无机材料学报 ›› 2018, Vol. 33 ›› Issue (7): 779-786.DOI: 10.15541/jim20170379 CSTR: 32189.14.10.15541/jim20170379
所属专题: 电催化研究
郭瑞华1,2,3, 莫逸杰2,3, 安胜利2,3, 张捷宇1, 周国治1
收稿日期:2017-08-07
修回日期:2017-11-17
出版日期:2018-07-10
网络出版日期:2018-06-19
基金资助:GUO Rui-hua1,2,3, MO Yi-Jie2,3, AN Sheng-Li2,3, ZHANG Jie-Yu1, ZHOU Guo-Zhi1
Received:2017-08-07
Revised:2017-11-17
Published:2018-07-10
Online:2018-06-19
摘要:
采用水热合成法, 以碳球为模板, 改变焙烧升温速率, 控制影响铈物种的扩散、渗透及碳球结构的收缩率, 制备了单、双壳层CeO2空心球。通过微波辅助乙二醇还原氯铂酸法制备了Pt-CeO2/RGO催化剂, 研究了CeO2空心球的添加对Pt基催化剂电催化性能的影响。利用X射线衍射仪(XRD)、比表面积及孔径分析仪(BET)、扫描电镜(SEM)和电子能谱(EDAX)、透射电镜(TEM)、X射线光电子能谱(XPS)对CeO2及催化剂的微观结构进行了表征, 利用电化学工作站对催化剂进行电化学性能测试。结果表明: 单、双壳层CeO2空心球的比表面积为124.44 m2/g、140.95 m2/g, 孔容为0.014427 cm3/(g·nm)、0.018605 cm3/(g·nm), 孔径分布在2~4 nm范围内。催化剂中的CeO2保持原有的球状形貌, Pt纳米粒子主要分布在CeO2附近; 当RGO∶CeO2=1∶2时, 添加了双壳层CeO2空心球的Pt-CeO2/RGO催化剂的电催化性能最优, 电化学活性表面积为94.27 m2/g, 对乙醇氧化的峰电流密度值为613.54 A/g, 1000 s的稳态电流密度值为135.45 A/g。
中图分类号:
郭瑞华, 莫逸杰, 安胜利, 张捷宇, 周国治. 氧化铈空心球可控合成及其对Pt基催化剂电催化性能的影响[J]. 无机材料学报, 2018, 33(7): 779-786.
GUO Rui-hua, MO Yi-Jie, AN Sheng-Li, ZHANG Jie-Yu, ZHOU Guo-Zhi. Cerium Oxide Hollow Sphere: Controllable Synthesis and Its Effect on Electrocatalytic Performance of Pt-based Catalysts[J]. Journal of Inorganic Materials, 2018, 33(7): 779-786.
