Colorful figure is available on website
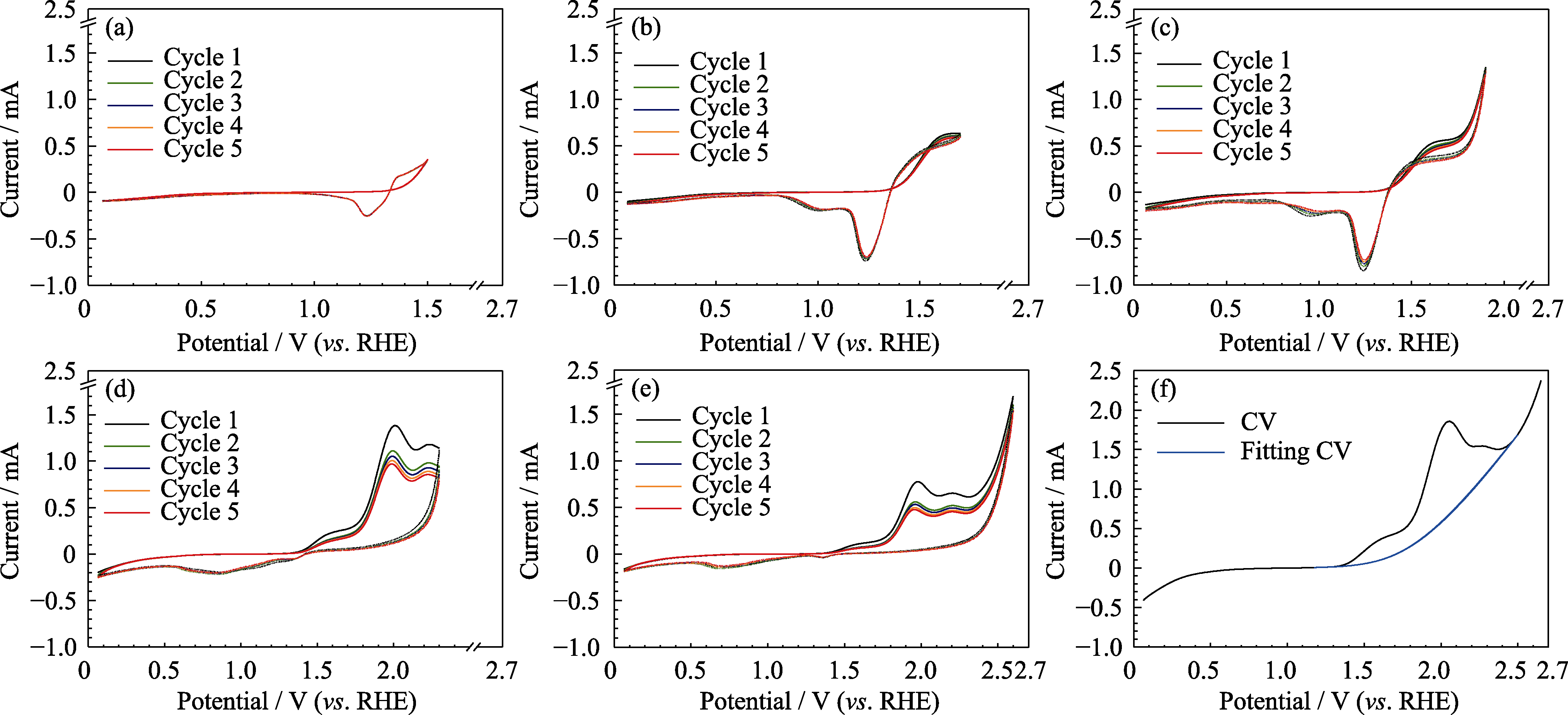
图5 滑动弧暖等离子体合成MnOx催化剂在截止电压为(a)1.5, (b)1.7, (c)1.9, (d)2.3, (e)2.6 V的CV曲线, (f)CV的测试和拟合曲线
Fig. 5 Cyclic voltammetric curves of MnOx catalysts synthesized by gliding arc warm plasma at ending potentials of (a) 1.5, (b) 1.7, (c) 1.9, (d) 2.3, and (e) 2.6 V, and (f) the comparison between the measured CV curve and the fitting curve educed by Bulter-Volmer equation (simple version) Colorful figures are available on website

图S4 滑动弧暖等离子体合成MnOx催化剂在不同截止电压(a)1.3, (b)1.4, (c)1.6, (d)1.8, (e)2.0, (f)2.1, (g)2.2, (h)2.4和(i)2.5 V的循环伏安图
Fig. S4 Cyclic voltammetry of MnOx catalyst synthesized by gliding arc warm plasma at ending potentials of (a) 1.3, (b) 1.4, (c) 1.6, (d) 1.8, (e) 2.0, (f) 2.1, (g) 2.2, (h) 2.4, and (i) 2.5V
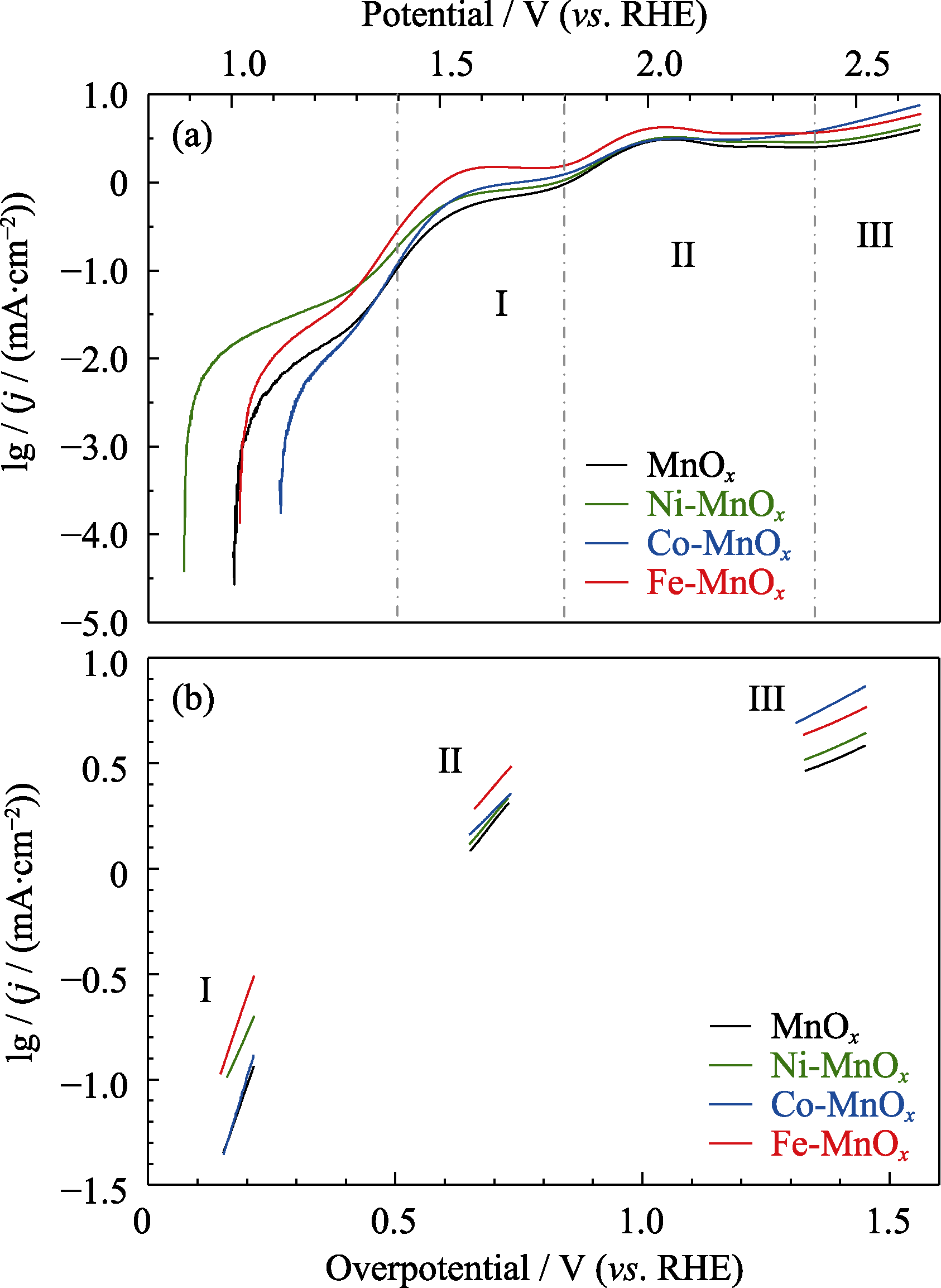
图6 MnOx、Ni-MnOx、Co-MnOx和Fe-MnOx催化剂的(a)lgj-E曲线和(b)类Tafel斜率图
Fig. 6 (a) lgj-E curves and (b) Tafel-type plots of MnOx, Ni-MnOx, Co-MnOx, and Fe-MnOx catalysts The data is derivative from Fig. 4; Colorful figures are available on website
| Catalyst | Potential/V | Slope/ (mV∙dec-1) | Starting Tafel (E, i)/(V, mA) |
|---|---|---|---|
| MnOx | 1.27-1.75I | 153 | (1.33, 0.02) |
| 1.75-2.42II | 359 | (1.82, 0.64) | |
| 2.42-2.65III | 893 | (2.55, 1.86) | |
| Ni-MnOx | 1.27-1.74I | 186 | (1.33, 0.51) |
| 1.74-2.42II | 356 | (1.82, 0.71) | |
| 2.42-2.65III | 879 | (2.52, 1.98) | |
| Co-MnOx | 1.27-1.74I | 131 | (1.31, 0.18) |
| 1.74-2.26II | 423 | (1.82, 0.80) | |
| 2.26-2.65III | 806 | (2.44, 2.41) | |
| Fe-MnOx | 1.25-1.74I | 144 | (1.30, 0.04) |
| 1.74-2.36II | 363 | (1.82, 1.03) | |
| 2.36-2.65III | 874 | (2.50, 2.52) |
