无机材料学报 ›› 2020, Vol. 35 ›› Issue (8): 867-881.DOI: 10.15541/jim20190561 CSTR: 32189.14.10.15541/jim20190561
所属专题: 封面文章; 生物材料论文精选(2020)
董少杰1,2( ),王旭东2,沈国芳2,3,王晓虹1(
),王旭东2,沈国芳2,3,王晓虹1( ),林开利2(
),林开利2( )
)
收稿日期:2019-11-04
修回日期:2019-11-24
出版日期:2020-08-20
网络出版日期:2020-01-20
作者简介:董少杰(1992-), 男, 博士研究生. E-mail: 基金资助:
DONG Shaojie1,2( ),WANG Xudong2,SHEN Steve Guofang2,3,WANG Xiaohong1(
),WANG Xudong2,SHEN Steve Guofang2,3,WANG Xiaohong1( ),LIN Kaili2(
),LIN Kaili2( )
)
Received:2019-11-04
Revised:2019-11-24
Published:2020-08-20
Online:2020-01-20
Supported by:摘要:
生物陶瓷支架具有良好的生物相容性和引导组织再生特性, 并可提供多孔的表面形貌和孔道结构, 以促进新生组织的长入, 在硬组织修复和骨组织工程支架领域获得了广泛的关注和临床应用。当前, 生物陶瓷支架仍然存在骨诱导活性差、生物功能单一、力学性能差等缺陷, 极大限制了它们的临床治疗效果和应用范围。本文从生物陶瓷支架的功能改性角度出发, 对材料实施表面功能涂层修饰、微纳结构改性、功能元素掺杂、力学增强等策略, 及其在改善植入体生物相容性、促进成骨活性、药物递送、抗肿瘤和抗菌等方面的应用进展进行了归纳和总结, 并对功能改性生物陶瓷支架的未来发展趋势作了展望。
中图分类号:
董少杰,王旭东,沈国芳,王晓虹,林开利. 生物陶瓷支架的功能改性及应用研究进展[J]. 无机材料学报, 2020, 35(8): 867-881.
DONG Shaojie,WANG Xudong,SHEN Steve Guofang,WANG Xiaohong,LIN Kaili. Research Progress on Functional Modifications and Applications of Bioceramic Scaffolds[J]. Journal of Inorganic Materials, 2020, 35(8): 867-881.
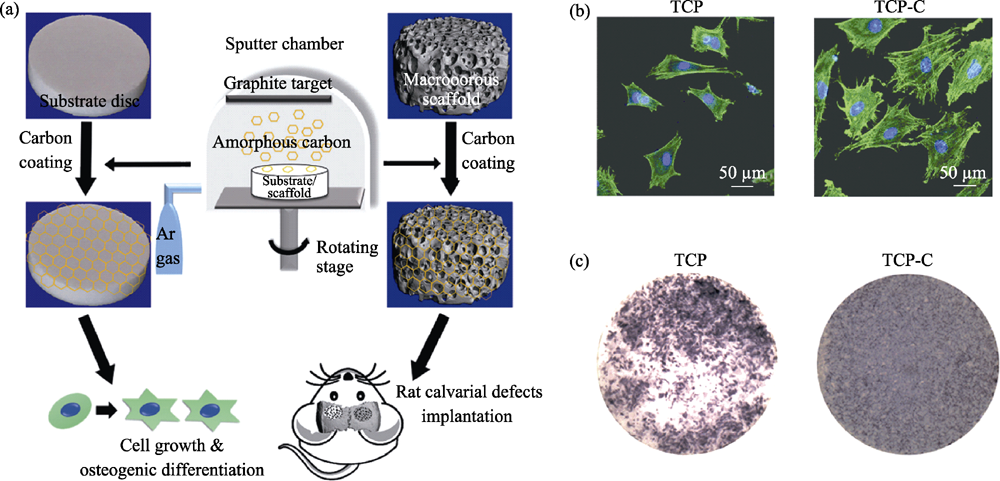
图1 无定型碳涂层β-TCP支架的制备流程图(a)及材料对BMSCs细胞黏附(b)、ALP表达(c)的调控[18]