| Number | Sample | CeO2 additive situation | Weight ratio of RGO to CeO2 |
|---|---|---|---|
| 1# | Pt-CeO2/ RGO | Add single shell CeO2 hollow sphere | RGO:CeO2=1:1 |
| 2# | Pt-CeO2/ RGO | Add single shell CeO2 hollow sphere | RGO:CeO2=1:2 |
| 3# | Pt-CeO2/ RGO | Add single shell CeO2 hollow sphere | RGO:CeO2=1:3 |
| 4# | Pt-CeO2/ RGO | Add double shell CeO2 hollow sphere | RGO:CeO2=1:1 |
| 5# | Pt-CeO2/ RGO | Add double shell CeO2 hollow sphere | RGO:CeO2=1:2 |
| 6# | Pt-CeO2/ RGO | Add double shell CeO2 hollow sphere | RGO:CeO2=1:3 |
| 7# | Pt/ RGO | Not add CeO2 | - |
表1 不同条件的七组催化剂
Table 1 Different conditions of seven groups of catalysts
| Number | Sample | CeO2 additive situation | Weight ratio of RGO to CeO2 |
|---|---|---|---|
| 1# | Pt-CeO2/ RGO | Add single shell CeO2 hollow sphere | RGO:CeO2=1:1 |
| 2# | Pt-CeO2/ RGO | Add single shell CeO2 hollow sphere | RGO:CeO2=1:2 |
| 3# | Pt-CeO2/ RGO | Add single shell CeO2 hollow sphere | RGO:CeO2=1:3 |
| 4# | Pt-CeO2/ RGO | Add double shell CeO2 hollow sphere | RGO:CeO2=1:1 |
| 5# | Pt-CeO2/ RGO | Add double shell CeO2 hollow sphere | RGO:CeO2=1:2 |
| 6# | Pt-CeO2/ RGO | Add double shell CeO2 hollow sphere | RGO:CeO2=1:3 |
| 7# | Pt/ RGO | Not add CeO2 | - |
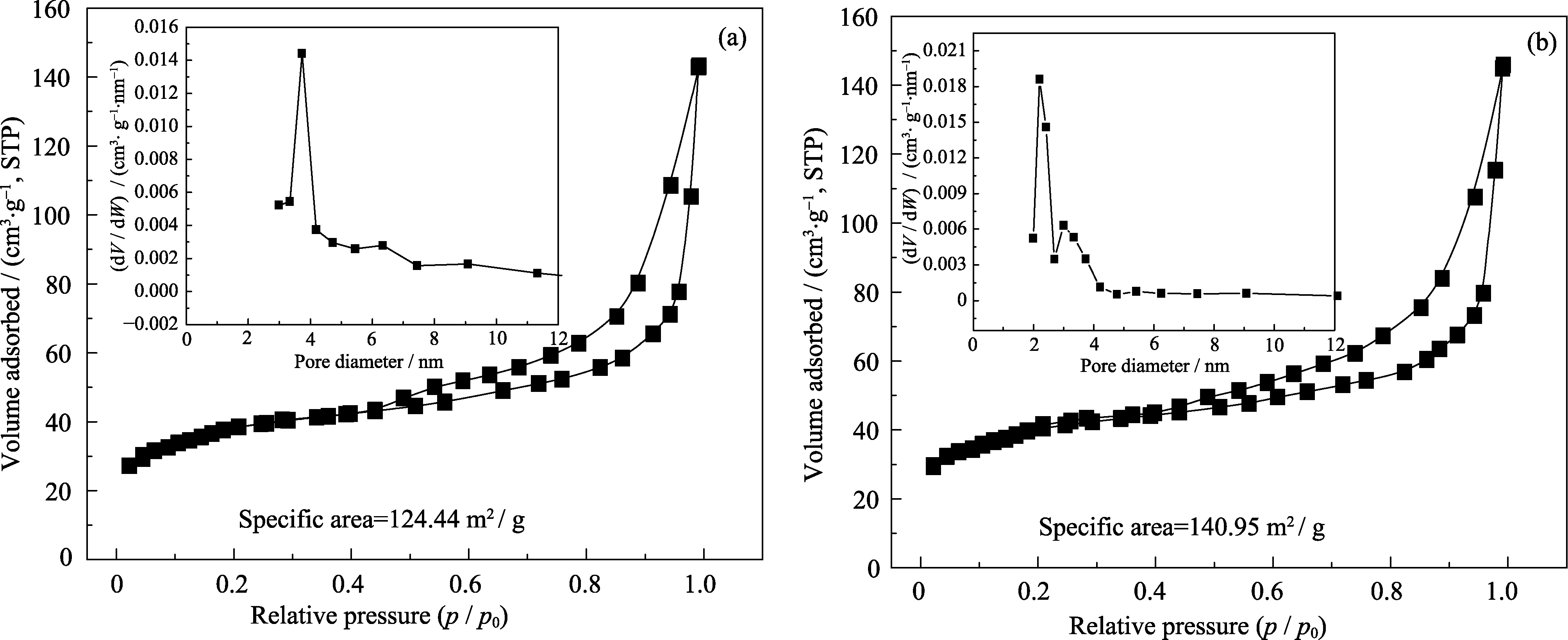
图4 两种CeO2的氮气吸脱附曲线与BJH孔径分布曲线
Fig. 4 Nitrogen sorption isotherms and BJH pore size distribution curves of two kinds of CeO2(a) Single shell CeO2; (b) Double shell CeO2
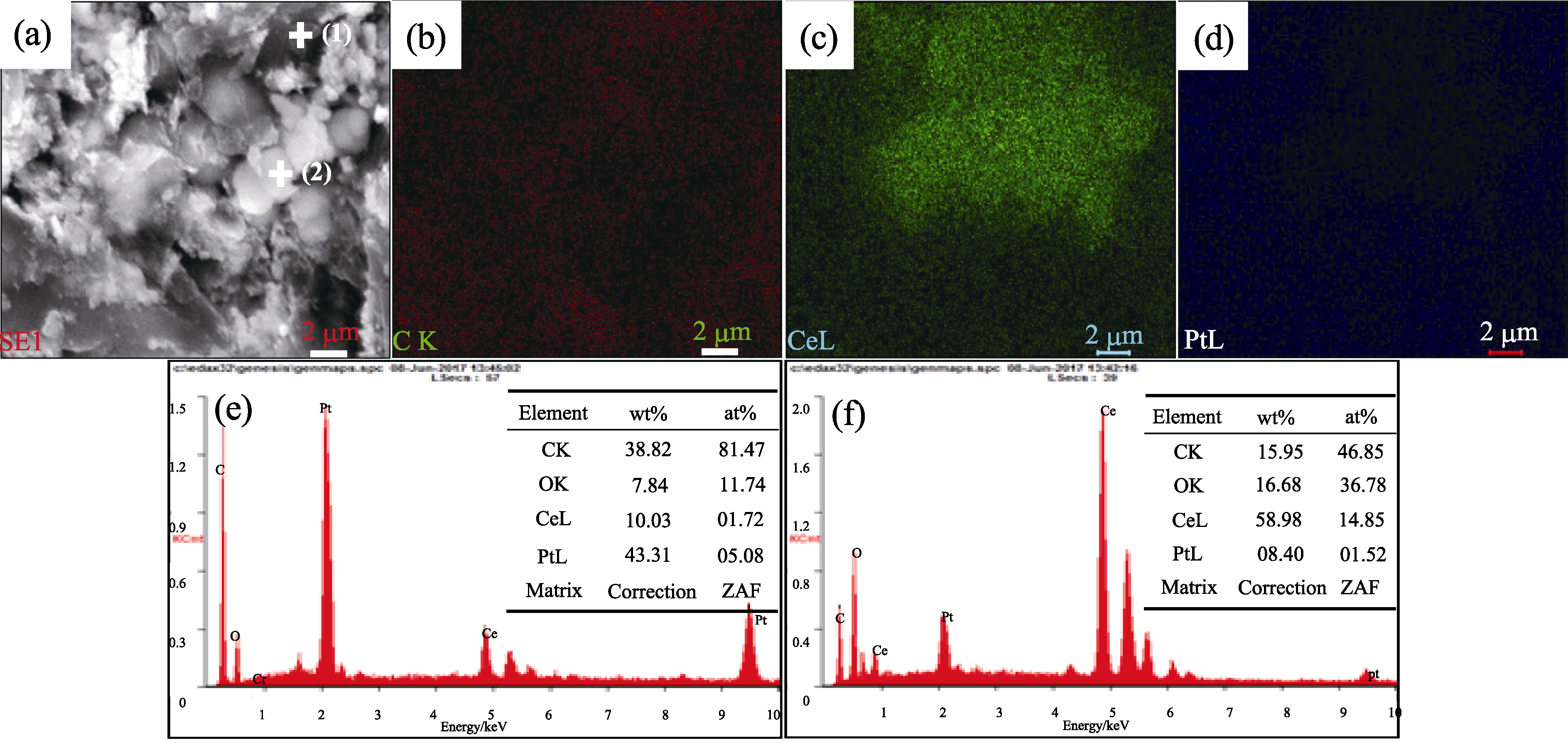