表S1 MnOx, Ni-MnOx, Co-MnOx和Fe-MnOx催化剂的电化学性能(源自图6)
Table S1 Electrochemical performances for MnOx, Ni-MnOx, Co-MnOx, and Fe-MnOx catalysts from Fig. 6
| Catalyst | Potential/V | Slope/ (mV∙dec-1) | Starting Tafel (E, i)/(V, mA) |
|---|---|---|---|
| MnOx | 1.27-1.75I | 153 | (1.33, 0.02) |
| 1.75-2.42II | 359 | (1.82, 0.64) | |
| 2.42-2.65III | 893 | (2.55, 1.86) | |
| Ni-MnOx | 1.27-1.74I | 186 | (1.33, 0.51) |
| 1.74-2.42II | 356 | (1.82, 0.71) | |
| 2.42-2.65III | 879 | (2.52, 1.98) | |
| Co-MnOx | 1.27-1.74I | 131 | (1.31, 0.18) |
| 1.74-2.26II | 423 | (1.82, 0.80) | |
| 2.26-2.65III | 806 | (2.44, 2.41) | |
| Fe-MnOx | 1.25-1.74I | 144 | (1.30, 0.04) |
| 1.74-2.36II | 363 | (1.82, 1.03) | |
| 2.36-2.65III | 874 | (2.50, 2.52) |
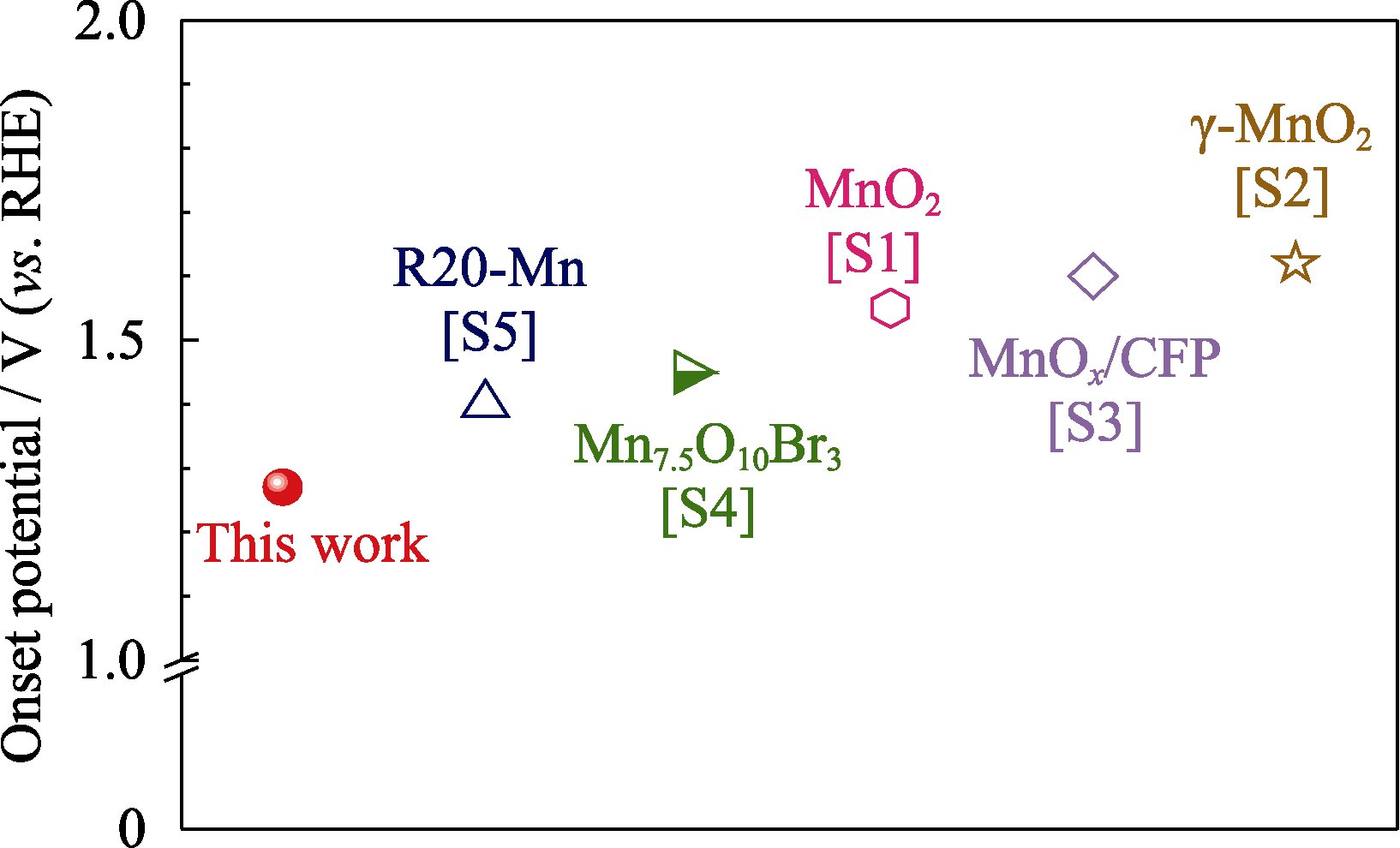
图S5 三维多孔电极中Co-MnOx催化剂与文献[S1-S5]报道的二维薄膜电极中氧化锰基催化剂在酸性条件下的析氧反应起始电位比较
Fig. S5 Comparison of onset potentials on Co-MnOx catalyst in this work with manganese oxides based electrocatalysts in literatures[S1-S5]

图7 MnOx, Ni-MnOx, Co-MnOx和Fe-MnOx催化剂的电化学阻抗谱图(插图为所测全部频率谱图)
Fig. 7 Electrochemical impedance spectra of MnOx, Ni-MnOx, Co-MnOx, and Fe-MnOx catalysts with insert showing full-range frequency measurement
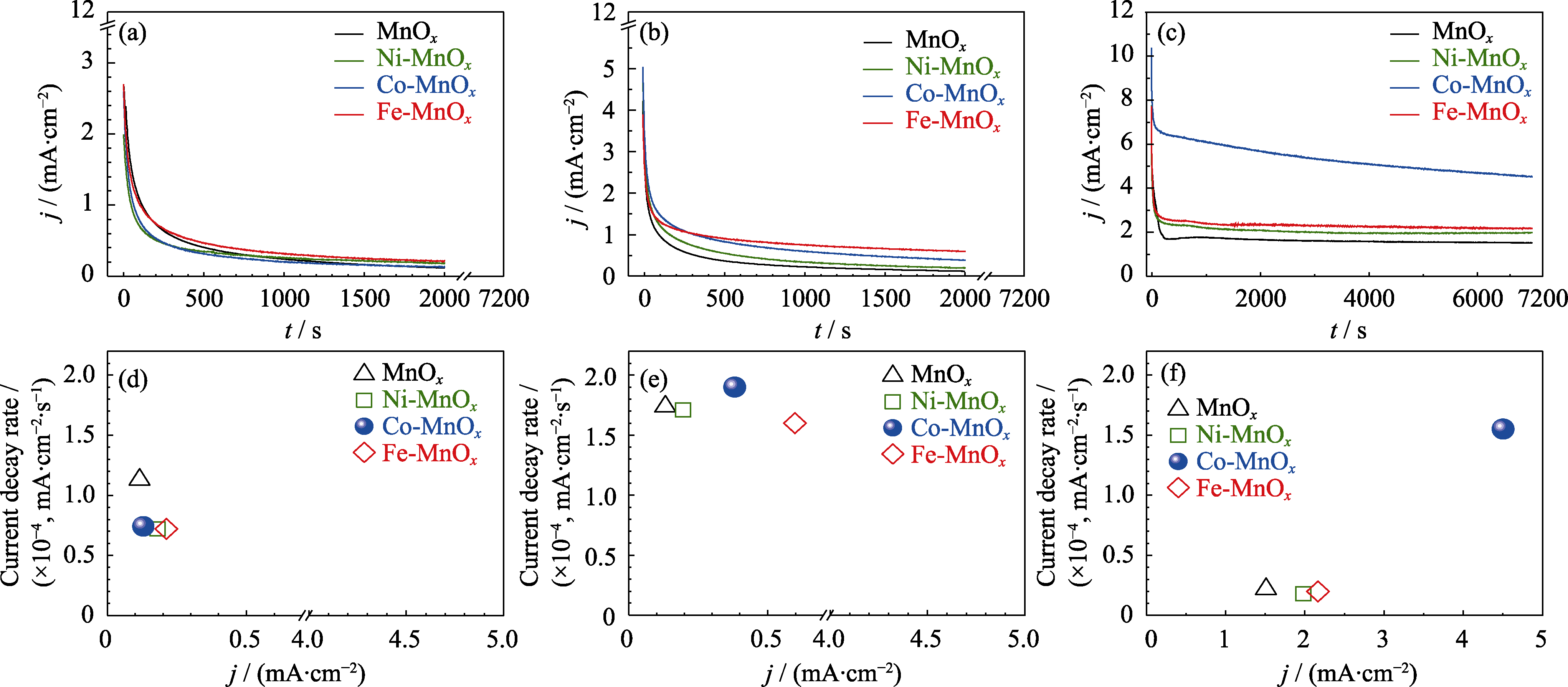
图8 MnOx, Ni-MnOx, Co-MnOx和Fe-MnOx催化剂在(a, d)1.5, (b, e) 1.9和(c, f) 2.5 V电压下的(a~c)电流密度-时间(j-t)的稳定性实验, 以及(d~f)电流衰减率与上述稳定性实验的末端电流密度的关系
Fig. 8 (a-c) Current density-time (j-t) dependence of stability tests, and (d-f) relationship between current decay rate and ending current density on MnOx, Ni-MnOx, Co-MnOx, and Fe-MnOx catalysts at (a, d) 1.5, (b, e) 1.9 and (c, f) 2.5 V Colorful figures are available on website
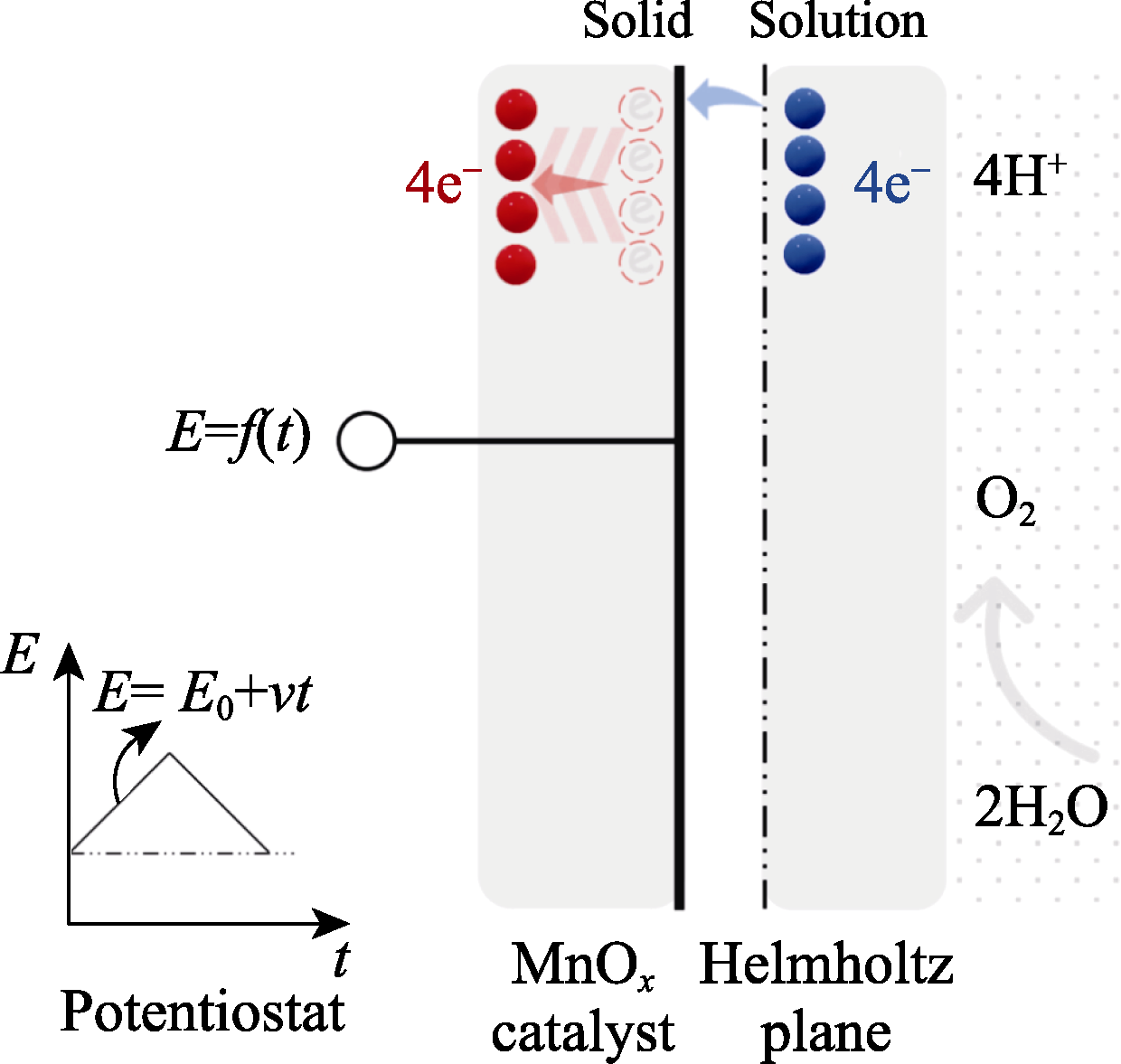
图9 电流阶跃特征的双电层解释示意图
