无机材料学报 ›› 2022, Vol. 37 ›› Issue (7): 697-709.DOI: 10.15541/jim20220128 CSTR: 32189.14.10.15541/jim20220128
• 综述 • 下一篇
孙炼( ), 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵(
), 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵( )
)
收稿日期:2022-03-08
修回日期:2022-04-12
出版日期:2022-07-20
网络出版日期:2022-04-26
通讯作者:
周新贵, 教授. E-mail: zhouxinguilmy@163.com作者简介:孙 炼(1993-), 男, 博士研究生. E-mail: sunlian12@nudt.edu.cn
基金资助:
SUN Lian( ), GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui(
), GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui( )
)
Received:2022-03-08
Revised:2022-04-12
Published:2022-07-20
Online:2022-04-26
Contact:
ZHOU Xingui, professor. E-mail: zhouxinguilmy@163.comAbout author:SUN Lian (1993-), male, PhD candidate. E-mail: sunlian12@nudt.edu.cn
Supported by:摘要:
氧还原(ORR)反应是燃料电池等清洁能源阴极的关键反应, 其反应动力学复杂, 阴极需使用Pt等贵金属催化剂。然而Pt价格昂贵, 且载体炭黑在高电位环境下稳定性欠佳, 导致电池部件成本高且寿命短。二维过渡金属硫属化合物(2D TMDs)具有高比表面积与可调节的电学性能, 且稳定性强, 有望在维持活性的同时提高燃料电池阴极的耐久性。本文梳理了近年来2D TMDs在ORR催化剂领域的最新研究进展: 首先概述了2D TMDs的结构、性质及ORR反应机理; 其次分析了调控2D TMDs的ORR性能策略, 包括异质元素掺杂、相转变、缺陷工程与应力工程等, 介绍了2D TMDs基异质结构对ORR性能的提升作用; 最后, 针对该领域目前存在的挑战进行展望与总结。
中图分类号:
孙炼, 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵. 二维过渡金属硫属化合物氧还原反应催化剂的研究进展[J]. 无机材料学报, 2022, 37(7): 697-709.
SUN Lian, GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui. Two-dimensional Transition Metal Dichalcogenides for Electrocatalytic Oxygen Reduction Reaction[J]. Journal of Inorganic Materials, 2022, 37(7): 697-709.
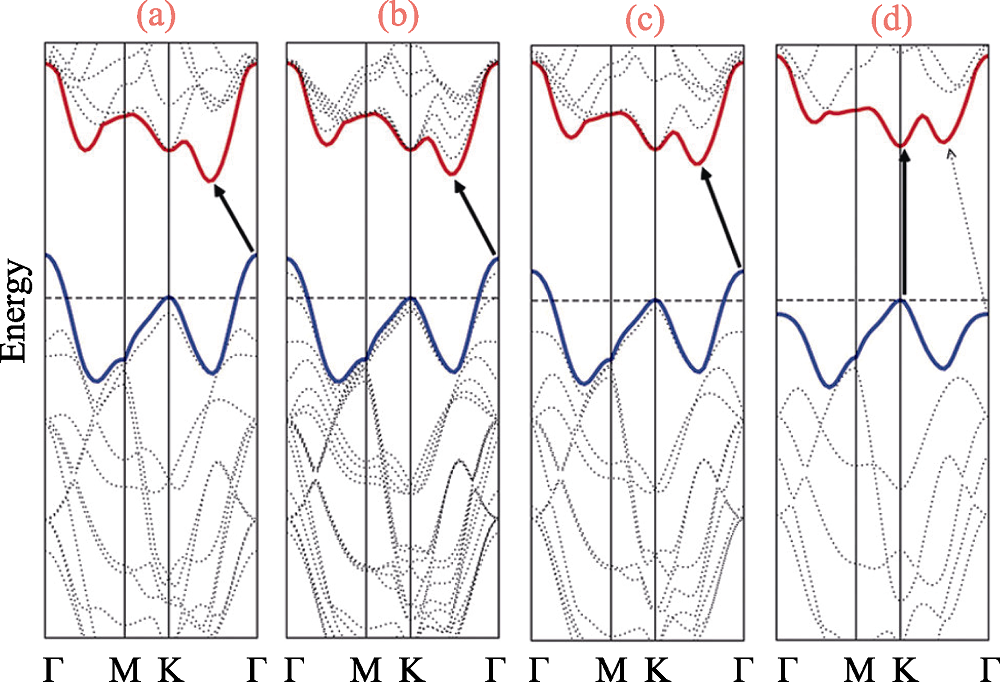
图2 (a) 块体结构MoS2, (b) 三层结构MoS2, (c) 双层结构MoS2与(d) 单层结构MoS2经过计算后得到的带隙[26]
