无机材料学报 ›› 2025, Vol. 40 ›› Issue (7): 735-746.DOI: 10.15541/jim20240333 CSTR: 32189.14.10.15541/jim20240333
• 综述 • 下一篇
朱文杰1,2,3( ), 唐璐1,2,3, 陆继长1,2,3, 刘江平1,2,3, 罗永明2,3,4
), 唐璐1,2,3, 陆继长1,2,3, 刘江平1,2,3, 罗永明2,3,4
收稿日期:2024-07-16
修回日期:2024-09-13
出版日期:2025-07-20
网络出版日期:2024-09-27
作者简介:朱文杰(1979-), 男, 教授. E-mail: zhuwenjie17@163.com
基金资助:
ZHU Wenjie1,2,3( ), TANG Lu1,2,3, LU Jichang1,2,3, LIU Jiangping1,2,3, LUO Yongming2,3,4
), TANG Lu1,2,3, LU Jichang1,2,3, LIU Jiangping1,2,3, LUO Yongming2,3,4
Received:2024-07-16
Revised:2024-09-13
Published:2025-07-20
Online:2024-09-27
About author:ZHU Wenjie (1979-), male, professor. E-mail: zhuwenjie17@163.com
Supported by:摘要:
控制与去除挥发性有机化合物(VOCs)一直是环境领域的热点问题, 催化氧化法因其低温、高效以及副产物无毒害等特点成为去除VOCs最有前景的技术之一。钙钛矿型氧化物(ABO3)是催化氧化VOCs的高效稳定催化剂。为了提高钙钛矿型催化剂的催化效率, 有必要针对性地分析钙钛矿型氧化物的设计, 以去除不同类型的VOCs。本文系统总结了近年来钙钛矿型氧化物催化氧化VOCs的研究进展。首先介绍了钙钛矿型氧化物在VOCs催化氧化中不同的设计策略, 包括形貌调控、A位和B位取代、缺陷工程和负载型钙钛矿催化剂, 阐明了钙钛矿型氧化物的催化性能与其材料组成、形貌、表面性质(氧物种、缺陷)和本身性质(氧空位浓度、晶格结构)之间的关系; 然后介绍了VOCs催化氧化的反应机制和降解途径, 并展望了钙钛矿型氧化物催化剂设计和反应机制研究的前景和挑战。
中图分类号:
朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746.
ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides[J]. Journal of Inorganic Materials, 2025, 40(7): 735-746.

图3 在100~600 ℃范围内LaFe0.8Cu0.2O3的抗水抗硫性能[25]
Fig. 3 Water and sulfur resistance over LaFe0.8Cu0.2O3 in the temperature range of 100-600 ℃[25] (a) C3H3N conversion; (b) CO2 yield; (c) CO yield; (d) N2 yield; (e) NH3 yield; (f) N2O yield; (g) NOx yield
| Catalyst | VOCs | ρVOCs/ (mg·L-1) | aGHSV/ (mL·g-1·h-1) | Preparation method | bT50/℃ | cT90/℃ | dR/(mol·g-1·s-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| La0.95Ce0.05CoO3 | Chlorobenzene | 4605 | 60000 | Reactive grinding method | 377 | 434 | 1.083×10-3 (200 ℃) | [ |
| Sol-Gel method | 313 | 425 | 6.775×10-9 (200 ℃) | |||||
| La0.9Ce0.1MnO3 | Styrene | 12780 | 25000 | Sol-Gel method | 306 | 328 | - | [ |
| LaFe0.8Cu0.2O3 | Acrylonitrile | 6512 | 120000 | Sol-Gel method | - | 250 | - | [ |
| LaFe0.8Co0.2O3 | Propylene | 1722 | 60000 | Sol-Gel method | 293 | 345 | - | [ |
| LaMn0.8Cu0.2O3 | Formaldehyde | 368 | 12000 | Sol-Gel method | 138 | 168 | - | [ |
| LaMn0.8Ni0.2O3 | 143 | 200 | ||||||
| LaMn0.8Zn0.2O3 | 146 | 200 | ||||||
| LaMn0.7Mg0.3O3 | Methane | 328 | 50000 | Sol-Gel method | 450 | 495 | 6.90×10-7 (400 ℃) | [ |