Fig. 9 Schematic double layer (of Helmholtz plane) for the featured current step Bottom left: A potentiostat that linearly loads voltage to double layer (at left side); Right: A double layer that consists of two sides of solid (MnOx catalyst) and solution
| [1] | LOISEL J, GALLEGO-SALA A V, AMESBURY M J, et al. Expert assessment of future vulnerability of the global peatland carbon sink. Nature Climate Change, 2021, 11(4): 70. |
| [2] | LAURENT A, ESPINOSA N. Environmental impacts of electricity generation at global, regional and national scales in 1980-2011: what can we learn for future energy planning? Energy & Environmental Science, 2015, 8(3): 689. |
| [3] | CHUNG D Y, LOPES P P, FARINAZZO P, et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nature Energy, 2020, 5(3): 222. |
| [4] | SUEN N T, HUNG, S F, QUAN Q, et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chemical Society Reviews, 2017, 46(2): 337. |
| [5] | URSUA A, GANDIA L M, SANCHIS P. Hydrogen production from water electrolysis: current status and future trends. Proceedings of the IEEE, 2012, 100(2): 410. |
| [6] | 王新东, 刘高阳, 许军元, 等. 质子交换膜水电解析氧电催化复合材料合成、微结构调控及性能研究. 中国科学: 化学, 2014(8): 1241. |
| [7] | DALY K M, JIMENEZ-VILLEGAS S, GODWIN B, et al. A comparison of photodeposited RuOx for alkaline water electrolysis. ACS Applied Energy Materials, 2023, 6(3): 1449. |
| [8] | GONG R, LIU B, WANG X, et al. Electronic structure modulation induced by cobalt-doping and lattice-contracting on armor-like ruthenium oxide drives pH-universal oxygen evolution. Small, 2022, 19(4): 2204889. |
| [9] | ZHAO F, WEN B, NIU W, et al. Increasing iridium oxide activity for the oxygen evolution reaction with hafnium modification. Journal of the American Chemical Society, 2021, 143(38): 15616. |