Fig. 2 Calculated band structures of (a) bulk MoS2, (b) quadrilayer MoS2, (c) bilayer MoS2, and (d) monolayer MoS2[26]
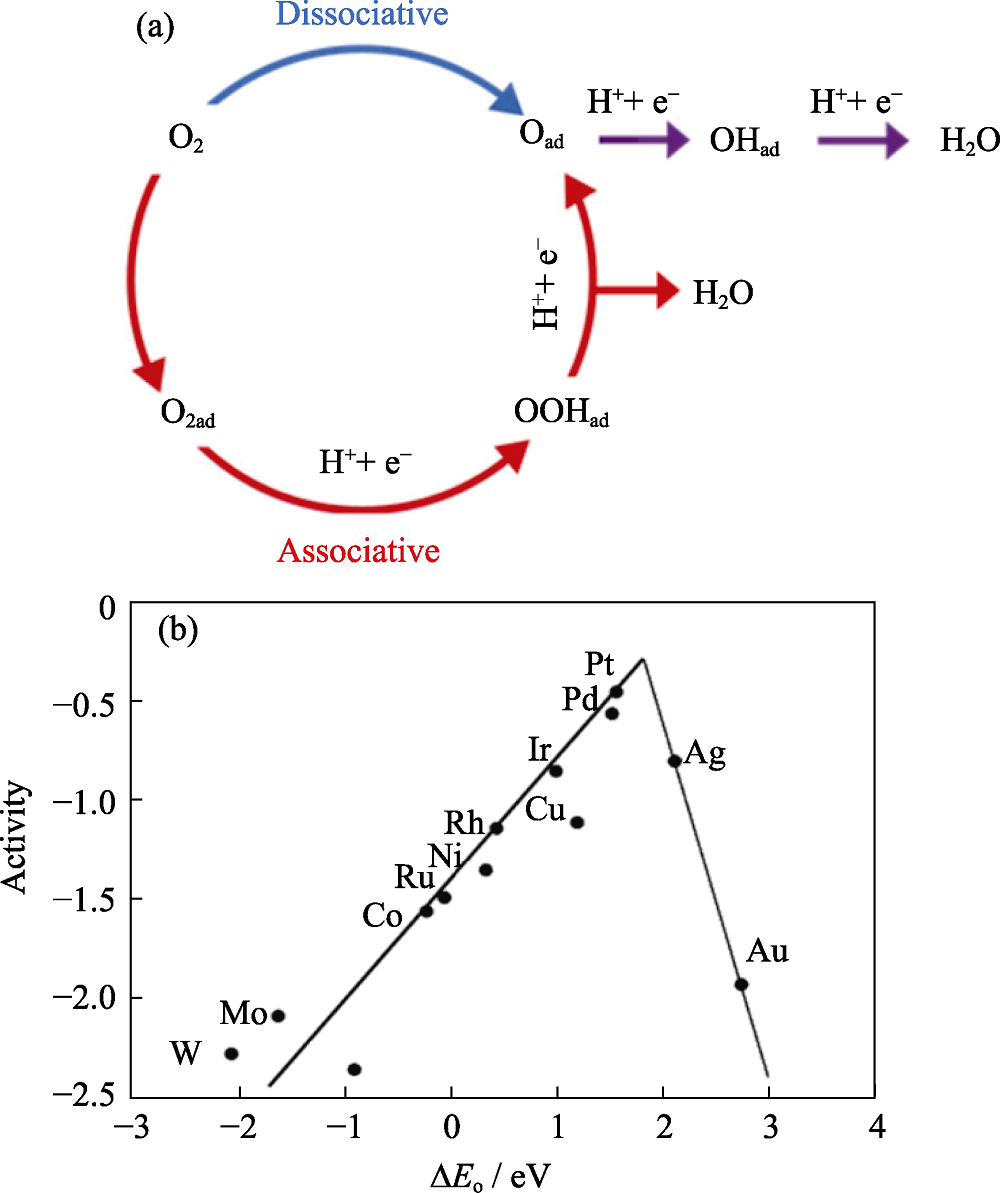
图3 ORR催化剂的设计理论[3,41]
Fig. 3 Designing strategy for ORR catalysts[3,41] (a) ORR mechanism with blue arrow representing dissociative mechanism, red arrows representing associative mechanism, and purple arrows representing the parts involving both mechanisms[3]; (b) “Volcano plots” showing relationship between oxygen binding energy and maximal activity[41] Colorful figures are available on website
| Catalyst | Electrolyte | Onset potential / V (vs. RHE) | Half-wave potential / V (vs. RHE) | Electrons transfer number, n | Ref. |
|---|---|---|---|---|---|
| P-MoS2-0.2 | 0.1 mol·L-1 KOH | 0.96 | 0.80 | 3.60 | [ |
| I-PdSe2-50 | 0.1 mol·L-1 KOH | - | 0.76 | 3.67 | [ |
| Ag/MoS2 | 0.1 mol·L-1 KOH | 0.90 | 0.83 | 3.98 | [ |
| 2H+1T-FeSe@NC | 1.0 mol·L-1 KOH | 0.97 | 0.80 | 3.90 | [ |
| O-MoS2-87 | 0.1 mol·L-1 KOH | 0.94 | 0.80 | 3.49 | [ |
表1 典型2D TMDs层状结构ORR催化剂的性能
Table 1 Properties of typical 2D TMDs-based ORR catalysts
| Catalyst | Electrolyte | Onset potential / V (vs. RHE) | Half-wave potential / V (vs. RHE) | Electrons transfer number, n | Ref. |
|---|---|---|---|---|---|
| P-MoS2-0.2 | 0.1 mol·L-1 KOH | 0.96 | 0.80 | 3.60 | [ |
| I-PdSe2-50 | 0.1 mol·L-1 KOH | - | 0.76 | 3.67 | [ |
| Ag/MoS2 | 0.1 mol·L-1 KOH | 0.90 | 0.83 | 3.98 | [ |
| 2H+1T-FeSe@NC | 1.0 mol·L-1 KOH | 0.97 | 0.80 | 3.90 | [ |
| O-MoS2-87 | 0.1 mol·L-1 KOH | 0.94 | 0.80 | 3.49 | [ |
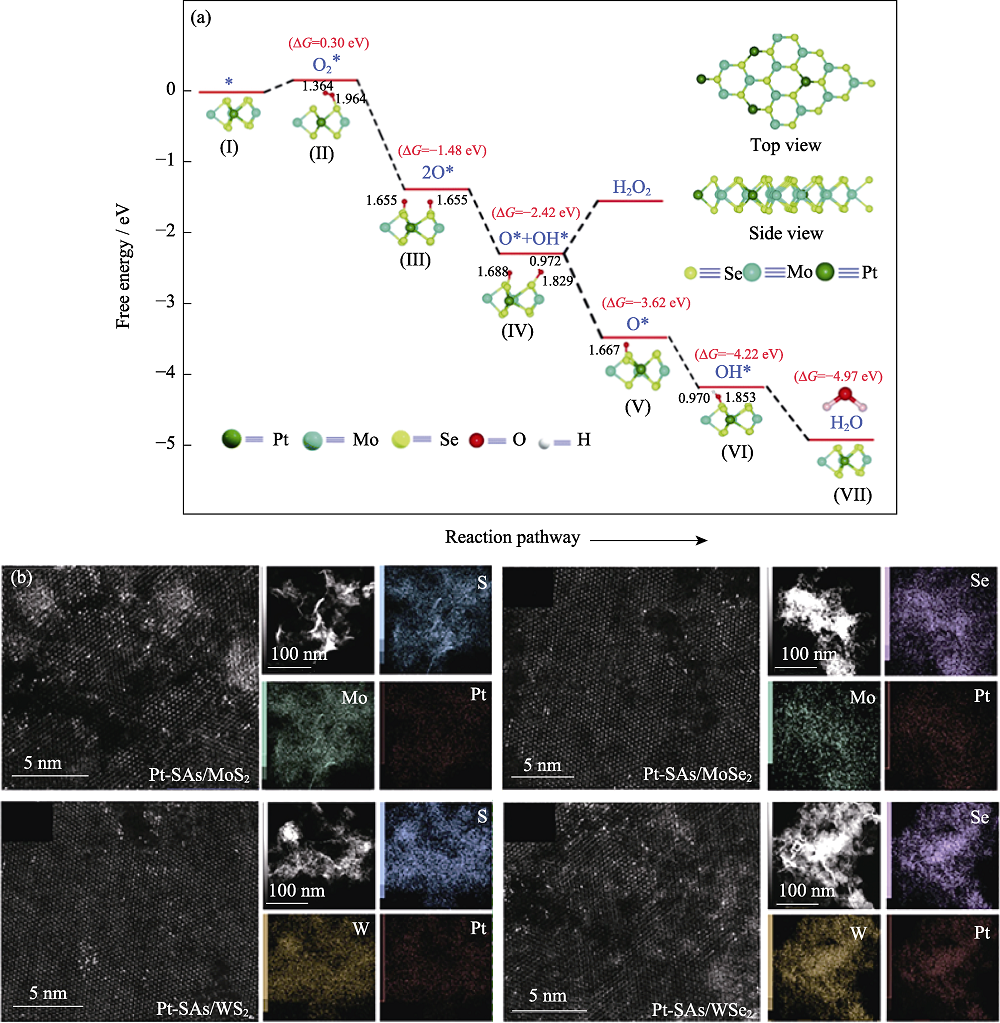
图4 2D TMDs的M位掺杂[53,55]