| La0.5Sr0.5Co0.8Fe0.2O3 | Toluene | 3770 | 30000 | Sol-Gel method | 251 | 270 | 8.43×10-8 (230 ℃) | [ |
| La0.8Ce0.2Mn0.8Ni0.2O3 | Trichloroethylene | 806 | 10000 | Sol-Gel method | 310 | 379 | - | [ |
| La0.8Ce0.2Mn0.8Ni0.2O3 | Toluene | 3770 | 18000 | Sol-Gel method | 260 | 295 | - | [ |
| La0.9Sr0.1Co0.9Mn0.1O3 | Benzene | 6390 | 30000 | PMMA template method | 272 | 328 | - | [ |
表1 A、B位掺杂改性的钙钛矿型催化剂及其在催化氧化VOCs中的应用
Table 1 A- and B-sites doped perovskite catalysts and their application in catalytic oxidation of VOCs
| Catalyst | VOCs | ρVOCs/ (mg·L-1) | aGHSV/ (mL·g-1·h-1) | Preparation method | bT50/℃ | cT90/℃ | dR/(mol·g-1·s-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| La0.95Ce0.05CoO3 | Chlorobenzene | 4605 | 60000 | Reactive grinding method | 377 | 434 | 1.083×10-3 (200 ℃) | [ |
| Sol-Gel method | 313 | 425 | 6.775×10-9 (200 ℃) | |||||
| La0.9Ce0.1MnO3 | Styrene | 12780 | 25000 | Sol-Gel method | 306 | 328 | - | [ |
| LaFe0.8Cu0.2O3 | Acrylonitrile | 6512 | 120000 | Sol-Gel method | - | 250 | - | [ |
| LaFe0.8Co0.2O3 | Propylene | 1722 | 60000 | Sol-Gel method | 293 | 345 | - | [ |
| LaMn0.8Cu0.2O3 | Formaldehyde | 368 | 12000 | Sol-Gel method | 138 | 168 | - | [ |
| LaMn0.8Ni0.2O3 | 143 | 200 | ||||||
| LaMn0.8Zn0.2O3 | 146 | 200 | ||||||
| LaMn0.7Mg0.3O3 | Methane | 328 | 50000 | Sol-Gel method | 450 | 495 | 6.90×10-7 (400 ℃) | [ |
| La0.5Sr0.5Co0.8Fe0.2O3 | Toluene | 3770 | 30000 | Sol-Gel method | 251 | 270 | 8.43×10-8 (230 ℃) | [ |
| La0.8Ce0.2Mn0.8Ni0.2O3 | Trichloroethylene | 806 | 10000 | Sol-Gel method | 310 | 379 | - | [ |
| La0.8Ce0.2Mn0.8Ni0.2O3 | Toluene | 3770 | 18000 | Sol-Gel method | 260 | 295 | - | [ |
| La0.9Sr0.1Co0.9Mn0.1O3 | Benzene | 6390 | 30000 | PMMA template method | 272 | 328 | - | [ |
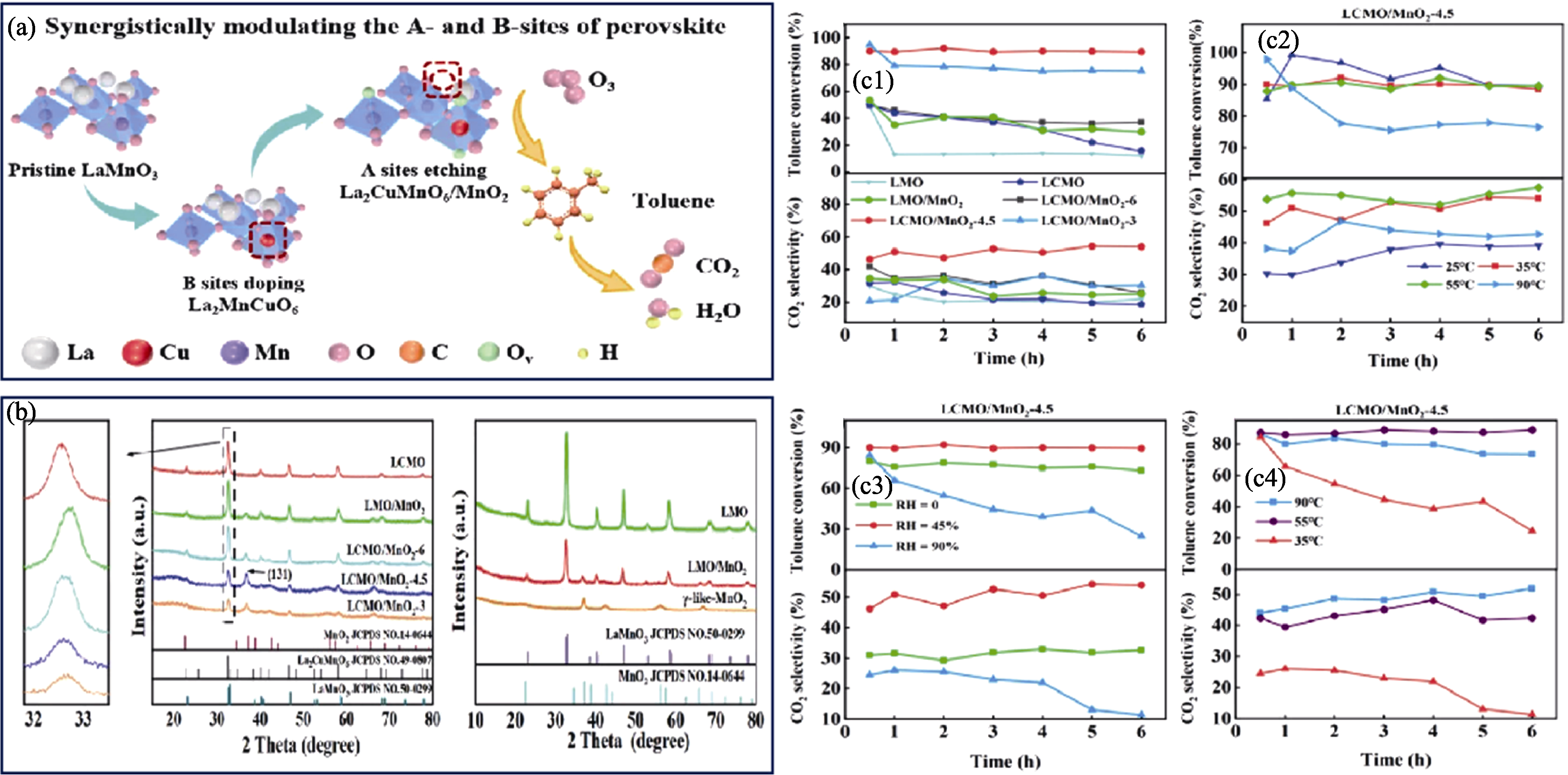
图4 选择性溶解技术配合Cu掺杂改性LaMnO3催化剂[37]
Fig. 4 Selective dissolution technique combined with Cu-doping modified LaMnO3 catalyst[37] (a) Synergistically modulating the A- and B-sites of perovskite; (b) XRD patterns of the synthesized catalysts; (c1-c4) Catalytic activities of the synthesized catalysts under different conditions

图5 含有A位阳离子缺陷的LaFeO3(LxFO)在甲苯催化氧化中的活性以及稳定性测试[46]