| [10] | SILVA C D F, CLAUDEL F, MARTIN V, et al. Oxygen evolution reaction activity and stability benchmarks for supported and unsupported IrOx electrocatalysts. ACS Catalysis, 2021, 11(7): 4107. |
| [11] | YANG X, SUN X, GAN L Y, et al. A CoOx/FeOx heterojunction on carbon nanotubes prepared by plasma-enhanced atomic layer deposition for the highly efficient electrocatalysis of oxygen evolution reactions. Journal of Materials Chemistry A, 2020, 8(30): 15140. |
| [12] | MOYSIADOU A, HU X. Stability profiles of transition metal oxides in the oxygen evolution reaction in alkaline medium. Journal of Materials Chemistry A, 2019, 7(45): 25865. |
| [13] | WU G, CHEN W, ZHENG X, et al. Hierarchical Fe-doped NiOx nanotubes assembled from ultrathin nanosheets containing trivalent nickel for oxygen evolution reaction. Nano Energy, 2017, 38: 167. |
| [14] | WANG P, ZHANG S, WANG Z, et al. Manganese-based oxide electrocatalysts for the oxygen evolution reaction: a review. Journal of Materials Chemistry A, 2023, 11(11): 5476. |
| [15] | LI A, OOKA H, BONNET N, et al. Stable potential windows for long-term electrocatalysis by manganese oxides under acidic conditions. Angewandte Chemie International Edition, 2019, 58(15): 5054. |
| [16] | BIGIANI L, MACCATO C, ANDREU T, et al. Quasi-1D Mn2O3 nanostructures functionalized with first-row transition-metal oxides as oxygen evolution catalysts. ACS Applied Nano Materials, 2020, 3(10): 9889. |
| [17] | FUJIMOTO K, UEDA Y, INOHARA D, et al. Cobalt-doped electrolytic manganese dioxide as an efficient bifunctional catalyst for oxygen evolution/reduction reactions. Electrochimica Acta, 2020, 354: 136592. |