Fig. 4 M-position doping for 2D TMDs[53,55] (a) ORR energy barrier for Pt-MoSe2 with insets showing crystal structures[53] ; (b) TEM images of Pt-SAs/2D TMDs prepared by galvanic replacement[55]
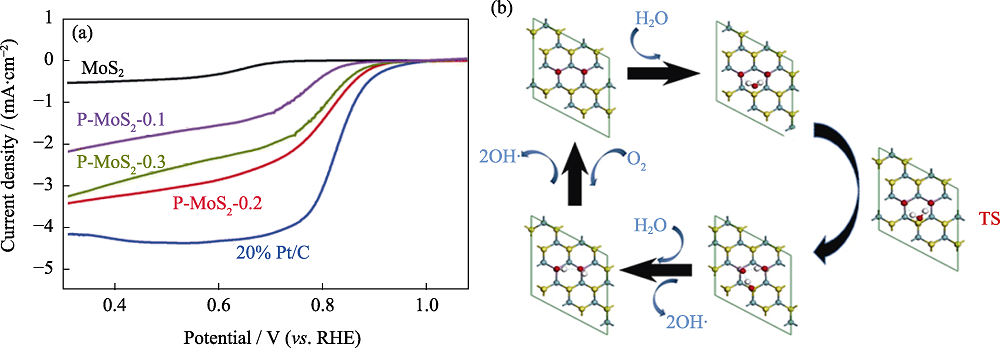
图5 2D TMDs的X位掺杂[45,61]
Fig. 5 X-position doping of 2D TMDs[45,61] (a) ORR polarization curves of P-MoS2 in 0.1 mol·L-1 KOH[45]; (b) Corresponding possible reaction mechanism[61]
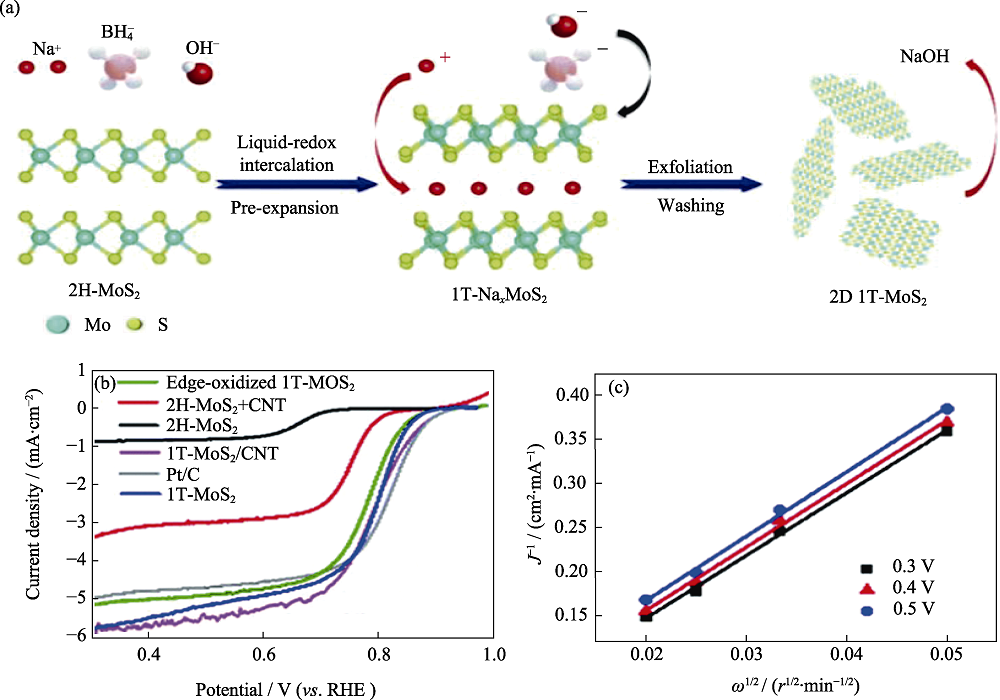
图6 由2H-MoS2相转变制备1T-MoS2纳米片的(a)示意图及其(b)在0.1 mol·L-1 KOH的ORR极化曲线与(c)K-L曲线[68]
Fig. 6 (a) Preparative schematic illustration by phase conversion from 2H-MoS2, (b) ORR polarization curves in 0.1 mol·L-1 KOH, and (c) corresponding K-L plots of 1T-MoS2[68] Colorful figures are available on website
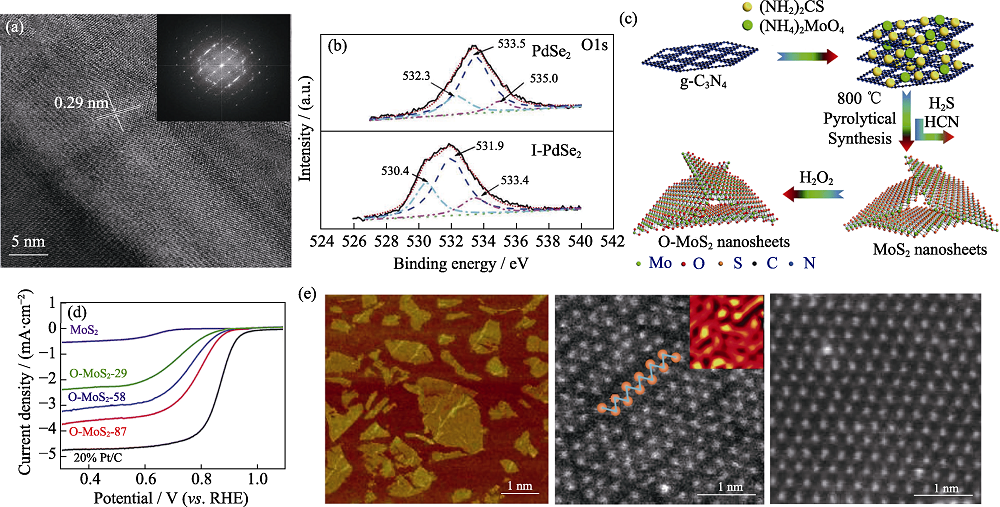
图7 2D TMDs的缺陷工程与应力工程调控[46,49,65]
Fig. 7 Defect and strain engineering of 2D TMDs[46,49,65] (a) TEM images and (b) O1s XPS spectra of defected I-PdSe2[46]; (c) Schematic illustration of synthesis of O-MoS2 (d) ORR polarization curves in 0.1 mol·L-1 KOH of O-MoS2[49]; (e) AFM (left) and TEM (middle and right) images of 2H-1T WS2 showing the formation of strain[65]
| Catalyst | Electrolyte | Onset potential / V (vs. RHE) | Half-wave potential / V (vs. RHE) | Electrons transfer number, n | Ref. |
|---|---|---|---|---|---|
| Pt/MoS2-rGO | 0.1 mol·L-1 HClO4 | 0.90 | 0.80 | - | [ |
| MoS2-CNT | 0.1 mol·L-1 KOH | 0.65 | - | ~4.00 | [ |
| MoS2/S-PC | 0.5 mol·L-1 H2SO4 | - | 0.86 | 4.00 | [ |
| Ni3S2/MoS2 | 0.1 mol·L-1 KOH | 0.95 | 0.88 | 3.99 | [ |
| hBN-MoS2 | 0.1 mol·L-1 KOH | 0.80 | 0.60 | 4.00 | [ |