Fig. 5 Activity and stability tests of LaFeO3 (LxFO) with A-site cation deficiency in catalytic oxidation of toluene[46] (a) Toluene conversion as a function of reaction temperature over LxFO samples (0.80≤x≤1.00) and commercial Fe2O3; (b) Effect of GHSV on the catalytic performance of L0.90FO sample; (c) Arrhenius plots of toluene oxidation over LxFO samples (0.80≤x≤1.00) under the conditions of toluene mass concentration 1000 mg·L−1 and GHSV 20000 mL·g−1·h−1; (d) Thermal stability test of toluene catalytic oxidation over L0.90FO sample at 300 ℃ (toluene mass concentration 1000 mg·L−1 and GHSV 20000 mL·g−1·h−1)

图6 LaxFeO3 (x=1.03, 1, 0.97)钙钛矿的结构及表征[47]
Fig. 6 Structure and characterization of LaxFeO3 (x=1.03, 1, 0.97) perovskite[47] (a) Unit cell of perovskite oxide (ABO3) with an ideal cubic structure (left), corresponding coordination state of bulk (middle) and surface (right) lattice oxygen (perovskite surface terminated with Fe cations); (b-d) EDS mappings and (e-g) corresponding HRTEM images of (b, e) La1.03FeO3, (c, f) LaFeO3, and (d, g) La0.97FeO3; (h) H2-TPR profiles of LaxFeO3 (x=1.03, 1, 0.97) oxides
| Catalyst | VOCs | ρVOCs/ (mg·L−1) | aGHSV/ (mL·g−1·h−1) | bT50/℃ | cT90/℃ | dR | Ref. |
|---|---|---|---|---|---|---|---|
| Ag/LaCoO3-250 | Toluene | 3770 | 30000 | 231 | 239 | 4.19×10−9 mol·m−2·s−1 (225 ℃) | [ |
| Ag/LaCoO3-450 | 260 | 278 | 3.09×10−9 mol·m−2·s−1 (225 ℃) | ||||
| Ag/LaCoO3-700 | 252 | 267 | 3.44×10−9 mol·m−2·s−1 (225 ℃) | ||||
| LaMnO3/CeO2 | 1,2-Dichloropropane | 4622 | 15000 | 171 | 260 | - | [ |
| LaMnO3/Al2O3 | 178 | 243 | |||||
| LaMnO3/TiO2 | 183 | 249 | |||||
| LaMnO3/YSZ | 189 | 243 | |||||
| 2%(in mass)Pd@N-L0.8S0.2MO | Toluene | 188 | 36000 | - | 166 | 9.61×10−9 mol·g−1·s−1 (105 ℃) | [ |
| 20%(in mass)LaCoO3/Ce0.9Zr0.1O2 | Toluene | 6406 | 60000 | 200 | - | 1.6×10−6 mol·g−1·s−1 (300 ℃) | [ |
| NiMnO3/CexZr1-xO2/cordierite | Benzene | 1834 | 15000 | - | 275 (eT95) | - | [ |
| CeO2-LaCo0.25Fe0.75O3 | Toluene | 3770 | 18000 | 150 | 205 | - | [ |
| CeO2-LaMn0.25Fe0.75O3 | 155 | 215 |
表2 负载型钙钛矿催化剂及其在催化氧化VOCs中的应用
Table 2 Perovskite-based supported catalysts and their application in catalytic oxidation of VOCs
| Catalyst | VOCs | ρVOCs/ (mg·L−1) | aGHSV/ (mL·g−1·h−1) | bT50/℃ | cT90/℃ | dR | Ref. |
|---|---|---|---|---|---|---|---|
| Ag/LaCoO3-250 | Toluene | 3770 | 30000 | 231 | 239 | 4.19×10−9 mol·m−2·s−1 (225 ℃) | [ |
| Ag/LaCoO3-450 | 260 | 278 | 3.09×10−9 mol·m−2·s−1 (225 ℃) | ||||
| Ag/LaCoO3-700 | 252 | 267 | 3.44×10−9 mol·m−2·s−1 (225 ℃) | ||||