| [18] | MELDER J, MEBS S, LESSING F, et al. Tuning electrocatalytic water oxidation by MnOx through the incorporation of abundant metal cations. Sustainable Energy & Fuels, 2023, 7(1): 92. |
| [19] | RUSANOV V D, FRIDMAN A A, SHOLIN G V. The physics of a chemically active plasma with nonequilibrium vibrational excitation of molecules. Soviet Physics Uspekhi, 1981, 24(6): 447. |
| [20] | LIU Y F, MU J S, XU X Y, et al. Microstructure and dry-sliding wear properties of TiC-reinforced composite coating prepared by plasma-transferred arc weld-surfacing process. Materials Science and Engineering: A, 2007, 458(1/2): 366. |
| [21] | FENG Z C, LIU Y F, LI Y, et al. Microstructure and high temperature reciprocating sliding wear properties of MoSi2/TiC/γ-Ni composite coating in-situ synthesized by co-axial powder feeding plasma transferred arc cladding. Tribology International, 2019, 129: 82. |
| [22] | CESCHINI L, LANZONI E, MARTINI C, et al. Comparison of dry sliding friction and wear of Ti6Al4V alloy treated by plasma electrolytic oxidation and PVD coating. Wear, 2008, 264(1/2): 86. |
| [23] | SABATINI G, CESCHINI L, MARTINI C, et al. Improving sliding and abrasive wear behaviour of cast A356 and wrought AA7075 aluminium alloys by plasma electrolytic oxidation. Materials & Design, 2010, 31(2): 816. |
| [24] | EFREMENKO V G, CHABAK Y G, FEDUN V I, et al. Formation mechanism, microstructural features and dry-sliding behaviour of “bronze/WC carbide” composite synthesised by atmospheric pulsed-plasma deposition. Vacuum, 2021, 185: 110031. |