图5 2#催化剂的SEM照片(a)和面扫分析结果(b)~(d)及图(a)中点(1)/(2)的EDS分析结果(e), (f)
Fig. 5 (a) SEM image , (b-d) plane scan analysis and (e, f) EDS analysis of dot(1) and (2) from (a) for 2# catalyst
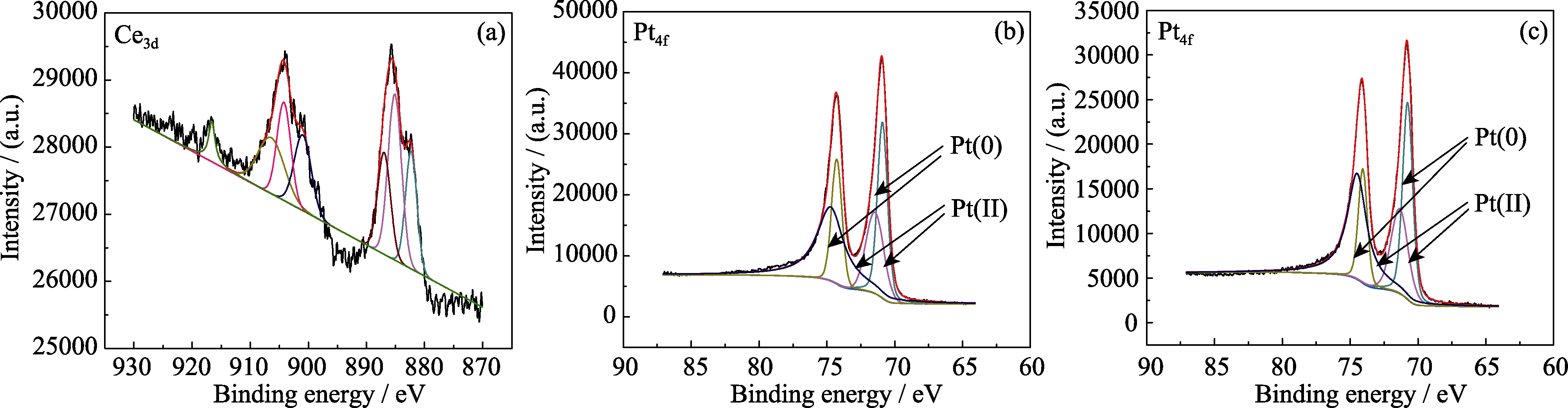
图6 催化剂的XPS图谱
Fig. 6 XPS spectra of catalysts(a) Ce3d XPS spectra of 2# catalyst; (b) Pt4f XPS spectra of 2# catalyst; (c) Pt4f XPS spectra of 5# catalyst
| Sample | Pt (0)/eV | Relative ratio/% | Pt (II)/eV | Relative ratio/% |
|---|---|---|---|---|
| 2# | 70.76, 74.07 | 74.73 | 71.46, 74.35 | 25.26 |
| 5# | 70.90, 74.27 | 81.93 | 71.46, 74.27 | 18.06 |
表2 催化剂的XPS图谱分析数据
Table 2 XPS data and the possible chemical states of catalysts
| Sample | Pt (0)/eV | Relative ratio/% | Pt (II)/eV | Relative ratio/% |
|---|---|---|---|---|
| 2# | 70.76, 74.07 | 74.73 | 71.46, 74.35 | 25.26 |
| 5# | 70.90, 74.27 | 81.93 | 71.46, 74.27 | 18.06 |
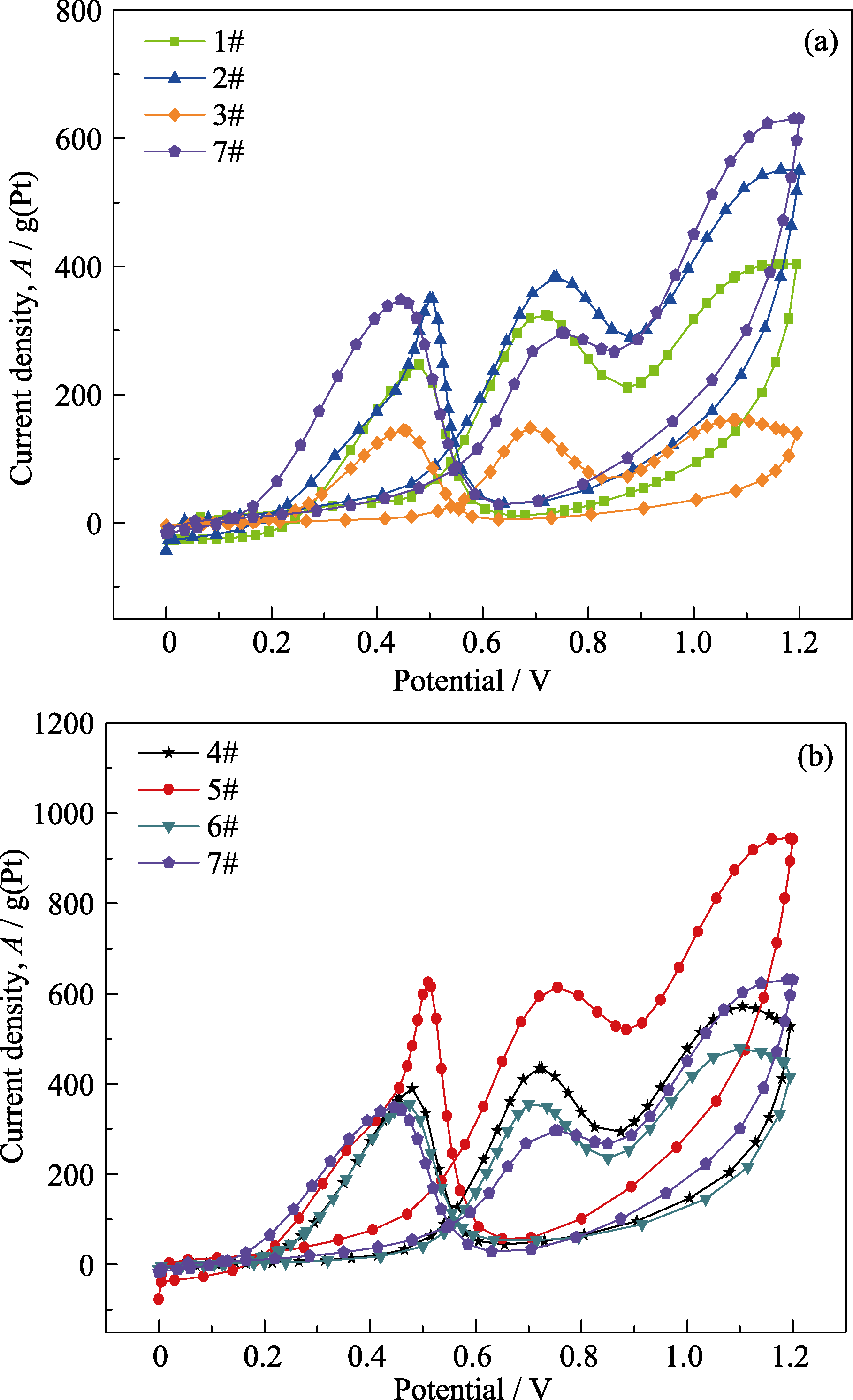
图9 催化剂在0.5 mol/L H2SO4+1 mol/L C2H5OH中的循环伏安曲线
Fig. 9 Cyclic voltammetry curves of catalysts in 0.5 mol/L H2SO4+1 mol/L C2H5OH(a) Add single shell CeO2; (b) Add double shell CeO2
| [1] | JEON M K, WON J Y, LEE K R,et al.Highly active PtRuFe/C catalyst for methanol electro-oxidation.Electrochemistry Communications, 2007, 9(9): 2163-2166. |
| [2] | RAHSEPAR M, PAKSHIR M, PIAO Y Z,et al.Synthesis and electrocatalytic performance of high loading active PtRu multiwalled carbon nanotube catalyst for methanol oxidation.Electrochimica Acta, 2012, 71(1): 246-251. |