| LaMnO3/CeO2 | 1,2-Dichloropropane | 4622 | 15000 | 171 | 260 | - | [ |
| LaMnO3/Al2O3 | 178 | 243 | |||||
| LaMnO3/TiO2 | 183 | 249 | |||||
| LaMnO3/YSZ | 189 | 243 | |||||
| 2%(in mass)Pd@N-L0.8S0.2MO | Toluene | 188 | 36000 | - | 166 | 9.61×10−9 mol·g−1·s−1 (105 ℃) | [ |
| 20%(in mass)LaCoO3/Ce0.9Zr0.1O2 | Toluene | 6406 | 60000 | 200 | - | 1.6×10−6 mol·g−1·s−1 (300 ℃) | [ |
| NiMnO3/CexZr1-xO2/cordierite | Benzene | 1834 | 15000 | - | 275 (eT95) | - | [ |
| CeO2-LaCo0.25Fe0.75O3 | Toluene | 3770 | 18000 | 150 | 205 | - | [ |
| CeO2-LaMn0.25Fe0.75O3 | 155 | 215 |
| [1] | GUO Y, WEN M, LI G, et al. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: a critical review. Applied Catalysis B: Environmental, 2021, 281: 119447. |
| [2] | ALMAIE S, VATANPOUR V, RASOULIFARD M H, et al. Volatile organic compounds (VOCs) removal by photocatalysts: a review. Chemosphere, 2022, 306: 135655. |
| [3] | ZHAO Z, MA S, GAO B, et al. A systematic review of intermediates and their characterization methods in VOCs degradation by different catalytic technologies. Separation and Purification Technology, 2023, 314: 123510. |
| [4] | ZHANG Y, LU J, ZHANG L, et al. Investigation into the catalytic roles of oxygen vacancies during gaseous styrene degradation process via CeO2 catalysts with four different morphologies. Applied Catalysis B: Environmental, 2022, 309: 121249. |
| [5] | ZHAO R, WANG H, ZHAO D, et al. Review on catalytic oxidation of VOCs at ambient temperature. International Journal of Molecular Sciences, 2022, 23(22): 13739. |
| [6] | ZHANG Y, ZHU W, LU J, et al. Induced synthesis of CeO2 with abundant crystal boundary for promoting catalytic oxidation of gaseous styrene. Applied Catalysis B: Environmental, 2024, 342: 123461. |
| [7] | LIAO W, ZHU W, LU J, et al. Revealing the equilibrium relationship between lattice oxygen mobility and styrene removal: sources of adsorption and activation by in situ experiments. Applied Surface Science, 2023, 629: 157434. |
| [8] | SU Y, FU K, PANG C, et al. Recent advances of chlorinated volatile organic compounds' oxidation catalyzed by multiple catalysts: reasonable adjustment of acidity and redox properties. Environmental Science & Technology, 2022, 56(14): 9854. |
| [9] |
PEÑA M A, FIERRO J L. Chemical structures and performance of perovskite oxides. Chemical Reviews, 2001, 101(7): 1981.