Fig. 1 Procedure for the fabricating of β-TCP scaffold coated with amorphous carbon (a), and adhesion (b) and ALP activity (c) of BMSCs cultured on the samples[18]
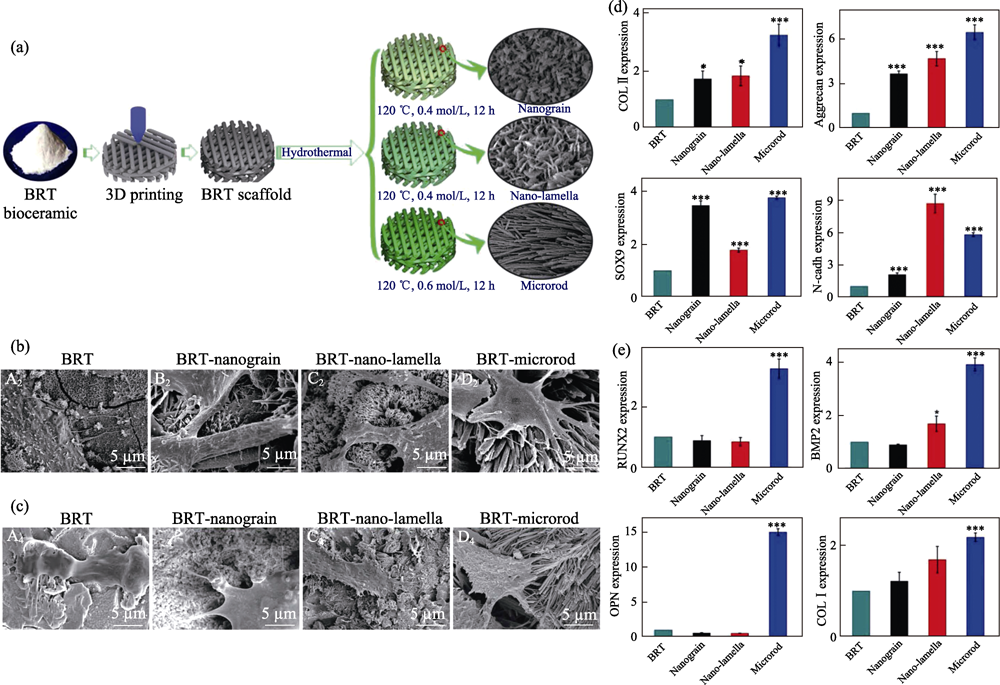
图2 白硅钙石支架的(a)制备及表面微纳结构改性, 促(b)软骨和(c)BMSCs细胞的黏附, 及其促进(d)成软骨和(e)成骨分化相关基因表达的能力[28]
Fig. 2 (a) Fabrication procedure of bredigite (BRT) scaffolds with modified micro/nanostructure on the surface, cell adhesion behavior of (b) chondrocytes and (c) BMSCs cultured on different scaffolds, and expression level of (d) chondrogenesis of chondrocytes and (e) osteogenesis related genes of BMSCs cultured on different scaffolds, respectively[28]
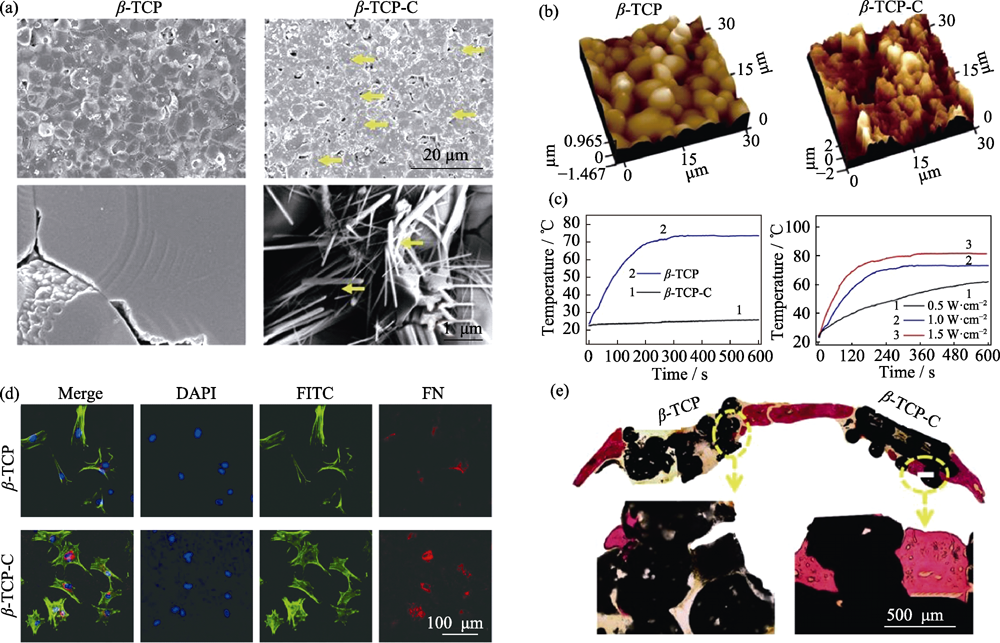
图3 β-TCP和CA修饰的β-TCP陶瓷的(a)SEM形貌(黄色箭头为碳气凝胶)和(b)原子力显微镜结构, (c)光热能力, (d)材料调控BMSCs黏附及FN表达和(e)大鼠颅骨缺损修复能力[32]
Fig. 3 Surface morphology of β-TCP and CA coated β-TCP (β-TCP-C) detected with (a) SEM (yellow, CA) and (b) atomic force microscope, (c) photothermal performance of β-TCP and β-TCP-C, (d) cell adhesion behavior and FN-expression of BMSCs cultured on β-TCP and β-TCP-C, and (e) osteogenesis capability of β-TCP and β-TCP-C scaffold in vivo[32]
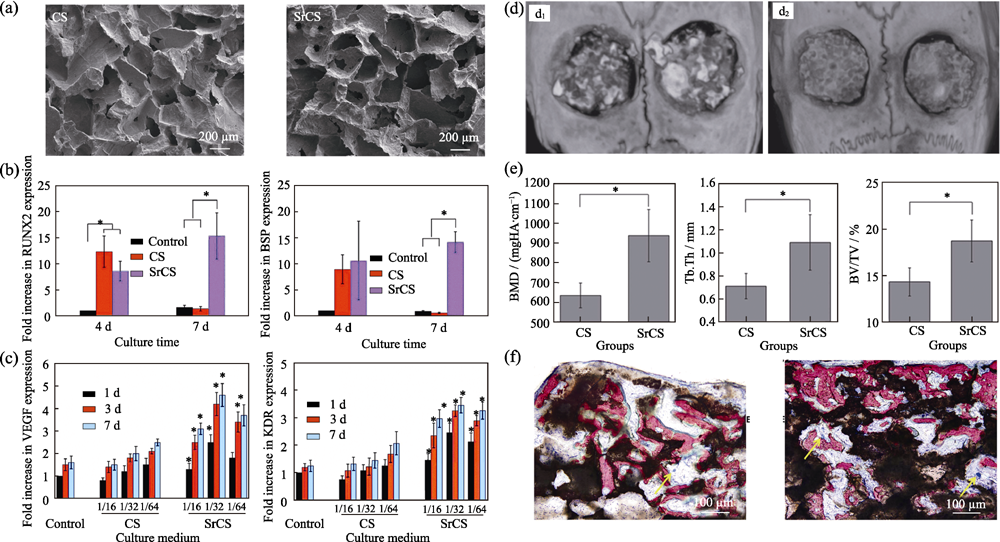
图4 CS和Sr-CS支架的(a)SEM照片, 材料浸提液对(b)BMSCs-OVX成骨分化和(c)HUVECs成血管分化的促进作用, (d)颅骨(d1, CS; d2, Sr-CS)micro-CT影像和(e)定量分析, 及(f)新生骨的VG染色结果(左: CS; 右: Sr-CS)[39]