| [3] | KANG S, LIM S, PECK D H,et al. Stability and durability of PtRu catalysts supported on carbon nanofibers for direct methanol fuel cells.International Journal of Hydrogen Energy, 2012, 37(5): 4685-4693. |
| [4] | FENG L G, ZHANG J, CAI W W,et al. Single passive direct methanol fuel cell supplied with pure methanol.Journal of Power Sources, 2011, 196(5): 2750-2753. |
| [5] | WANG ZHAO-MING, WEI XING, PENG WEI,et al. On-line electrochemical transmission infrared spectroscopic study of Pb2+ enhanced C-C bond breaking in the ethanol oxidation reaction.Acta Phys. -Chim. Sin, 2016, 32(6): 1467-1472. |
| [6] | JENNINGS P C, POLLET B G, JOHNSTON R L.Theoretical studies of Pt-Ti nanoparticles for potential use as PEMFC electrocatalysts.Physical Chemistry Chemical Physics, 2012, 14(9): 3134-3139. |
| [7] | COCHELL T, MANTHIRAM A.Pt@PdxCuy/C core-shell electrocatalysts for oxygen reduction reaction in fuel cells.Langmuir, 2012, 28(2): 1579-1587. |
| [8] | STASSI A, GATTO I, MONFORTE G,et al. The effect of thermal treatment on structure and surface composition of PtCo electro- catalysts for application in PEMFCs operating under automotive conditions.Journal of Power Sources, 2012, 208(15): 35-45. |
| [9] | BLIZNAKOV S T, VUKMIROVIC M B, YANG L,et al. Pt monolayer on electrodeposited Pd nanostructures: advanced cathode catalysts for PEM fuel cells.Journal of the Electrochemical Society, 2012, 159(9): F501-F506. |
| [10] | FENG L G, ZHAO X, YANG J,et al. Electrocatalytic activity of Pt/C catalysts for methanol electrooxidation promoted by molybdovanadophosphoric acid.Catalysis Communications, 2011, 14(1): 10-14. |