PMID |
| [10] | GRABOWSKA E. Selected perovskite oxides: characterization, preparation and photocatalytic properties—a review. Applied Catalysis B: Environmental, 2016, 186: 97. |
| [11] | WANG K, HAN C, SHAO Z, et al. Perovskite oxide catalysts for advanced oxidation reactions. Advanced Functional Materials, 2021, 31(30): 2102089. |
| [12] | YANG L, LI Y, SUN Y, et al. Perovskite oxides in catalytic combustion of volatile organic compounds: recent advances and future prospects. Energy & Environmental Materials, 2022, 5(3): 751. |
| [13] |
HUMAYUN M, ULLAH H, USMAN M, et al. Perovskite-type lanthanum ferrite based photocatalysts: preparation, properties, and applications. Journal of Energy Chemistry, 2022, 66: 314.
DOI |
| [14] |
ARANDIYAN H, WANG Y, SUN H, et al. Ordered meso- and macroporous perovskite oxide catalysts for emerging applications. Chemical Communications, 2018, 54(50): 6484.
DOI PMID |
| [15] | WEI Y, LENG Y, WANG R, et al. Peroxydisulfate activation by LaNiO3 nanoparticles with different morphologies for the degradation of organic pollutants. Water Science and Technology, 2022, 85(1): 39. |
| [16] | WU M, LI H, MA S, et al. Boosting the surface oxygen activity for high performance iron-based perovskite oxide. Science of the Total Environment, 2021, 795: 148904. |
| [17] | 郭晓青.钙钛矿催化剂的结构优化与掺杂改性及其对含甲烷废气的催化氧化性能研究. 青岛: 青岛科技大学硕士学位论文, 2022. |
| [18] | SHI Z, DONG F, TANG Z, et al. Design Sr, Mn-doped 3DOM LaCoO3 perovskite catalysts with excellent SO2 resistance for benzene catalytic combustion. Chemical Engineering Journal, 2023, 473: 145476. |
| [19] | LIU L, JIA J, SUN T, et al. A facile method for scalable preparation of mesoporous structured SmMnO3 perovskites sheets for efficient catalytic oxidation of toluene. Materials Letters, 2018, 212: 107. |
| [20] | CHEN S, HAO Y, CHEN R, et al. Hollow multishelled spherical PrMnO3 perovskite catalyst for efficient catalytic oxidation of CO and toluene. Journal of Alloys and Compounds, 2021, 861: 158584. |
| [21] | LIU J, WANG J, ZHAO Z, et al. Synthesis of LaxK1-xCoO3 nanorod and their catalytic performances for CO oxidation. Journal of Rare Earths, 2014, 32(2): 170. |
| [22] | ŞAHIN R Z Y, DUPLANČIĆ M, TOMAŠIĆ V, et al. Essential role of B metal species in perovskite type catalyst structure and activity on toluene oxidation. International Journal of Environmental Science and Technology, 2022, 19(1): 553. |
| [23] | TARJOMANNEJAD A, FARZI A, NIAEI A, et al. An experimental and kinetic study of toluene oxidation over LaMn1-xBxO3 and La0.8A0.2Mn0.3B0.7O3 (A=Sr, Ce and B=Cu, Fe) nano-perovskite catalysts. Korean Journal of Chemical Engineering, 2016, 33(9): 2628. |
| [24] | PAN F, ZHANG W, FERRONATO C, et al. Boosting propene oxidation activity over LaFeO3 perovskite catalysts by cobalt substitution. Applied Catalysis A: General, 2022, 643: 118779. |
| [25] | ZHANG R, LI P, XIAO R, et al. Insight into the mechanism of catalytic combustion of acrylonitrile over Cu-doped perovskites by an experimental and theoretical study. Applied Catalysis B: Environmental, 2016, 196: 142. |