Fig. 4 (a) Morphologies of CS and Sr-CS scaffolds, expression level of (b) osteogenic genes of BMSCs-OVX and (c) angiogenic genes of HUVECs cultured with the extracts of CS and Sr-CS scaffolds, (d) micro-CT images (d1, CS; d2, Sr-CS), (e) morphometric analysis and (f) VG staining results of the new formed bone (left: CS; right: Sr-CS)[39]
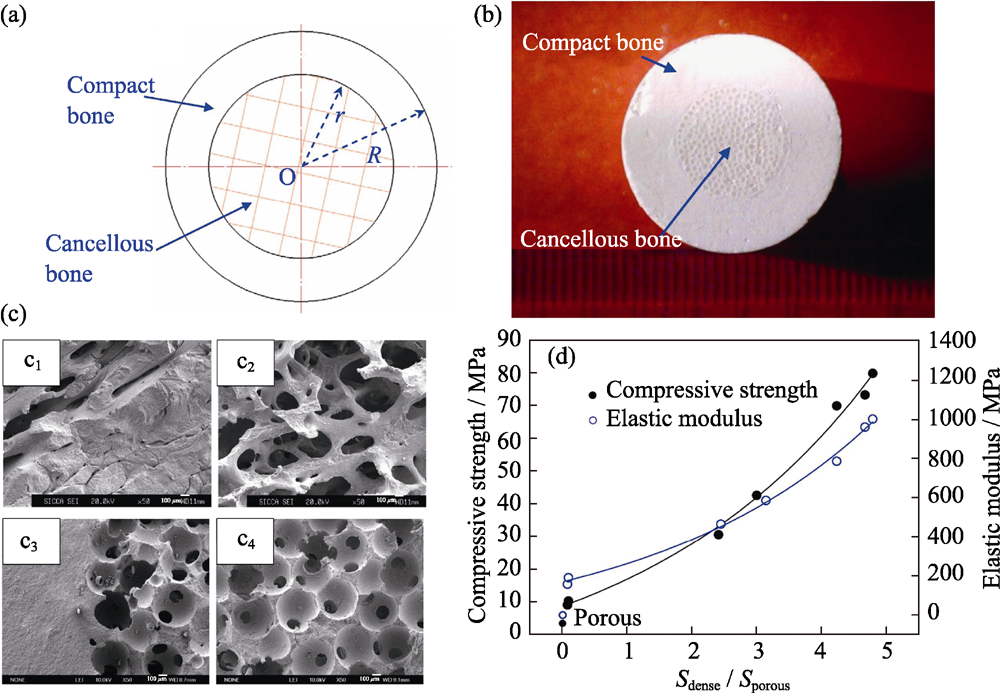
图5 仿生梯度多孔结构β-TCP支架的(a)结构示意图及(b)数码照片, (c)天然骨(c1~c2)皮质-松质交界区与支架(c3~c4)高密度-低密度交界区SEM照片, (d)高密度/低密度区域面积比与材料压缩强度的定量函数关系[61]
Fig. 5 (a) Structure-diagram and (b) digital images of biomimetic β-TCP scaffolds (c) SEM images of (c1-c2) compact/cancellous interface of natural bone and (c3-c4) dense/porous interface of scaffold, (d) function curves of compression strength, modulus of elasticity and dense/porous cross-sectional area ratio[61]
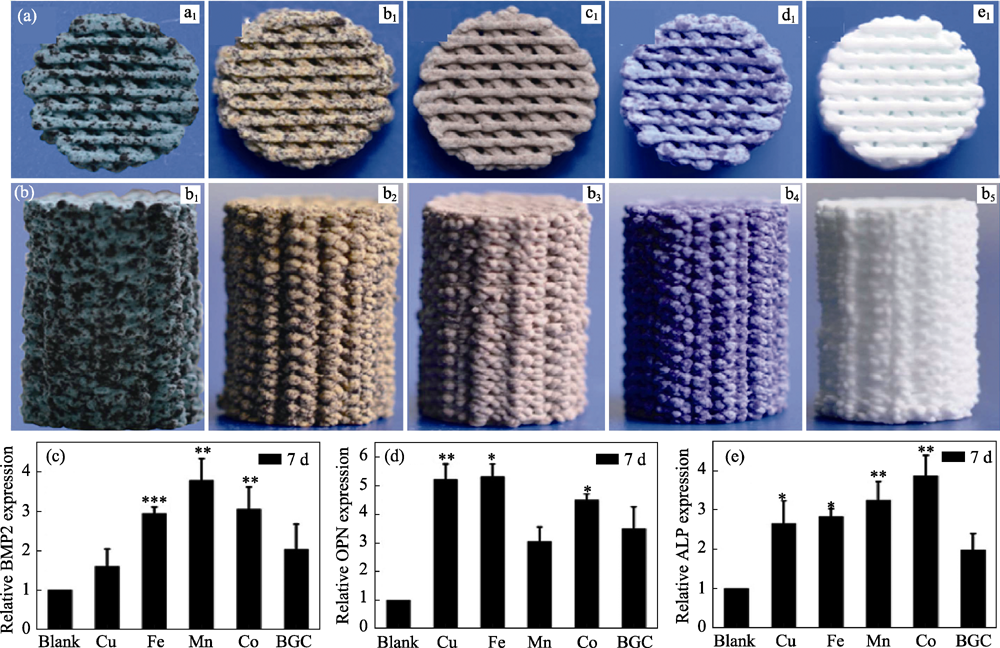
图6 Cu, Fe, Mn, Co元素掺杂生物玻璃支架的截面(a1~a5)和侧面(b1~b5)形貌及(c~e)促成骨分化能力[55]
Fig. 6 Morphologies (top view (a) and side view (b)) of 3D printing bioglass scaffolds containing Cu, Fe, Mn, Co elements and pure bioglass, (c-e) expression of osteogenic genes of BMSCs cultured on culture plate, 3D printing bioglass scaffolds containing Cu, Fe, Mn, Co elements doping and pure bioglass[55]
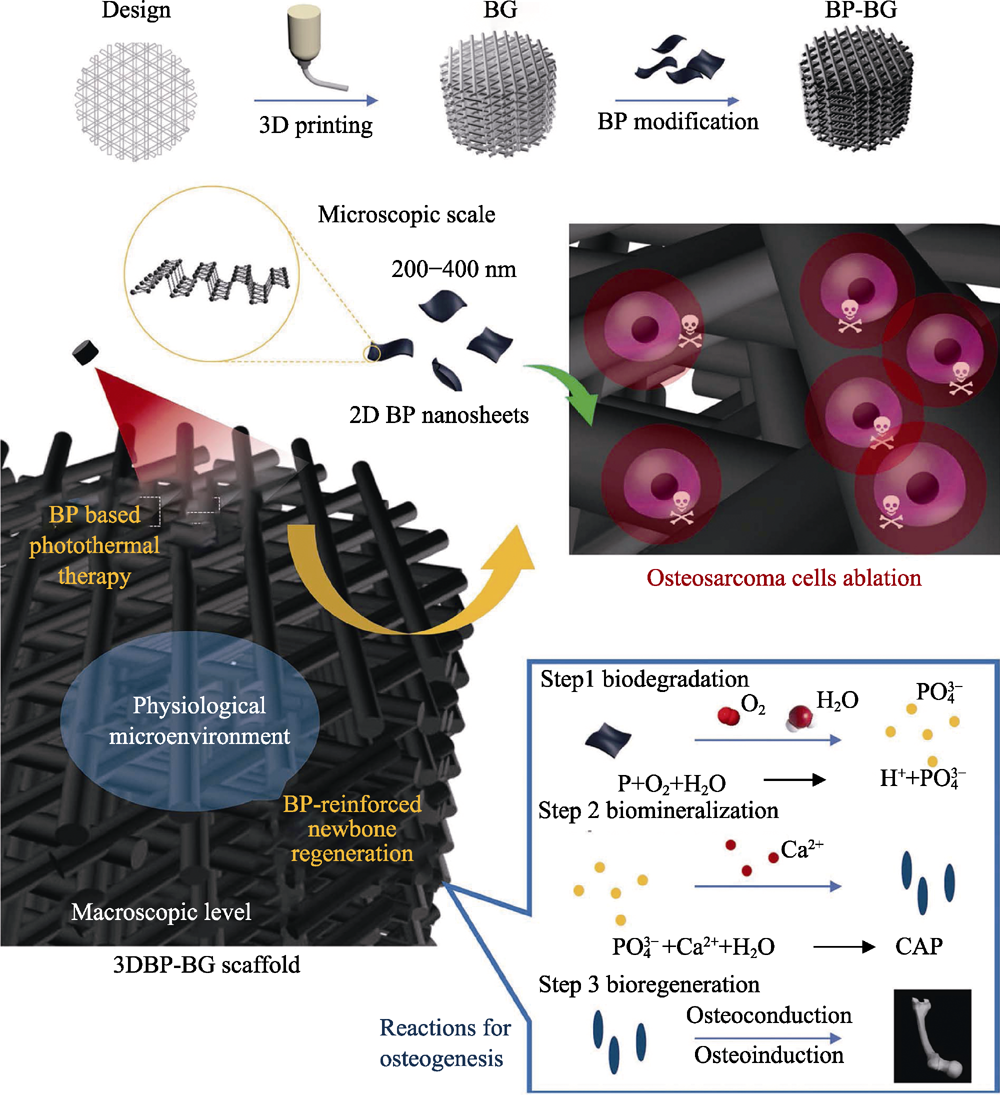