| FePc-MoS2 | 0.1 mol·L-1 KOH | - | 0.89 | 4.00 | [ |
表2 典型2D TMDs异质结构ORR催化剂的性能
Table 2 Properties of typical 2D TMDs heterostructures-based ORR catalysts
| Catalyst | Electrolyte | Onset potential / V (vs. RHE) | Half-wave potential / V (vs. RHE) | Electrons transfer number, n | Ref. |
|---|---|---|---|---|---|
| Pt/MoS2-rGO | 0.1 mol·L-1 HClO4 | 0.90 | 0.80 | - | [ |
| MoS2-CNT | 0.1 mol·L-1 KOH | 0.65 | - | ~4.00 | [ |
| MoS2/S-PC | 0.5 mol·L-1 H2SO4 | - | 0.86 | 4.00 | [ |
| Ni3S2/MoS2 | 0.1 mol·L-1 KOH | 0.95 | 0.88 | 3.99 | [ |
| hBN-MoS2 | 0.1 mol·L-1 KOH | 0.80 | 0.60 | 4.00 | [ |
| FePc-MoS2 | 0.1 mol·L-1 KOH | - | 0.89 | 4.00 | [ |
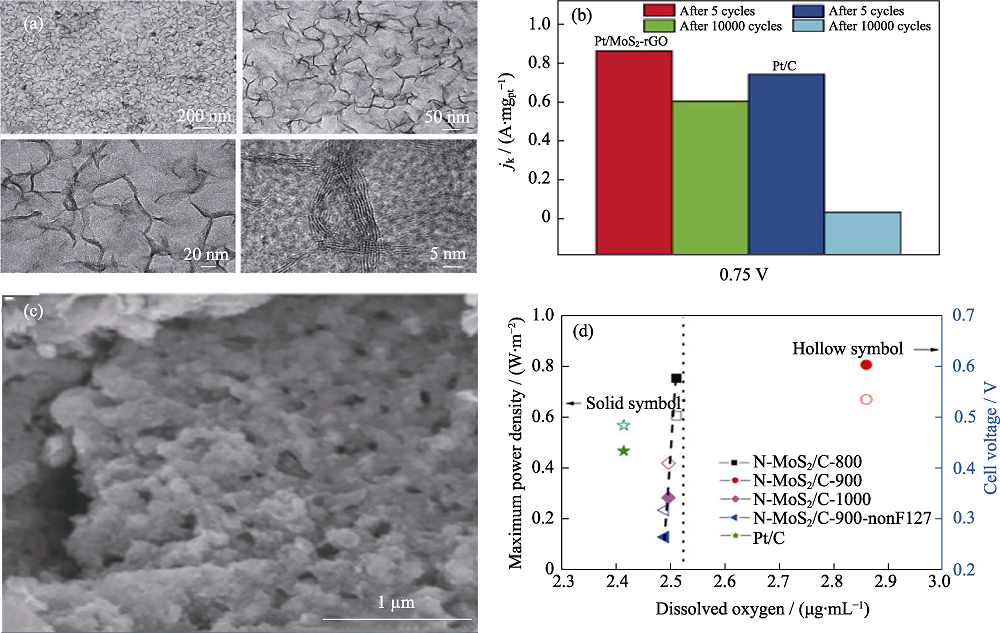
图8 2D TMDs@碳材料异质结ORR催化剂[85,92]
Fig. 8 2D TMDs@carbon materials heterostructure ORR catalysts[85,92] (a) TEM images and (b) activity variations of Pt/MoS2-rGO[85]; (c) SEM image and (d) power density of microbial fuel cells based on N-MoS2/C catalysts[92] Colorful figures are available on website
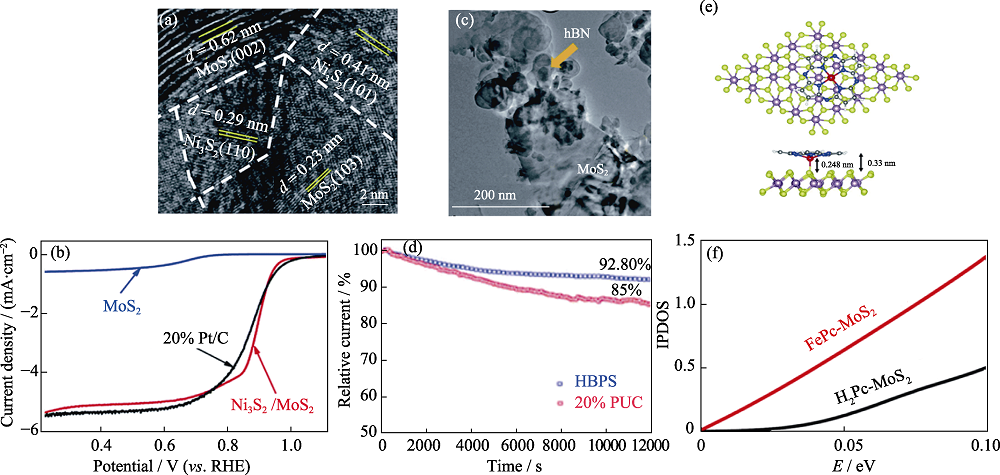
图9 其它2D TMDs基异质结构ORR催化剂[88⇓-90]
Fig. 9 Other 2D TMDs-based heterostructure ORR catalysts[88⇓-90] (a) TEM image of Ni3S2/MoS2 and (b) corresponding ORR polarization curves in 0.1 mol·L-1 KOH[88]; (c) TEM image of hBN/MoS2 and its (d) ORR durability test[89]; (e) Structure of FePc-MoS2 and (f) its integrated partial density of states IPDOS[90]
| [1] |
HAO C, LIU Z R, LIU W, et al. Research progress of carbon- supported metal single atom catalysts for oxygen reduction reaction. Journal of Inorganic Materials, 2021, 36(8): 820-834.