| [26] | CAO X, LU J, ZHENG X, et al. Regulation of the reaction pathway to design the high sulfur/coke-tolerant Ce-based catalysts for decomposing sulfur-containing VOCs. Chemical Engineering Journal, 2022, 429: 132473. |
| [27] | LI X, ZHAO H, LIANG J, et al. A-site perovskite oxides: an emerging functional material for electrocatalysis and photocatalysis. Journal of Materials Chemistry A, 2021, 9(11): 6650. |
| [28] | CHANG H, BJØRGUM E, MIHAI O, et al. Effects of oxygen mobility in La-Fe-based perovskites on the catalytic activity and selectivity of methane oxidation. ACS Catalysis, 2020, 10(6): 3707. |
| [29] | ANSARI A A, AHMAD N, ALAM M, et al. Physico-chemical properties stalytic activity of the Sol-Gel prepared Ce-ion doped LaMnO3 perovskites. Scientific Reports, 2019, 9: 7747. |
| [30] | ANSARI A A, ADIL S F, ALAM M, et al. Catalytic performance of the Ce-doped LaCoO3 perovskite nanoparticles. Scientific Reports, 2020, 10: 15012. |
| [31] | PÉREZ H A, LÓPEZ C A, CADÚS L E, et al. Catalytic feasibility of Ce-doped LaCoO3 systems for chlorobenzene oxidation: an analysis of synthesis method. Journal of Rare Earths, 2022, 40(6): 897. |
| [32] | SHIN H, BAEK M, KIM D H. Sulfur resistance of Ca-substituted LaCoO3 catalysts in CO oxidation. Molecular Catalysis, 2019, 468: 148. |
| [33] | LI X, LI M, MA X, et al. Nonstoichiometric perovskite for enhanced catalytic oxidation through excess A-site cation. Chemical Engineering Science, 2020, 219: 115596. |
| [34] | YUAN B, TAO Y, QI S, et al. Effect of A, B-site cation on the catalytic activity of La1-xAxMn1-yByO3 (A=Ce, B=Ni) perovskite-type oxides for toluene oxidation. Environmental Science and Pollution Research International, 2023, 30(13): 36993. |
| [35] |
HE C B, PAN K L, CHANG M B. Catalytic oxidation of trichloroethylene from gas streams by perovskite-type catalysts. Environmental Science and Pollution Research International, 2018, 25(12): 11584.
DOI PMID |
| [36] | LI Y, LIU S, YIN K, et al. Understanding the mechanisms of catalytic enhancement of La-Sr-Co-Fe-O perovskite-type oxides for efficient toluene combustion. Journal of Environmental Chemical Engineering, 2023, 11(1): 109050. |
| [37] | WANG D, LUO K, TIAN H, et al. Transforming plain LaMnO3 perovskite into a powerful ozonation catalyst: elucidating the mechanisms of simultaneous A and B sites modulation for enhanced toluene degradation. Environmental Science & Technology, 2024, 58(27): 12167. |
| [38] | 辛松.改性钙钛矿催化燃烧苯乙烯的稳定性与耐久性研究. 武汉: 华中科技大学硕士学位论文, 2020. |
| [39] | 丁俊彦.镧锰钙钛矿掺杂改性对甲醛催化氧化脱除的实验与机理研究. 武汉: 华中科技大学博士学位论文, 2022. |
| [40] | FENG M, LIN J, LI J, et al. Magnesium-enhanced redox property and surface acidity-basicity of LaMnO3 perovskites for efficient methane purification. Separation and Purification Technology, 2024, 330: 125391. |
| [41] |
WEXLER R B, GAUTAM G S, STECHEL E B, et al. Factors governing oxygen vacancy formation in oxide perovskites. Journal of the American Chemical Society, 2021, 143(33): 13212.