图7 BP-BG支架的制备以及骨肉瘤治疗-骨缺损修复机制示意图[16]
Fig. 7 Schematic illustration of fabrication process of BP-BG scaffold and stepwise therapeutic strategy for the elimination of osteosarcoma followed by osteogenesis by BP-BG[16]
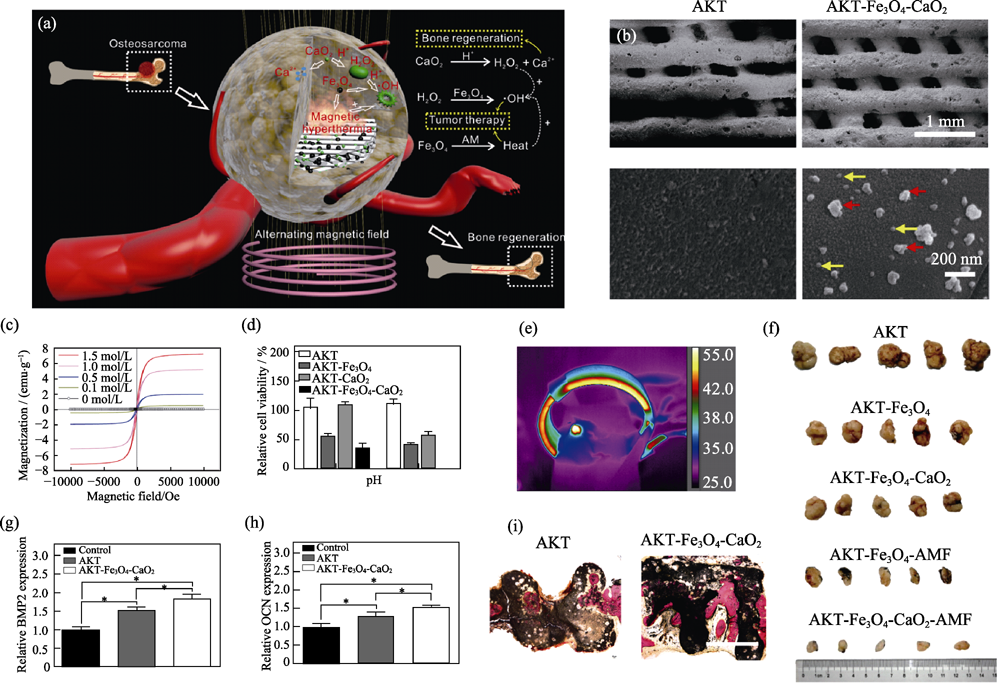
图8 功能改性的多功能AKT-Fe3O4-CaO2支架的(a)作用机制、(b)表面形貌、(c)可调控磁化强度、(d~f)骨肿瘤的磁热-催化联合治疗及(g~i)促进成骨基因表达和肿瘤治疗后的骨缺损修复[7]
Fig. 8 (a) Schematic diagram of AKT-Fe3O4-CaO2 scaffold functioning to obtain efficient tumor ablation and enhanced bone regeneration, (b) SEM images of AKT and AKT-Fe3O4-CaO2 scaffold (red arrows: Fe3O4 nanoparticles; yellow arrows: CaO2 nanoparticles), (c) magnetization curves of AKT-Fe3O4-CaO2 scaffolds soaked in Fe3O4 suspensions with various concentrations, (d) in vitro therapeutic effect of AKT-Fe3O4-CaO2 scaffold, (e) infrared images of nude mice in alternating magnetic fields, (f) in vivo therapeutic effect of AKT-Fe3O4-CaO2 scaffold, (g, h) expression of osteogenic genes of BMSCs, and (i) regeneration of cranium defects implanted with AKT and AKT-Fe3O4-CaO2 scaffolds[7] (1 emu?g-1= 1×103 A?m-1?g-1, 1 Oe=80 A?m-1)
| [1] |
GEORGAKILAS V, TIWARI J N, KEMP K C, et al. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and ciomedical applications. Chemical Reviews, 2016,116(9):5464-5519.