| [25] | ÇELIK O N. Microstructure and wear properties of WC particle reinforced composite coating on Ti6Al4V alloy produced by the plasma transferred arc method. Applied Surface Science, 2013, 274: 334. |
| [26] | FRIDMAN A, NESTER S, KENNEDY L A, et al. Gliding arc gas discharge. Progress in Energy and Combustion Science, 1999, 25(2): 211. |
| [27] | 孙进桃, 赵旭腾, 陈琪, 等. 等离子催化重整CH4/CO2中的协同效应及积碳动力学. 工程热物理学报, 2023, 44(5): 1428. |
| [28] | ZHANG S Y, LI X S, LIU J B, et al. Dimensionless factors for an alternating-current non-thermal arc plasma. Physics of Plasmas, 2016, 23(12): 120707. |
| [29] | CHEN D, QIAO M, LU Y R, et al. Preferential cation vacancies in perovskite hydroxide for the oxygen evolution reaction. Angewandte Chemie International Edition, 2018, 57(28): 8691. |
| [30] | WANG X, ZHUANG L, JIA Y, et al. Plasma-triggered synergy of exfoliation, phase transformation, and surface engineering in cobalt diselenide for enhanced water oxidation. Angewandte Chemie International Edition, 2018, 57(50): 16421. |
| [31] | XU L, JIANG Q, XIAO Z, et al. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angewandte Chemie International Edition, 2016, 55(17): 5277. |
| [32] | LIU S X, LI X S, ZHU X, et al. Gliding arc plasma synthesis of crystalline TiO2 nanopowders with high photocatalytic activity. Plasma Chemistry and Plasma Processing, 2013, 33(5): 827. |
| [33] | ZHU X, LI J J, LIU M T, et al. Mesoporous TiO2 electrocatalysts synthesized by gliding arc plasma for oxygen evolution reaction. Journal of Physics D: Applied Physics, 2021, 54(48): 484003. |
| [34] | ZHANG S Y, LI X S, LIU J L, et al. Plasmochemical approach to template-free synthesis of highly crystalline mesoporous TiO2 within milliseconds. ChemNanoMat, 2019, 5(4): 403. |