DOI PMID |
| [42] | LI S F, ZHENG J, YAN D. Cationic defect engineering in perovskite La2CoMnO6 for enhanced electrocatalytic oxygen evolution. Inorganic Chemistry, 2023, 62(28): 11009. |
| [43] | LUO Y, WU Y. Defect engineering of nanomaterials for catalysis. Nanomaterials, 2023, 13(6): 1116. |
| [44] | FENG C, GAO Q, XIONG G, et al. Defect engineering technique for the fabrication of LaCoO3 perovskite catalyst via urea treatment for total oxidation of propane. Applied Catalysis B: Environmental, 2022, 304: 121005. |
| [45] | XU Y, DHAINAUT J, DACQUIN J P, et al. On the role of cationic defects over the surface reactivity of manganite-based perovskites for low temperature catalytic oxidation of formaldehyde. Applied Catalysis B: Environmental, 2024, 342: 123400. |
| [46] | WU M, CHEN S, XIANG W. Oxygen vacancy induced performance enhancement of toluene catalytic oxidation using LaFeO3 perovskite oxides. Chemical Engineering Journal, 2020, 387: 124101. |
| [47] |
HE J, WANG T, BI X, et al. Subsurface A-site vacancy activates lattice oxygen in perovskite ferrites for methane anaerobic oxidation to syngas. Nature Communications, 2024, 15: 5422.
DOI PMID |
| [48] | YANG J, SHI L, LI L, et al. Surface modification of macroporous La0.8Sr0.2CoO3 perovskite oxides integrated monolithic catalysts for improved propane oxidation. Catalysis Today, 2021, 376: 168. |
| [49] | DAI L, LU X B, CHU G H, et al. Surface tuning of LaCoO3 perovskite by acid etching to enhance its catalytic performance. Rare Metals, 2021, 40(3): 555. |
| [50] | BAI S, ZHANG N, GAO C, et al. Defect engineering in photocatalytic materials. Nano Energy, 2018, 53: 296. |
| [51] | FANG Z, BUEKEN B, DE VOS D E, et al. Defect-engineered metal-organic frameworks. Angewandte Chemie International Edition, 2015, 54(25): 7234. |
| [52] |
ARANDIYAN H, MOFARAH S S, SORRELL C C, et al. Defect engineering of oxide perovskites for catalysis and energy storage: synthesis of chemistry and materials science. Chemical Society Reviews, 2021, 50(18): 10116.
DOI PMID |
| [53] | CHEN H, WEI G, LIANG X, et al. The distinct effects of substitution and deposition of Ag in perovskite LaCoO3 on the thermally catalytic oxidation of toluene. Applied Surface Science, 2019, 489: 905. |
| [54] | ZHANG C H, WANG C, GIL S, et al. Catalytic oxidation of 1,2-dichloropropane over supported LaMnOx oxides catalysts. Applied Catalysis B: Environmental, 2017, 201: 552. |
| [55] | CHEN B, WEN Y, GAO S, et al. Mechanistic insights into the role of acidity to activity and anti-poisoning over Nb based catalysts for CVOCs combustion. Applied Catalysis A: General, 2022, 636: 118581. |
| [56] | LI X, CHEN D, LI N, et al. Highly efficient Pd catalysts loaded on La1-xSrxMnO3 perovskite nanotube support for low-temperature toluene oxidation. Journal of Alloys and Compounds, 2021, 871: 159575. |
| [57] | GIROIR-FENDLER A, ALVES-FORTUNATO M, RICHARD M, et al. Synthesis of oxide supported LaMnO3 perovskites to enhance yields in toluene combustion. Applied Catalysis B: Environmental, 2016, 180: 29. |
| [58] | ALIFANTI M, FLOREA M, SOMACESCU S, et al. Supported perovskites for total oxidation of toluene. Applied Catalysis B: Environmental, 2005, 60(1/2): 33. |
| [59] | DENG L, HUANG C, KAN J, et al. Effect of coating modification of cordierite carrier on catalytic performance of supported NiMnO3 catalysts for VOCs combustion. Journal of Rare Earths, 2018, 36(3): 265. |