DOI URL PMID |
| [2] |
HABRAKEN W, HABIBOVIC P, EPPLE M, et al. Calcium phosphates in biomedical applications: materials for the future? Materials Today, 2016,19(2):69-87.
DOI URL |
| [3] |
LIN K L, WU C T, CHANG J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomaterialia, 2014,10(10):4071-4102.
DOI URL |
| [4] |
WANG X L, REN Z M, CHANG J. Synthesis and orientation of Fe-doped hydroxyapatite in high magnetic field. Journal of Inorganic Materials, 2018,33(1):75-80.
DOI URL |
| [5] |
ZHANG B, YANG C A, SHI P. Synthesis of graphene/ hydroxyapatite composite bioceramics via plasma Activated sintering, Journal of Inorganic Materials, 2018,33(12):1355-1359.
DOI URL |
| [6] |
WU C T, CHANG J. Silicate bioceramics for bone tissue regeneration. Journal of Inorganic Materials, 2013,28(1):29-39.
DOI URL |
| [7] | DONG S, CHEN Y, YU L, et al. Magnetic hyperthermia- synergistic H2O2 self-sufficient catalytic suppression of osteosarcoma with enhanced bone-regeneration bioactivity by 3D-printing composite scaffolds. Advanced Functional Materials, 2019, 30(4): 1907071-1-15. |
| [8] |
SHI Z Y, LI Q, TANG S C, et al. Surface modification on property of mesoporous calcium magnesium silicate/polyetheretherketone composites. Journal of Inorganic Materials, 2018,33(1):67-74.
DOI URL |
| [9] |
BAI F, WANG Z, LU J, et al. The correlation between the internal structure and vascularization of controllable porous bioceramic aterials in vivo: a quantitative study. Tissue Engineering Part A, 2010,16(12):3791-3803.
DOI URL PMID |
| [10] |
LIN K L, CHANG J, ZENG Y, et al. Preparation of macroporous calcium silicate ceramics. Materials Letters, 2004,58(15):2109-2113.
DOI URL |
| [11] |
BAINO F, FIUME E, BARBERI J, et al. Processing methods for making porous bioactive glass-based scaffolds-a state-of-the-art review. International Journal of Applied Ceramic Technology, 2019,16(5):1762-1796.
DOI URL |
| [12] |
XIN C, QI X, ZHU M, et al. Hydroxyapatite whisker-reinforced composite scaffolds through 3D printing for bone repair. Journal of Inorganic Materials, 2017,32(8):837-844.
DOI URL |
| [13] |
MA H, FENG C, CHANG J, et al. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomaterialia, 2018,79:37-59.
DOI URL PMID |
| [14] |
VON ERLACH T C, BERTAZZO S, WOZNIAK M A, et al. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nature Materials, 2018,17(3):237-242.
DOI URL PMID |
| [15] | ROGOWSKA-TYLMAN J, LOCS J, SALMA I, et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Materials Science & Engineering: C, 2019,99:669-684. |
| [16] | YANG B W, YIN J H, CHEN Y, et al. 2D-black-phosphorus- reinforced 3D-printed scaffolds: a stepwise countermeasure for osteosarcoma. Advanced Materials, 2018, 30(10): 1705611-1-12. |
| [17] | ZHANG Y L, ZHAI D, XU M C, et al. 3D-printed bioceramic scaffolds with antibacterial and osteogenic activity. Biofabrication, 2017, 9(2): 025037-1-12. |
| [18] |
ZHANG X, LI H, LIU J, et al. Amorphous carbon modification on implant surface: a general strategy to enhance osteogenic differentiation for diverse biomaterials via FAK/ERK1/2 signaling pathways. Journal of Materials Chemistry B, 2019,7(15):2518-2533.
DOI URL PMID |
| [19] |
TOURI M, MOZTARZADEH F, OSMAN N A A, et al. 3D- printed biphasic calcium phosphate scaffolds coated with an oxygen generating system for enhancing engineered tissue survival. Materials Science and Engineering: C, 2018,84:236-242.
DOI URL |
| [20] |
WANG C, LIN K, CHANG J, et al. Osteogenesis and angiogenesis induced by porous beta-CaSiO3/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials, 2013,34(1):64-77.
DOI URL |
| [21] |
LIN K, XIA L, GAN J, et al. Tailoring the nanostructured surfaces of hydroxyapatite bioceramics to promote protein adsorption, osteoblast growth, and osteogenic differentiation. ACS Applied Materials & Interfaces, 2013,5(16):8008-8017.
URL PMID |
| [22] |
ZHAO C, XIA L, ZHAI D, et al. Designing ordered micropatterned hydroxyapatite bioceramics to promote the growth and osteogenic differentiation of bone marrow stromal cells. Journal of Materials Chemistry B, 2015,3(6):968-976.
DOI URL PMID |
| [23] |
MAO L, LIU J, ZHAO J, et al. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. International Journal of Nanomedicine, 2015,10:7031-7044.
DOI URL PMID |
| [24] |
XIA L, LIN K, JIANG X, et al. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells. Biomaterials, 2014,35(30):8514-8527.
DOI URL |
| [25] |
WANG X, ZHOU Y, XIA L, et al. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids and Surfaces B-Biointerfaces, 2015,126:358-366.
DOI URL |
| [26] |
XIA L, XIE Y, FANG B, et al. In situ modulation of crystallinity and nano-structures to enhance the stability and osseointegration of hydroxyapatite coatings on Ti-6Al-4V implants. Chemical Engineering Journal, 2018,347:711-720.
DOI URL |
| [27] |
XIA L, ZHANG N, WANG X, et al. The synergetic effect of nano-structures and silicon-substitution on the properties of hydroxyapatite scaffolds for bone regeneration. Journal of Materials Chemistry B, 2016,4(19):3313-3323.
DOI URL PMID |
| [28] |
DENG C J, LIN R C, ZHANG M, et al. Micro/nanometer- structured scaffolds for regeneration of both cartilage and subchondral bone. Advanced Functional Materials, 2019,29(4):1806068-15.
DOI URL |
| [29] |
YANG C, WANG X Y, MA B, et al. 3D-printed bioactive Ca3SiO5 bone cement scaffolds with nano surface structure for bone regeneration. ACS Applied Materials & Interfaces, 2017,9(7):5757-5767.