DOI |
| [2] | RAHMAN M A, WANG X J, WEN C E. High energy density metal-air batteries: a review. Journal of The Electrochemical Society, 2013, 160(10): A1759-A1771. |
| [3] |
LIU Z Y, ZHAO Z P, PENG B S, et al. Beyond extended surfaces: understanding the oxygen reduction reaction on nanocatalysts. Journal of the American Chemical Society, 2020, 142(42): 17812-17827.
DOI URL |
| [4] |
XIANG J, LIU B, LIU B, et al. A self-terminated electrochemical fabrication of electrode pairs with angstrom-sized gaps. Electrochemistry Communications, 2006, 8(4): 577-580.
DOI URL |
| [5] |
TIAN X L, ZHAO X, SU Y Q, et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science, 2019, 366(6467): 850-856.
DOI URL |
| [6] |
LI M F, ZHAO Z P, CHENG T, et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science, 2016, 354(6318): 1414-1419.
DOI URL |
| [7] |
ZENG R R, WANG K, SHAO W, et al. Investigation on the coordination mechanism of Pt-containing species and qualification of the alkaline content during Pt/C preparation via a solvothermal polyol method. Chinese Journal of Catalysis, 2020, 41(5): 820-829.
DOI URL |
| [8] |
WANG J J, YIN G P, SHAO Y Y, et al. Effect of carbon black support corrosion on the durability of Pt/C catalyst. Journal of Power Sources, 2007, 171(2): 331-339.
DOI URL |
| [9] |
JIMÉNEZ-MORALES I, REYES-CARMONA A, DUPONT M, et al. Correlation between the surface characteristics of carbon supports and their electrochemical stability and performance in fuel cell cathodes. Carbon Energy, 2021, 3(4): 654-665.
DOI URL |
| [10] | KONG F P, SHI W Z, SONG Y J, et al. Surface/near-surface structure of highly active and durable Pt-based catalysts for oxygen reduction reaction: a review. Advanced Energy and Sustainability Research, 2021, 2(7): 2100025. |
| [11] |
JIANG Y F, YANG L J, SUN T, et al. Significant contribution of intrinsic carbon defects to oxygen reduction activity. ACS Catalysis, 2015, 5(11): 6707-6712.
DOI URL |
| [12] |
LAI Q X, ZHENG J, TANG Z M, et al. Optimal configuration of N-doped carbon defects in 2D turbostratic carbon nanomesh for advanced oxygen reduction electrocatalysis. Angewandte Chemie International Edition, 2020, 59(29): 11999-12006.
DOI URL |
| [13] |
LANG X Y, HAN G F, XIAO B B, et al. Mesostructured intermetallic compounds of platinum and non-transition metals for enhanced electrocatalysis of oxygen reduction reaction. Advanced Functional Materials, 2015, 25(2): 230-237.
DOI URL |
| [14] |
NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films. Science, 2004, 306(5696): 666-669.
DOI URL |
| [15] | LI D D, LI T, HAO G Y, et al. IrO2 nanoparticle-decorated single- layer NiFe LDHs nanosheets with oxygen vacancies for the oxygen evolution reaction. Chemical Engineering Journal, 2020, 399: 125738. |
| [16] |
LI R, WANG S H, CHEN X X, et al. Highly anisotropic and water molecule-dependent proton conductivity in a 2D homochiral copper(II) metal-organic framework. Chemistry of Materials, 2017, 29(5): 2321-2331.
DOI URL |
| [17] |
WANG Z T, LI H, YAN S C, et al. Synthesis of a two-dimensional covalent organic framework with the ability of conducting proton along skeleton. Acta Chimica Sinica, 78(1): 63-68.
DOI URL |
| [18] | WANG X, RAGHUPATHY R K M, QUEREBILLO C J, et al. Interfacial covalent bonds regulated electron-deficient 2D black phosphorus for electrocatalytic oxygen reactions. Advanced Materials, 2021, 33(20): 2008752. |
| [19] |
LIN Y, CONNELL J W. Advances in 2D boron nitride nanostructures: nanosheets, nanoribbons, nanomeshes, and hybrids with graphene. Nanoscale, 2012, 4(22): 6908-6939.
DOI URL |
| [20] |
LI Y B, QIN Y Q, CHEN K, et al. Molten salt synthesis of nanolaminated Sc2SnC MAX phase. Journal of Inorganic Materials, 2021, 36(7): 773-778.
DOI URL |
| [21] |
WANG S S, YU Y, ZHANG S Q, et al. Atomic-scale studies of overlapping grain boundaries between parallel and quasi-parallel grains in low-symmetry monolayer ReS2. Matter, 2020, 3(6): 2108-2123.
DOI URL |
| [22] |
DING J H, ZHAO H R, ZHAO X P, et al. How semiconductor transition metal dichalcogenides replaced graphene for enhancing anticorrosion. Journal of Materials Chemistry A, 2019, 7(22): 13511-13521.
DOI URL |
| [23] |
ZHU C R, GAO D Q, DING J, et al. TMD-based highly efficient electrocatalysts developed by combined computational and experimental approaches. Chemical Society Reviews, 2018, 47(12): 4332-4356.