| [60] | QI S, TAO Y, JIANG S, et al. CeO2 supported MOFs derived LaBxFeyO3 (B=Mn, Co) perovskite catalysts for degradation of toluene. Environmental Science and Pollution Research, 2023, 30(15): 45414. |
| [61] | 耿俊, 柯权力, 周文茜, 等. 催化燃烧催化剂抗硫性的研究进展. 燃料化学学报, 2022, 50(5): 564. |
| [62] | YANG C, MIAO G, PI Y, et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: a review. Chemical Engineeing Journal, 2019, 370: 1128. |
| [63] | HE C, LI P, CHENG J, et al. A comprehensive study of deep catalytic oxidation of benzene, toluene, ethyl acetate, and their mixtures over Pd/ZSM-5 catalyst: mutual effects and kinetics. Water, Air, & Soil Pollution, 2010, 209: 365. |
| [64] | MARS P, VAN KREVELEN D W. Oxidations carried out by means of vanadium oxide catalysts. Chemical Engineering Science, 1954, 3: 41. |
| [65] | BEHAR S, GÓMEZ-MENDOZA N A, GÓMEZ-GARCÍA M Á, et al. Study and modelling of kinetics of the oxidation of VOC catalyzed by nanosized Cu-Mn spinels prepared via an alginate route. Applied Catalysis A: General, 2015, 504: 203. |
| [66] | LENG Y, CAO X, SUN X, et al. Catalytic oxidation mechanism of toluene on the Ce0.875Zr0.125O2 (110) surface. Catalysts, 2023, 14(1): 22. |
| [67] | LI X, WANG X, DING J, et al. Engineering active surface oxygen sites of cubic perovskite cobalt oxides toward catalytic oxidation reactions. ACS Catalysis, 2023, 13(9): 6338. |
| [68] | ZHANG Z, JIANG Z, SHANGGUAN W. Low-temperature catalysis for VOCs removal in technology and application: a state-of-the-art review. Catalysis Today, 2016, 264: 270. |
| [69] | ZONOUZ P R, NIAEI A, TARJOMANNEJAD A. Kinetic modeling of CO oxidation over La1-xAxMn0.6Cu0.4O3 (A=Sr and Ce) nanoperovskite-type mixed oxides. International Journal of Environmental Science and Technology, 2016, 13(7): 1665. |
| [70] | ZABIHI M, KHORASHEH F, SHAYEGAN J. Studies on the catalyst preparation methods and kinetic behavior of supported cobalt catalysts for the complete oxidation of cyclohexane. Reaction Kinetics, Mechanisms and Catalysis, 2015, 114(2): 611. |
| [71] | ARANZABAL A, AYASTUY-ARIZTI J L, GONZÁLEZ- MARCOS J A, et al. The reaction pathway and kinetic mechanism of the catalytic oxidation of gaseous lean TCE on Pd/alumina catalysts. Journal of Catalysis, 2003, 214(1): 130. |
| [72] | AO R, MA L, GUO Z, et al. NO oxidation performance and kinetics analysis of BaMO3 (M=Mn, Co) perovskite catalysts. Environmental Science and Pollution Research International, 2021, 28(6): 6929. |
| [1] | 余升阳, 苏海军, 姜浩, 余明辉, 姚佳彤, 杨培鑫. 激光增材制造超高温氧化物陶瓷孔隙缺陷形成及抑制研究进展[J]. 无机材料学报, 2025, 40(9): 944-956. |
| [2] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [3] | 肖晓琳, 王玉祥, 谷佩洋, 朱圳荣, 孙勇. 二维无机材料调控病损皮肤组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 860-870. |
| [4] | 马景阁, 吴成铁. 无机生物材料用于毛囊和毛发再生的研究[J]. 无机材料学报, 2025, 40(8): 901-910. |
| [5] | 张洪健, 赵梓壹, 吴成铁. 无机生物材料调控神经细胞功能及神经化组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 849-859. |
| [6] | 艾敏慧, 雷波. 微纳米生物活性玻璃: 功能化设计与血管化皮肤再生[J]. 无机材料学报, 2025, 40(8): 921-932. |
| [7] | 王宇彤, 常江, 徐合, 吴成铁. 硅酸盐生物陶瓷/玻璃促创面修复的研究进展:作用、机制和应用方式[J]. 无机材料学报, 2025, 40(8): 911-920. |
| [8] | 马文平, 韩雅卉, 吴成铁, 吕宏旭. 无机活性材料在类器官研究领域的应用[J]. 无机材料学报, 2025, 40(8): 888-900. |
| [9] | 罗晓民, 乔志龙, 刘颍, 杨晨, 常江. 无机生物活性材料调控心肌再生的研究进展[J]. 无机材料学报, 2025, 40(8): 871-887. |
| [10] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [11] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [12] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [13] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [14] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [15] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||