| [11] | LI MIN, LUO YUAN, XU WEI-JIA,et al. DMFC anode catalyst Fe3O4@Pt particles: synthesis and catalytic performance.Journal of Inorganic Materials, 2017, 9(32): 917-921. |
| [12] | ZHOU C M, WANG H J, PENG F,et al. MnO2/CNT supported Pt and PtRu nanocatalysts for direct methanol fuel cells.Langmuir, 2009, 25(13): 7711-7717. |
| [13] | TIMPERMAN L, FENG Y J, VOGEL W,et al. Substrate effect on oxygen reduction electrocatalysis.Electrochimica Acta, 2010, 26(1): 7558-7563. |
| [14] | DHAVALE V M, KURUNGOT S.Tuning the performance of low-Pt polymer electrolyte membrane fuel cell electrodes derived from Fe2O3@Pt/C core-shell catalyst prepared by anin situ anchoring strategy.The Journal of Physical Chemistry C, 2012, 116(13): 7318-7326. |
| [15] | FENG L G, YAN L, CUI Z M,et al. High activity of Pd-WO3/C catalyst as anodic catalyst for direct formic acid fuel cell.Journal of Power Sources, 2011, 196(5): 2469-2474. |
| [16] | TSIOUVARAS N, MARTINEZ-HUERTA M V, MOLINER R,et al. CO tolerant PtRu-MoOx nanoparticles supported on carbon nanofibers for direct methanol fuel cells.Journal of Power Sources, 2009, 186(2): 299-304. |
| [17] | ELEZOVIĆ N R, BABIĆ B M, RADMILOVIĆ V R,et al. Pt/C doped by MoOx as the electrocatalyst for oxygen reduction and methanol oxidation.Journal of Power Sources, 2008, 175(1): 250-255. |