DOI URL |
| [24] |
XIAO Y, ZHOU M Y, LIU J L, et al. Phase engineering of two- dimensional transition metal dichalcogenides. Science China Materials, 2019, 62(6): 759-775.
DOI URL |
| [25] | LIU D Y, HONG J H, LI X B, et al. Synthesis of 2H-1T′ WS2-ReS2 heterophase structures with atomically sharp interface via hydrogen- triggered one-pot growth. Advanced Functional Materials, 2020, 30(16): 1910169. |
| [26] |
SPLENDIANI A, SUN L, ZHANG Y B, et al. Emerging photoluminescence in monolayer MoS2. Nano Letters, 2010, 10(4): 1271-1275.
DOI URL |
| [27] |
BALASUBRAMANYAM S, SHIRAZI M, BLOODGOOD M A, et al. Edge-site nanoengineering of WS2 by low-temperature plasma- enhanced atomic layer deposition for electrocatalytic hydrogen evolution. Chemistry of Materials, 2019, 31(14): 5104-5115.
DOI URL |
| [28] |
SARMA P V, KAYAL A, SHARMA C H, et al. Electrocatalysis on edge-rich spiral WS2 for hydrogen evolution. ACS Nano, 2019, 13(9): 10448-10455.
DOI URL |
| [29] |
LIU J Y, JIANG X, LI X T, et al. Time- and momentum-resolved image-potential states of 2H-MoS2 surface. Physical Chemistry Chemical Physics, 2021, 23(46): 26336-26342.
DOI URL |
| [30] |
JIANG X, ZHENG Q J, LAN Z G, et al. Real-time GW-BSE investigations on spin-valley exciton dynamics in monolayer transition metal dichalcogenide. Science Advances, 7(10): eabf3759.
DOI URL |
| [31] | JING Q H, ZHANG H, HUANG H, et al. Ultrasonic exfoliated ReS2nanosheets: fabrication and use as co-catalyst for enhancing photocatalytic efficiency of TiO2 nanoparticles under sunlight. Nanotechnology, 2019, 30(18): 184001. |
| [32] |
LI H, YIN Z Y, HE Q Y, et al. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small, 2012, 8(1): 63-67.
DOI URL |
| [33] |
ZHANG Q Y, MEI L, CAO X H, et al. Intercalation and exfoliation chemistries of transition metal dichalcogenides. Journal of Materials Chemistry A, 2020, 8(31): 15417-15444.
DOI URL |
| [34] |
LI S W, LIU Y C, ZHAO X D, et al. Molecular engineering on MoS2 enables large interlayers and unlocked basal planes for high- performance aqueous Zn-ion storage. Angewandte Chemie International Edition, 2021, 60(37): 20286-20293.
DOI URL |
| [35] |
CHEN X Y, WANG Z M, WEI Y Z, et al. High phase-purity 1T-MoS2 ultrathin nanosheets by a spatially confined template. Angewandte Chemie International Edition, 2019, 58(49): 17621-17624.
DOI URL |
| [36] |
VAN DER ZANDE A M, HUANG P Y, CHENET D A, et al. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nature Materials, 2013, 12(6): 554-561.
DOI URL |
| [37] |
ZHOU J D, LIN J H, HUANG X W, et al. A library of atomically thin metal chalcogenides. Nature, 2018, 556(7701): 355-359.
DOI URL |
| [38] |
WANG S S, RONG Y M, FAN Y, et al. Shape evolution of monolayer MoS2 crystals grown by chemical vapor deposition. Chemistry of Materials, 2014, 26(22): 6371-6379.
DOI URL |
| [39] | ZHANG Y, YAO Y Y, SENDEKU M G, et al. Recent progress in CVD growth of 2D transition metal dichalcogenides and related heterostructures. Advanced Materials, 2019, 31(41): 1901694. |
| [40] |
HUANG H W, LI K, CHEN Z, et al. Achieving remarkable activity and durability toward oxygen reduction reaction based on ultrathin Rh-doped Pt nanowires. Journal of the American Chemical Society, 2017, 139(24): 8152-8159.
DOI URL |
| [41] |
NØRSKOV J K, ROSSMEISL J, LOGADOTTIR A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. The Journal of Physical Chemistry B, 2004, 108(46): 17886-17892.
DOI URL |
| [42] |
CUI Y, ZHOU C W, LI X Z, et al. High performance electrocatalysis for hydrogen evolution reaction using nickel-doped CoS2 nanostructures: experimental and DFT insights. Electrochimica Acta, 2017, 228: 428-435.
DOI URL |
| [43] | WU L F, DZADE N Y, CHEN N, et al. Cu electrodeposition on nanostructured MoS2 and WS2and implications for HER active site determination. Journal of The Electrochemical Society, 2020, 167(11): 116517. |
| [44] | WANG Z W, LI W L, ZHENG Y P, et al. How does the active site in the MoSe2 surface affect its electrochemical performance as anode material for metal-ion batteries? Applied Surface Science, 2020, 526: 146637. |
| [45] |
HUANG H, FENG X, DU C C, et al. High-quality phosphorus- doped MoS2 ultrathin nanosheets with amenable ORR catalytic activity. Chemical Communications, 2015, 51(37): 7903-7906.
DOI URL |
| [46] |
KOH S W, HU J, HWANG J M, et al. Two-dimensional palladium diselenide for the oxygen reduction reaction. Materials Chemistry Frontiers, 2021, 5(13): 4970-4980.
DOI URL |
| [47] | VATTIKUTI S V P, NAGAJYOTHI P C, DEVARAYAPALLI K C, et al. Hybrid Ag/MoS2 nanosheets for efficient electrocatalytic oxygen reduction. Applied Surface Science, 2020, 526: 146751. |
| [48] |
CAO Y F, HUANG S C, PENG Z Q, et al. Phase control of ultrafine FeSe nanocrystals in a N-doped carbon matrix for highly efficient and stable oxygen reduction reaction. Journal of Materials Chemistry A, 2021, 9(6): 3464-3471.
DOI URL |
| [49] |
HUANG H, FENG X, DU C C, et al. Incorporated oxygen in MoS2 ultrathin nanosheets for efficient ORR catalysis. Journal of Materials Chemistry A, 2015, 3(31): 16050-16056.
DOI URL |
| [50] |
SARKAR D, XIE X J, KANG J H, et al. Functionalization of transition metal dichalcogenides with metallic nanoparticles: implications for doping and gas-sensing. Nano Letters, 2015, 15(5): 2852-2862.