| [35] | ASL H Z, ROZATI S M. High-quality spray-deposited fluorine- doped tin oxide: effect of film thickness on structural, morphological, electrical, and optical properties. Applied Physics A, 2019, 125(10): 689. |
| [36] | WANG Z, XU K, RUAN S, et al. Mesoporous Co-Mn spinel oxides as efficient catalysts for low temperature propane oxidation. Catalysis Letters, 2021, 152(9): 2695. |
| [37] | HAMMER B, NORSKOV J K. Why gold is the noblest of all the metals. Nature, 1995, 376 (6537): 238. |
| [38] | DONG C, QU Z, QIN Y, et al. Revealing the highly catalytic performance of spinel CoMn2O4 for toluene oxidation: involvement and replenishment of oxygen species using in situ designed-TP techniques. ACS Catalysis, 2019, 9(8): 6698. |
| [39] | HUANG H, ZHAO Y, BAI Y, et al. Conductive metal-organic frameworks with extra metallic sites as an efficient electrocatalyst for the hydrogen evolution reaction. Advanced Science, 2020, 7(9): 2000012. |
| [40] | KISSLINGER R, RIDDELL S, SAVELA S, et al. Transparent nanoporous P-type NiO films grown directly on non-native substrates by anodization. Journal of Materials Science: Materials in Electronics, 2019, 30(12): 11327. |
| [41] | HOLM E A, FOILES S M. How grain growth stops: a mechanism for grain-growth stagnation in pure materials. Science, 2010, 328(5982): 1138. |
| [42] | NAJAFKHANI F, KHEIRI S, POURBAHARI B, et al. Recent advances in the kinetics of normal/abnormal grain growth: a review. Archives of Civil and Mechanical Engineering, 2021, 21: 1. |
| [43] | SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A, 1976, 32(5): 751. |