| [18] | BAI Y X, WU J J, XI J Y,et al. Electrochemical oxidation of ethanol on Pt-ZrO2/C catalyst.Electrochemistry Communications, 2005, 7(11): 1087-1090. |
| [19] | FENG L G, YANG J, HU Y,et al. Electrocatalytic properties of PdCeOx/C anodic catalyst for formic acid electrooxidation.International Journal of Hydrogen Energy, 2012, 37(6): 4812-4818. |
| [20] | ZHOU J H, HE J P, WANG T,et al. Synergistic effect of Re2O3 (Re=Sm, Eu and Gd) on Pt/mesoporous carbon catalyst for methanol electro-oxidation.Electrochimica Acta, 2009, 54(11): 3103-3108. |
| [21] | SKORODUMOVA N V, BAUDIN M, HERMANSSON K.Surface properties of CeO2 from first principles.Physical Review B, 2004, 69(7): 1-8. |
| [22] | SCIBIOH M A, KIM S K, CHO E A,et al. Pt-CeO2/C anode catalyst for direct methanol fuel cells.Applied Catalysis B: Environmental, 2008, 84(3/4): 773-782. |
| [23] | ZHOU Y, GAO Y F, LIU Y C,et al. High efficiency Pt-CeO2/ carbon nanotubes hybrid composite as an anode electrocatalyst for direct methanol fuel cells.Journal of Power Sources, 2010, 195(6): 1605-1609. |
| [24] | MASUDA T, FUKUMITSU H, FUGANE K,et al. Role of cerium oxide in the enhancement of activity for the oxygen reduction reaction at Pt-CeOx nanocomposite electrocatalyst - an in situ electrochemical X-ray absorption fine structure study.The Journal of Physical Chemistry C, 2012, 116(18): 10098-10102. |
| [25] | LIN R, CAO C H, ZHANG H Y,et al. Electro-catalytic activity of enhanced CO tolerant cerium-promoted Pt/C catalyst for PEM fuel cell anode.International Journal of Hydrogen Energy, 2012, 37(5): 4648-4656. |