DOI URL |
| [51] | CHEN E, XU W, CHEN J, et al. 2D layered noble metal dichalcogenides (Pt, Pd, Se, S) for electronics and energy applications. Materials Today Advances, 2020, 7: 100076. |
| [52] | SOLOMON G, KOHAN M G, VAGIN M, et al. Decorating vertically aligned MoS2 nanoflakes with silver nanoparticles for inducing a bifunctional electrocatalyst towards oxygen evolution and oxygen reduction reaction. Nano Energy, 2021, 81: 105664. |
| [53] |
UPADHYAY S N, PAKHIRA S. Mechanism of electrochemical oxygen reduction reaction at two-dimensional Pt-doped MoSe2 material: an efficient electrocatalyst. Journal of Materials Chemistry C, 2021, 9(34): 11331-11342.
DOI URL |
| [54] |
HWANG J, NOH S H, HAN B. Design of active bifunctional electrocatalysts using single atom doped transition metal dichalcogenides. Applied Surface Science, 2019, 471: 545-552.
DOI URL |
| [55] |
SHI Y, MA Z R, XIAO Y Y, et al. Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nature Communications, 2021, 12(1): 3021.
DOI URL |
| [56] |
SHI Y, WANG J, WANG C, et al. Hot electron of Au nanorods activates the electrocatalysis of hydrogen evolution on MoS2 nanosheets. Journal of the American Chemical Society, 2015, 137(23): 7365-7370.
DOI URL |
| [57] |
CHEN Z X, LENG K, ZHAO X X, et al. Interface confined hydrogen evolution reaction in zero valent metal nanoparticles-intercalated molybdenum disulfide. Nature Communications, 2017, 8(1): 14548.
DOI URL |
| [58] |
QI K, YU S S, WANG Q Y, et al. Decoration of the inert basal plane of defect-rich MoS2 with Pd atoms for achieving Pt-similar HER activity. Journal of Materials Chemistry A, 2016, 4(11): 4025-4031.
DOI URL |
| [59] |
TIAN S F, TANG Q. Activating transition metal dichalcogenide monolayers as efficient electrocatalysts for the oxygen reduction reaction via single atom doping. Journal of Materials Chemistry C, 2021, 9(18): 6040-6050.
DOI URL |
| [60] |
ZHANG H Y, TIAN Y, ZHAO J X, et al. Small dopants make big differences: enhanced electrocatalytic performance of MoS2 monolayer for oxygen reduction reaction (ORR) by N- and P-doping. Electrochimica Acta, 2017, 225: 543-550.
DOI URL |
| [61] |
LIU C, DONG H L, JI Y J, et al. Origin of the catalytic activity of phosphorus doped MoS2 for oxygen reduction reaction (ORR) in alkaline solution: a theoretical study. Scientific Reports, 2018, 8(1): 13292.
DOI URL |
| [62] |
WANG H T, TSAI C, KONG D S, et al. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Research, 2015, 8(2): 566-575.
DOI URL |
| [63] |
GAO C, RAO D W, YANG H, et al. Dual transition-metal atoms doping: an effective route to promote the ORR and OER activity on MoTe2. New Journal of Chemistry, 2021, 45(12): 5589-5595.
DOI URL |
| [64] |
GONG Y J, YUAN H T, WU C L, et al. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Nature Nanotechnology, 2018, 13(4): 294-299.
DOI URL |
| [65] |
VOIRY D, YAMAGUCHI H, LI J W, et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nature Materials, 2013, 12(9): 850-855.
DOI URL |
| [66] |
WANG Y Y, WANG M R, LU Z S, et al. Enabling multifunctional electrocatalysts by modifying the basal plane of unifunctional 1T′- MoS2 with anchored transition metal single atoms. Nanoscale, 2021, 13(31): 13390-13400.
DOI URL |
| [67] | ZHAO B, SHEN D Y, ZHANG Z C, et al. 2D metallic transition- metal dichalcogenides: structures, synthesis, properties, and applications. Advanced Functional Materials, 2021, 31(48): 2105132. |
| [68] |
SADIGHI Z, LIU J P, ZHAO L, et al. Metallic MoS2 nanosheets: multifunctional electrocatalyst for the ORR, OER and Li-O2 batteries. Nanoscale, 2018, 10(47): 22549-22559.
DOI URL |
| [69] |
LIN Y C, DUMCENCO D O, HUANG Y S, et al. Atomic mechanism of the semiconducting-to-metallic phase transition in single- layered MoS2. Nature Nanotechnology, 2014, 9(5): 391-396.
DOI URL |
| [70] |
LIN Y C, DUMCENCO D O, KOMSA H P, et al. Properties of individual dopant atoms in single-layer MoS2: atomic structure, migration, and enhanced reactivity. Advanced Materials, 2014, 26(18): 2857-2861.
DOI URL |
| [71] | DING W, HU L, DAI J M, et al. Highly ambient-stable 1T-MoS2 and 1T-WS2 by hydrothermal synthesis under high magnetic fields. ACS Nano, 2019, 13(2): 1694-1702. |
| [72] |
PRABHU P, JOSE V, LEE J M. Design strategies for development of TMD-based heterostructures in electrochemical energy systems. Matter, 2020, 2(3): 526-553.
DOI URL |
| [73] | WANG S, ZHANG D, LI B, et al. Ultrastable in-plane 1T-2H MoS2 heterostructures for enhanced hydrogen evolution reaction. Advanced Energy Materials, 2018, 8(25): 1801345. |
| [74] |
YIN Y, HAN J C, ZHANG Y M, et al. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets. Journal of the American Chemical Society, 2016, 138(25): 7965-7972.
DOI URL |
| [75] |
ZHU J, WANG Z C, DAI H, et al. Boundary activated hydrogen evolution reaction on monolayer MoS2. Nature Communications, 2019, 10(1): 1348.
DOI URL |
| [76] |
MENG Y N, GAO Y, LI K, et al. Vacancy-induced oxygen reduction activity in Janus transition metal dichalcogenides. ChemElectroChem, 2020, 7(20): 4233-4238.
DOI URL |
| [77] |
YANG J, WANG Z Y, HUANG C X, et al. Compressive strain modulation of single iron sites on helical carbon support boosts electrocatalytic oxygen reduction. Angewandte Chemie International Edition, 2021, 60(42): 22722-22728.
DOI URL |
| [78] | XU X, LIANG T, KONG D, et al. Strain engineering of two- dimensional materials for advanced electrocatalysts. Materials Today Nano, 2021, 14: 100111. |
| [79] |
ZHAO S Y, WANG K, ZOU X L, et al. Group VB transition metal dichalcogenides for oxygen reduction reaction and strain-enhanced activity governed by p-orbital electrons of chalcogen. Nano Research, 2019, 12(4): 925-930.