DOI URL PMID |
| [30] | WANG X C, LI T, MA H S, et al. A 3D-printed scaffold with MoS2 nanosheets for tumor therapy and tissue regeneration. NPG Asia Materials, 2017, 9: e376-1-14. |
| [31] |
XIA L, LIN K, JIANG X, et al. Enhanced osteogenesis through nano-structured surface design of macroporous hydroxyapatite bioceramic scaffolds via activation of ERK and p38 MAPK signaling pathways. Journal of Materials Chemistry B, 2013,1(40):5403-5416.
DOI URL |
| [32] |
DONG S, ZHANG Y-N, WANG J, et al. A novel multifunctional carbon aerogel coated platform for osteosarcoma therapy and enhanced bone regeneration. Journal of Materials Chemistry B, 2020,8(3):368-379.
DOI URL PMID |
| [33] |
WANG C, LIN K, CHANG J, et al. The stimulation of osteogenic differentiation of mesenchymal stem cells and vascular endothelial growth factor secretion of endothelial cells by beta-CaSiO3/beta- Ca3(PO4)(2) scaffolds. Journal of Biomedical Materials Research Part A, 2014,102(7):2096-2104.
DOI URL |
| [34] |
WITTE F, KAESE V, HAFERKAMP H, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials, 2005,26(17):3557-3563.
DOI URL |
| [35] |
YOSHIZAWA S, BROWN A, BARCHOWSKY A, et al. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomaterialia, 2014,10(6):2834-2842.
DOI URL |
| [36] | XIA L, YIN Z, MAO L, et al. Akermanite bioceramics promote osteogenesis,angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Scientific Reports, 2016, 6: 22005- 1-17. |
| [37] |
AMIN N, CLARK C C T, TAGHIZADEH M, et al. Zinc supplements and bone health: the role of the RANKL-RANK axis as a therapeutic target. Journal of Trace Elements in Medicine and Biology, 2019, DOI: 10.1016/j.jtemb.2019.126417.
DOI URL PMID |
| [38] |
QIAO Y Q, ZHANG W J, TIAN P, et al. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials, 2014,35(25):6882-6897.
DOI URL |
| [39] |
LIN K L, XIA L G, LI H Y, et al. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials, 2013,34(38):10028-10042.
DOI URL |
| [40] |
LIU W, WANG T, YANG C, et al. Alkaline biodegradable implants for osteoporotic bone defects-importance of microenvironment pH. Osteoporosis International, 2016,27(1):93-104.
DOI URL PMID |
| [41] |
GUO X, WEI S, LU M, et al. Dose-dependent effects of strontium ranelate on ovariectomy rat bone marrow mesenchymal stem cells and human umbilical vein endothelial cells. International Journal of Biological Sciences, 2016,12(12):1511-1522.
DOI URL PMID |
| [42] | GUO X, WEI S, LU M, et al. RNA-Seq investigation and in vivo study the effect of strontium ranelate on ovariectomized rat via the involvement of ROCK1. Artificial Cells Nanomedicine and Biotechnology, 2018,46:S629-S641. |
| [43] |
LIN K, WANG X, ZHANG N, et al. Strontium (Sr) strengthens the silicon (Si) upon osteoblast proliferation, osteogenic differentiation and angiogenic factor expression. Journal of Materials Chemistry B, 2016,4(21):3632-3638.
DOI URL PMID |
| [44] | WANG C Y, CHEN B, WANG W, et al. Strontium released bi-lineage scaffolds with immunomodulatory properties induce a pro-regenerative environment for osteochondral regeneration. Materials Science & Engineering: C, 2019, 103: 109833-1-12. |
| [45] |
ZHANG X, LI H, LIN C, et al. Synergetic topography and chemistry cues guiding osteogenic differentiation in bone marrow stromal cells through ERK1/2 and p38 MAPK signaling pathway. Biomaterials Science, 2018,6(2):418-430.
DOI URL PMID |
| [46] |
FIELDING G, BOSE S. SiO2 and ZnO dopants in three- dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomaterialia, 2013,9(11):9137-9148.
DOI URL PMID |
| [47] |
WU C, ZHAI D, MA H, et al. Stimulation of osteogenic and angiogenic ability of cells on polymers by pulsed laser deposition of uniform akermanite-glass nanolayer. Acta Biomaterialia, 2014,10(7):3295-3306.
DOI URL |
| [48] |
KONG N, LIN K, LI H, et al. Synergy effects of copper and silicon ions on stimulation of vascularization by copper-doped calcium silicate. Journal of Materials Chemistry B, 2014,2(8):1100-1110.
DOI URL |
| [49] |
SHI M C, CHEN Z T, FARNAGHI S, et al. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomaterialia, 2016,30:334-344.
DOI URL PMID |
| [50] |
LI J Y, ZHAI D, LV F, et al. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomaterialia, 2016,36:254-266.
DOI URL PMID |
| [51] |
LI H, LI J Y, JIANG J, et al. An osteogenesis/angiogenesis- stimulation artificial ligament for anterior cruciate ligament reconstruction. Acta Biomaterialia, 2017,54:399-410.
DOI URL PMID |
| [52] |
ZHOU Y H, HAN S W, XIAO L, et al. Accelerated host angiogenesis and immune responses by ion release from mesoporous bioactive glass. Journal of Materials Chemistry B, 2018,6(20):3274-3284.
DOI URL PMID |
| [53] |
LIN R C, DENG C J, LI X X, et al. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics, 2019,9(21):6300-6313.
DOI URL PMID |
| [54] |
DANG W T, WANG X Y, LI J Y, et al. 3D printing of Mo- containing scaffolds with activated anabolic responses and bi-lineage bioactivities. Theranostics, 2018,8(16):4372-4392.
DOI URL PMID |
| [55] |
LIU Y Q, LI T, MA H S, et al. 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomaterialia, 2018,73:531-546.
DOI URL PMID |
| [56] |
MEININGER S, MANDAL S, KUMAR A, et al. Strength reliability and in vitro degradation of three-dimensional powder printed strontium-substituted magnesium phosphate scaffolds. Acta Biomaterialia, 2016,31:401-411.
DOI URL PMID |
| [57] |
MEININGER S, MOSEKE C, SPATZ K, et al. Effect of strontium substitution on the material properties and osteogenic potential of 3D powder printed magnesium phosphate scaffolds. Materials Science and Engineering: C, 2019,98:1145-1158.
DOI URL |
| [58] |
XIE J, SHAO H, HE D, et al. Ultrahigh strength of three- dimensional printed diluted magnesium doping wollastonite porous scaffolds. MRS Communications, 2015,5(4):631-639.