| [26] | LIU C W, WEI Y C, WANG K W.Surface condition manipulation and oxygen reduction enhancement of PtAu/C catalysts synergistically modified by CeO2 addition and N2 treatment.The Journal of Physical Chemistry C, 2011, 115(17): 8702-8708. |
| [27] | LI J Y, XIONG S L, PAN J.Hydrothermal synthesis and electrochemical properties of urchin-like core-shell copper oxide nanostructures.The Journal of Physical Chemistry C, 2010, 114(21): 9645-9650. |
| [28] | DENG D, LEE J Y.Hollow core-shell mesospheres of crystalline SnO2 nanoparticle aggregates for high capacity Li+ ion storage.Chemistry of Material, 2008, 20(5): 1841-1846. |
| [29] | XU P F, YU R B, REN H,et al. Hierarchical nanoscale multi-shell Au/CeO2 hollow spheres.Chem Sci, 2014, 5(11): 4221-4226. |
| [30] | DEIVARAJ T C, CHEN W X, LEE J Y.Preparation of PtNi nanoparticles for the electrocatalytic oxidation of methanol.Journal of Materials Chemistry, 2003, 13(10): 2555-2560. |
| [31] | HUANG M H, JIANG Y Y, JIN C H,et al. Pt-Cu alloy with high density of surface Pt defects for efficient catalysis of breaking C-C bond in ethanol.Electrochimica Acta, 2014, 125(10): 29-37. |
| [32] | ZHOU Y, GAO Y F, LIU Y C,et al. High efficiency Pt-CeO2/ carbon nanotubes hybrid composite as an anode electrocatalyst for direct methanol fuel cells.Journal of Power Sources, 2010, 195(6): 1605-1609. |
| [33] | YU S P, LIU Q B, YANG W S,et al. Graphene-CeO2 hybrid support for Pt nanoparticles as potential electrocatalyst for direct methanol fuel cells.Electrochimica Acta, 2013, 94(1): 245-251. |