DOI URL |
| [80] |
LI H, CONTRYMAN A W, QIAN X F, et al. Optoelectronic crystal of artificial atoms in strain-textured molybdenum disulphide. Nature Communications, 2015, 6(1): 7381.
DOI URL |
| [81] | TIWARI A P, YOON Y, NOVAK T G, et al. Lattice strain formation through spin-coupled shells of MoS2 on Mo2C for bifunctional oxygen reduction and oxygen evolution reaction electrocatalysts. Advanced Materials Interfaces, 2019, 6(22): 1900948. |
| [82] |
HAN C, WANG Y D, LEI Y P. Recent progress on nano-heterostructure photocatalysts for solar fuels generation. Journal of Inorganic Materials, 2015, 30(11): 1121-1130.
DOI URL |
| [83] |
MAO Y H, MA X C, WU D X, et al. Interfacial polarons in van der Waals heterojunction of monolayer SnSe2 on SrTiO3 (001). Nano Letters, 2020, 20(11): 8067-8073.
DOI URL |
| [84] | LIU Y, ZHAO G J, ZHANG J X, et al. First-principles investigation on the interfacial interaction and electronic structure of BiVO4/WO3 heterostructure semiconductor material. Applied Surface Science, 2021, 549: 149309. |
| [85] |
ANWAR M T, YAN X H, ASGHAR M R, et al. MoS2-rGO hybrid architecture as durable support for cathode catalyst in proton exchange membrane fuel cells. Chinese Journal of Catalysis, 2019, 40(8): 1160-1167.
DOI URL |
| [86] |
LEE C, OZDEN S, TEWARI C S, et al. MoS2-carbon nanotube porous 3D network for enhanced oxygen reduction reaction. ChemSusChem, 2018, 11(17): 2960-2966.
DOI URL |
| [87] |
PARK H S, HAN S B, KWAK D H, et al. Sulfur-doped porphyrinic carbon nanostructures synthesized with amorphous MoS2 for the oxygen reduction reaction in an acidic medium. ChemSusChem, 2017, 10(10): 2202-2209.
DOI URL |
| [88] |
MAO J X, LIU P, DU C C, et al. Tailoring 2D MoS2 heterointerfaces for promising oxygen reduction reaction electrocatalysis. Journal of Materials Chemistry A, 2019, 7(15): 8785-8789.
DOI URL |
| [89] |
ROY D, PANIGRAHI K, DAS B K, et al. Boron vacancy: a strategy to boost the oxygen reduction reaction of hexagonal boron nitride nanosheet in hBN-MoS2 heterostructure. Nanoscale Advances, 2021, 3(16): 4739-4749.
DOI URL |
| [90] |
KWON I S, KWAK I H, KIM J Y, et al. Two-dimensional MoS2/Fe-phthalocyanine hybrid nanostructures as excellent electrocatalysts for hydrogen evolution and oxygen reduction reactions. Nanoscale, 2019, 11(30): 14266-14275.
DOI URL |
| [91] |
XIN S L, LIU Z Q, MA L, et al. Visualization of the electrocatalytic activity of three-dimensional MoSe2@reduced graphene oxide hybrid nanostructures for oxygen reduction reaction. Nano Research, 2016, 9(12): 3795-3811.
DOI URL |
| [92] |
HAO L, YU J, XU X, et al. Nitrogen-doped MoS2/carbon as highly oxygen-permeable and stable catalysts for oxygen reduction reaction in microbial fuel cells. Journal of Power Sources, 2017, 339: 68-79.
DOI URL |
| [93] |
SHANG X, YAN K L, LIU Z Z, et al. Oxidized carbon fiber supported vertical WS2 nanosheets arrays as efficient 3D nanostructure electrocatalyts for hydrogen evolution reaction. Applied Surface Science, 2017, 402: 120-128.
DOI URL |
| [94] | CHENG C, HE B W, FAN J J, et al. An inorganic/organic S-scheme heterojunction H2-production photocatalyst and its charge transfer mechanism. Advanced Materials, 2021, 33(22): 2100317. |
| [95] | CHEN J L, QIAN G F, ZHANG H, et al. PtCo@PtSn heterojunction with high stability/activity for pH-universal H2 evolution. Advanced Functional Materials, 2022, 32(5): 2107597. |
| [96] | SUN L, WANG B, WANG Y D. High-temperature gas sensor based on novel Pt single atoms@SnO2 nanorods@SiC nanosheets multi- heterojunctions. ACS Applied Materials & Interfaces, 2020, 12(19): 21808-21817. |
| [97] | HE L H, CUI B B, LIU J M, et al. Fabrication of porous CoOx/mC@MoS2 composite Loaded on g-C3N4 nanosheets as a highly efficient dual electrocatalyst for oxygen reduction and hydrogen evolution reactions. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 9257-9268. |
| [98] | CHUONG N D, THANH T D, KIM N H, et al. Hierarchical heterostructures of ultrasmall Fe2O3-encapsulated MoS2/N-graphene as an effective catalyst for oxygen reduction reaction. ACS Applied Materials & Interfaces, 2018, 10(29): 24523-24532. |
| [99] | BAI J M, MENG T, GUO D L, et al. Co9S8@MoS2 core-shell heterostructures as trifunctional electrocatalysts for overall water splitting and Zn-air batteries. ACS Applied Materials & Interfaces, 2018, 10(2): 1678-1689. |
| [100] |
LI W M, YU A P, HIGGINS D C, et al. Biologically inspired highly durable iron phthalocyanine catalysts for oxygen reduction reaction in polymer electrolyte membrane fuel cells. Journal of the American Chemical Society, 2010, 132(48): 17056-17058.
DOI URL |
| [101] |
SAMANTA M, GHOSH S, MUKHERJEE M, et al. Enhanced electrocatalytic oxygen reduction reaction from organic-inorganic heterostructure. International Journal of Hydrogen Energy, 2022, 47(10): 6710-6720.
DOI URL |
| [102] |
ZHOU X L, HAO H, ZHANG Y J, et al. Patterning of transition metal dichalcogenides catalyzed by surface plasmons with atomic precision. Chem, 2021, 7(6): 1626-1638.
DOI URL |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [8] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [9] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [10] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [11] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [12] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [13] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [14] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| [15] | 刘磊, 郭瑞华, 王丽, 王艳, 张国芳, 关丽丽. Pt3Co高指数晶面氧还原过程的密度泛函理论研究[J]. 无机材料学报, 2025, 40(1): 39-46. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||