DOI URL |
| [59] |
KE X, ZHANG L, YANG X, et al. Low-melt bioactive glass- reinforced 3D printing akermanite porous cages with highly improved mechanical properties for lumbar spinal fusion. J Tissue Eng. Regen. Med., 2018,12(5):1149-1162.
DOI URL PMID |
| [60] |
FU S Y, YU B, DING H F, et al, Zirconia incorporation in 3D printed beta-Ca2SiO4 scaffolds on their physicochemical and biological property. Journal of Inorganic Materials, 2019,34(4):444-454.
DOI URL |
| [61] |
ZHANG F, CHANG J, LU J, et al. Bioinspired structure of bioceramics for bone regeneration in load-bearing sites. Acta Biomaterialia, 2007,3(6):896-904.
DOI URL |
| [62] | FENG C, ZHANG W, DENG C, et al. 3D Printing of lotus root-like biomimetic materials for cell delivery and tissue regeneration. Advanced Science, 2017, 4(12): 1700401-1-9. |
| [63] |
ALMELA T, BROOK I M, KHOSHROO K, et al. Simulation of cortico-cancellous bone structure by 3D printing of bilayer calcium phosphate-based scaffolds. Bioprinting, 2017,6:1-7.
DOI URL |
| [64] |
JUNG-BIN L, WOO-YOUL M, YOUNG-HAG K, et al. Porous calcium phosphate ceramic scaffolds with tailored pore orientations and mechanical properties using lithography-based ceramic 3D printing technique. Materials, 11(9):1711-1718.
DOI URL |
| [65] |
MATHARU G S, DANIEL J, ZIAEE H, et al. Failure of a novel ceramic-on-ceramic hip resurfacing prosthesis. Journal of Arthroplasty, 2015,30(3):416-418.
DOI URL PMID |
| [66] |
MANNY P, JAVAD P, SHARKEY P F, et al. Causes of failure of ceramic-on-ceramic and metal-on-metal hip arthroplasties. Clinical Orthopaedics & Related Research, 2012,470(2):382-387.
DOI URL PMID |
| [67] |
ZHAO L, WU C T, LIN K L, et al. The effect of poly(lactic-co- glycolic acid)(PLGA) coating on the mechanical, biodegradable, bioactive properties and drug release of porous calcium silicate scaffolds. Bio-medical Materials and Engineering, 2012,22(5):289-300.
DOI URL |
| [68] |
LI CHEN, AI FANRONG, MIAO XINXIN, et al. “The return of ceramic implants”: rose stem inspired dual layered modification of ceramic scaffolds with improved mechanical and anti-infective properties. Materials Science and Engineering: C, 2018,93:873-879.
DOI URL |
| [69] | KIM B S, YANG S S, PARK H, et al. Improvement of mechanical strength and osteogenic potential of calcium sulfate-based hydroxyapatite 3-dimensional printed scaffolds by epsilon- polycarbonate coating. Journal of Biomaterials Science-Polymer Edition, 2017,28(13):1256-1270. |
| [70] |
DíAZ-RODRíGUEZ P, GONZáLEZ P, SERRA J, et al. Key parameters in blood-surface interactions of 3D bioinspired ceramic materials. Materials Science and Engineering: C, 2014,41:232-239.
DOI URL |
| [71] |
LIENEMANN P S, LUTOLF M P, MARTIN E. Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Advanced Drug Delivery Reviews, 2012,64(12):1078-1089.
DOI URL PMID |
| [72] |
LI M, CHENG Y, ZHENG Y F, et al. Surface characteristics and corrosion behaviour of WE43 magnesium alloy coated by SiC film. Applied Surface Science. 2012,258(7):3074-3081.
DOI URL |
| [73] |
YANG Y, LAI Y, ZHANG Q, et al. A novel electrochemical strategy for improving blood compatibility of titanium-based biomaterials. Colloids & Surfaces B Biointerfaces, 2010,79(1):309-313.
DOI URL PMID |
| [74] |
ZHAO L, LIN K L, ZHANG M L, et al. The influences of poly(lactic-co-glycolic acid) (PLGA) coating on the biodegradability, bioactivity, and biocompatibility of calcium silicate bioceramics. Journal of Materials Science, 2011,46(14):4986-4993.
DOI URL |
| [75] |
WEI T, DANG-SHENG X. Bioinspired superhydrophobic progress and recent advances of its functional application. Journal of Inorganic Materials, 2019,34(11):1133-1144.
DOI URL |
| [76] |
ARIMA Y, IWATA H, Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials, 2007,28(20):3074-3082.
DOI URL |
| [77] |
TENG Y Q, ZHANG Y Q, HENG L P, et al. Conductive polymer porous film with tunable wettability and adhesion. Materials, 2015,8(4):1817-1830.
DOI URL PMID |
| [78] |
CHEN Z, ZHANG D, PENG E, et al. 3D-printed ceramic structures with in situ grown whiskers for effective oil/water separation. Chemical Engineering Journal, 2019,373:1223-1232.
DOI URL |
| [79] |
SONG Y, LIN K, HE S, et al. Nano-biphasic calcium phosphate/polyvinyl alcohol composites with enhanced bioactivity for bone repair via low-temperature three-dimensional printing and loading with platelet-rich fibrin. International Journal of Nanomedicine, 2018,13:505-523.
DOI URL PMID |
| [80] |
TROMBETTA R, INZANA J A, SCHWARZ E M, et al. 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Annals of Biomedical Engineering, 2017,45(1):1-22.
DOI URL PMID |
| [81] |
BOSE S, VAHABZADEH S, BANDYOPADHYAY A. Bone tissue engineering using 3D printing. Materials Today, 2013,16(12):496-504.
DOI URL |
| [82] |
ZHANG Y L, ZHAI D, XU M C, et al. 3D-printed bioceramic scaffolds with a Fe3O4/graphene oxide nanocomposite interface for hyperthermia therapy of bone tumor cells. Journal of Materials Chemistry B, 2016,4(17):2874-2886.
DOI URL PMID |
| [83] |
TORRES P M C, VIEIRA S I, CERQUEIRA A R, et al. Effects of Mn-doping on the structure and biological properties of β-tricalcium phosphate. Journal of Inorganic Biochemistry, 2014,136:57-66.
DOI URL |
| [84] |
MIN Z, SHICHANG Z, CHEN X, et al. 3D-printed dimethyloxallyl glycine delivery scaffolds to improve angiogenesis and osteogenesis. Biomater. Sci., 2015,3(8):1236-1244.