| [1] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [2] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [3] | 信震宇, 郭瑞华, 乌仁托亚, 王艳, 安胜利, 张国芳, 关丽丽. Pt-Fe/GO纳米催化剂的制备及其电催化乙醇氧化性能研究[J]. 无机材料学报, 2025, 40(4): 379-387. |
| [4] | 瞿牡静, 张淑兰, 朱梦梦, 丁浩杰, 段嘉欣, 代恒龙, 周国红, 李会利. CsPbBr3@MIL-53纳米复合荧光粉的合成、性能及其白光LEDs应用[J]. 无机材料学报, 2024, 39(9): 1035-1043. |
| [5] | 潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919. |
| [6] | 苗鑫, 闫世强, 韦金豆, 吴超, 樊文浩, 陈少平. Te基热电器件反常界面层生长行为及界面稳定性研究[J]. 无机材料学报, 2024, 39(8): 903-910. |
| [7] | 陈甜, 罗媛, 朱刘, 郭学益, 杨英. 有机-无机共添加增强柔性钙钛矿太阳能电池机械弯曲及环境稳定性能[J]. 无机材料学报, 2024, 39(5): 477-484. |
| [8] | 杨博, 吕功煊, 马建泰. 镍铁氢氧化物-磷化钴复合电极电催化分解水研究[J]. 无机材料学报, 2024, 39(4): 374-382. |
| [9] | 张宇晨, 陆知遥, 赫晓东, 宋广平, 朱春城, 郑永挺, 柏跃磊. 硫族MAX相硼化物的物相稳定性和性能预测[J]. 无机材料学报, 2024, 39(2): 225-232. |
| [10] | 王煜, 熊浩, 黄孝坤, 江琳沁, 吴波, 黎健生, 杨爱军. 低剂量异辛酸亚锡调控两步法制备Sn-Pb混合钙钛矿太阳能电池[J]. 无机材料学报, 2024, 39(12): 1339-1347. |
| [11] | 周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158. |
| [12] | 方万丽, 沈黎丽, 李海艳, 陈薪羽, 陈宗琦, 寿春晖, 赵斌, 杨松旺. NiOx介孔层的成膜过程对碳电极钙钛矿太阳能电池性能的影响[J]. 无机材料学报, 2023, 38(9): 1103-1109. |
| [13] | 陈雨, 林埔安, 蔡冰, 张文华. 钙钛矿太阳能电池无机空穴传输材料的研究进展[J]. 无机材料学报, 2023, 38(9): 991-1004. |
| [14] | 胡忠良, 傅赟天, 蒋蒙, 王连军, 江莞. Nb/Mg3SbBi界面层热稳定性研究[J]. 无机材料学报, 2023, 38(8): 931-937. |
| [15] | 刘建, 王凌坤, 许保亮, 赵倩, 王耀萱, 丁艺, 张胜泰, 段涛. 熔盐法低温合成掺钕ZrSiO4陶瓷的物相演变和化学稳定性[J]. 无机材料学报, 2023, 38(8): 910-916. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||