| [44] | CHEN J, YAO M, WANG X. Investigation of transition metal ion doping behaviors on TiO2 nanoparticles. Journal of Nanoparticle Research, 2007, 10(1): 163. |
| [45] | XIONG L P, HU S, HOU J W, et al. Preparation and catalytic activity of Pt based hydrophobic catalysts adulterated with Fe series elements. Journal of Inorganic Materials, 2011, 26(1): 91. |
| [46] | LIDE D R. CRC handbook of chemistry and physics, 90th ed. Boca Raton: CRC Press, 2010: 1215. |
| [47] | LARMINIE J. Fuel cell systems explained, 2nd ed. New York: Wiley, 2003: 48. |
| [48] | BARD A J, FAULKNER L R. Electrochemical methods:fundamentals and applications, 2nd ed. New York: Wiley, 2001: 15. |
| [49] | FAN X, LIANG X, ZHAO Z, et al. Enhanced treatment performance of phenol wastewater by electrochemical reactor with MnOx/Ti membrane electrode modified with Sb-SnO2 interlayer People's Republic of China. Journal of Materials Science: Materials in Electronics, 2020, 31(21): 19044. |
| [50] | SUN Z, LIU C, LI X S, et al. Semi-transparent nanofilms of plasmonic Au/TiO2 for visible-light photocatalysis. Materials Chemistry and Physics, 2022, 280: 125773. |
| [51] | 朱晓兵, 李佳佳, 李怡宁, 等. 氧化锰电催化析氧反应及其电极界面特性. 化工学报, 2021, 72(S1): 398. |
| [1] | 岳全鑫, 郭瑞华, 王瑞芬, 安胜利, 张国芳, 关丽丽. 3D核壳结构NiMoO4@CoFe-LDH纳米棒的高效析氧及全解水性能研究[J]. 无机材料学报, 2024, 39(11): 1254-1264. |
| [2] | 王鹏, 靳遵龙, 陈宁光, 刘勇豪. Mo掺杂α-MnO2电催化析氧反应的理论研究[J]. 无机材料学报, 2022, 37(5): 541-546. |
| [3] | 付永胜, 毕敏, 李春, 孙敬文, 汪信, 朱俊武. 非贵金属/碳氮复合材料电催化析氧反应的研究进展[J]. 无机材料学报, 2022, 37(2): 163-172. |
| [4] | 张盛, 蒋亿, 纪媛媛, 杜莹, 盛振环, 殷竟洲, 李乔琦, 张莉莉. 凹凸棒石/g-C3N4复合材料的制备及其电催化析氧性能研究[J]. 无机材料学报, 2019, 34(8): 803-810. |
| [5] | 龚云,刘艳,顾萍,朱钰方,周晓霞. 超声辅助合成纳米氧化锰及其低浓度NO去除性能研究[J]. 无机材料学报, 2019, 34(2): 186-192. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||