DOI URL PMID |
| [85] | DIAZ-RODRIGUEZ P, SANCHEZ M, LANDIN M. Drug-loaded biomimetic ceramics for tissue engineering. Pharmaceutics, 2018, 10(4): 272-1-20. |
| [86] |
MEIßNER R, BERTOL L, REHMAN M A U, et al. Bioprinted 3D calcium phosphate scaffolds with gentamicin releasing capability. Ceramics International, 2019,45(6):7090-7094.
DOI URL |
| [87] |
LI T, ZHAI D, MA B, et al. 3D printing of hot dog-like biomaterials with hierarchical architecture and distinct bioactivity. Advanced Science, 2019,6(19):1901146-8.
DOI URL PMID |
| [88] |
SONG J-J, CHEN B, LIN K-L. Core-shell structured hydroxyapatite/mesoporous silica nanoparticle: preparation and application in drug delivery. Journal of Inorganic Materials, 2018,33(6):623-628.
DOI URL |
| [89] |
CHEN G, ROY I, YANG C, et al. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chemical Reviews, 2016,116(5):2826-2885.
DOI URL PMID |
| [90] | ZHEN F, YAN C, CUI C, et al. Near infrared fluorescent peptide nanoparticles for enhancing esophageal cancer therapeutic efficacy. Nature Communications, 2018, 9(1): 2605-1-11. |
| [91] |
WANG S, CHEN Y, LI X, et al. Injectable 2D MoS2-integrated drug delivering implant for highly efficient NIR-triggered synergistic tumor hyperthermia. Advanced Materials, 2015,27(44):7117-7122.
DOI URL PMID |
| [92] | CHANDRAWATI D R, CHANG D J Y H, REINATORRES D E, et al. Localized and controlled delivery of nitric oxide to the conventional outflow pathway via enzyme biocatalysis: toward therapy for glaucoma. Advanced Materials, 2017, 29(16): 1604932-1-7. |
| [93] |
BADGWELL B, BLUM M, ESTRELLA J, et al. Personalised therapy for localised gastric and gastro-oesophageal adenocarcinoma. Lancet Oncology, 2016,17(12):1628-1629.
DOI URL PMID |
| [94] |
YANG B W, GU Z, CHEN Y. Nanomedicine-augmented cancer-localized treatment by 3D theranostic implants. Journal of Biomedical Nanotechnology, 2017,13(8):871-890.
DOI URL |
| [95] |
MA H, JIANG C, ZHAI D, et al. A bifunctional biomaterial with photothermal effect for tumor therapy and bone regeneration. Advanced Functional Materials, 2016,26(8):1197-1208.
DOI URL |
| [96] |
WU P, GRAINGER D W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials, 2006,27(11):2450-2467.
DOI URL |
| [97] |
ARIJIT KUMAR C, RUCHIRA C, TARAKDAS B. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology, 2014, 25(13): 135101-1-13.
DOI URL PMID |
| [98] |
SCHRAND A M, RAHMAN M F, HUSSAIN S M, et al. Metal-based nanoparticles and their toxicity assessment. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 2010,2(5):544-568.
DOI URL PMID |
| [99] |
LIAO F, MA J Q, GE H G. Preparation, characterization and antimicrobial activity of core-satellite Ag/PDA@SiO2@CoFe2O4 magnetic composites. Journal of Inorganic Materials, 2017,32(5):523-528.
DOI URL |
| [100] |
LIU Y L, WANG R L, LI N, et al. Preparation of zinc oxide mesocrystal filler and the properties of dental composite resins. Journal of Inorganic Materials, 2019,34(10):1077-1084.
DOI URL |
| [101] |
WANG Q, TANG P F, GE X, et al. Experimental and simulation studies of strontium/zinc-codoped hydroxyapatite porous scaffolds with excellent osteoinductivity and antibacterial activity. Applied Surface Science, 2018,462:118-126.
DOI URL |
| [102] |
VALAPPIL S P, COOMBES M, WRIGHT L, et al. Role of gallium and silver from phosphate-based glasses on in vitro dual species oral biofilm models of porphyromonas gingivalis and Streptococcus gordonii. Acta Biomaterialia, 2012,8(5):1957-1965.
DOI URL |
| [103] |
GOH Y F, ALSHEMARY A Z, AKRAM M, et al. In-vitro characterization of antibacterial bioactive glass containing ceria. Ceramics International, 2014,40(1):729-737.
DOI URL |
| [104] |
CHATZISTAVROU X, FENNO J C, FAULK D, et al. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomaterialia, 2014,10(8):3723-3732.
DOI URL |
| [105] | BRAUER D S, KARPUKHINA N, KEDIA G, et al. Bactericidal strontium-releasing injectable bone cements based on bioactive glasses. Journal of the Royal Society Interface, 2013, 10(78): 20120647-1-8. |
| [106] |
WU Y, XIA L, ZHOU Y, et al. Evaluation of osteogenesis and angiogenesis of icariin loaded on micro/nano hybrid structured hydroxyapatite granules as a local drug delivery system for femoral defect repair. Journal of Materials Chemistry B, 2015,3(24):4871-4883.
DOI URL PMID |
| [107] |
ZHOU Y, WU Y, MA W, et al. The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. Journal of Materials Chemistry B, 2017,5(3):612-625.
DOI URL PMID |
| [108] | ZHANG F M, CHANG J, LIN K L, et al. Preparation, mechanical properties and in vitro degradability of wollastonite/tricalcium phosphate macroporous scaffolds from nanocomposite powders. Journal of Materials Science-Materials in Medicine, 2008,19(1):167-173. |
| [109] |
ZHANG F, LIN K, CHANG J, et al. Spark plasma sintering of macroporous calcium phosphate scaffolds from nanocrystalline powders. Journal of The European Ceramic Society, 2008,28(3):539-545.
DOI URL |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 孙晶, 李翔, 毛小建, 章健, 王士维. 月桂酸改性剂对氮化铝粉体抗水解性能的影响[J]. 无机材料学报, 2025, 40(7): 826-832. |
| [3] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [4] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [5] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [6] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [7] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [8] | 陈曦, 袁媛, 谭业强, 刘昌胜. 无机非金属生物材料发展战略研究[J]. 无机材料学报, 2025, 40(5): 449-456. |
| [9] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [10] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [11] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [12] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [13] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [14] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [15] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||