无机材料学报 ›› 2022, Vol. 37 ›› Issue (8): 883-890.DOI: 10.15541/jim20220097 CSTR: 32189.14.10.15541/jim20220097
所属专题: 【能源环境】金属有机框架材料(202309); 【信息功能】电致变色与热致变色材料(202312)
张笑宇( ), 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿(
), 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿( ), 王宏志(
), 王宏志( )
)
收稿日期:2022-02-28
修回日期:2022-05-31
出版日期:2022-08-20
网络出版日期:2022-06-03
通讯作者:
王宏志, 教授. E-mail: wanghz@dhu.edu.cn;作者简介:张笑宇(1998-), 男, 硕士研究生. E-mail: dhuzxyu@163.com
基金资助:
ZHANG Xiaoyu( ), LIU Yongsheng, LI Ran, LI Yaogang, ZHANG Qinghong, HOU Chengyi, LI Kerui(
), LIU Yongsheng, LI Ran, LI Yaogang, ZHANG Qinghong, HOU Chengyi, LI Kerui( ), WANG Hongzhi(
), WANG Hongzhi( )
)
Received:2022-02-28
Revised:2022-05-31
Published:2022-08-20
Online:2022-06-03
Contact:
WANG Hongzhi, professor. E-mail: wanghz@dhu.edu.cn;About author:ZHANG Xiaoyu(1998-), male, Master candidate. E-mail: dhuzxyu@163.com
Supported by:摘要:
室温离子液体具有宽电化学窗口和良好的环境稳定性, 是电致变色器件的理想电解质。然而传统电致变色材料的晶格间隙较窄, 限制了离子液体中大尺寸离子的扩散, 且大离子反复脱/嵌会破坏传统电致变色材料的结构, 导致性能衰减。金属有机框架材料(MOFs)是一种具有拓扑结构的多孔晶态材料, 有望为离子液体中大尺寸离子的传输提供通道。本工作在导电玻璃表面制备了三亚苯类Cu3(HHTP)2 (HHTP=2,3,6,7,10,11-六羟基三苯并菲) MOF薄膜, 并研究了Cu3(HHTP)2薄膜在离子液体电解质中电化学和电致变色行为和性能。结果表明, 相对于传统的LiClO4/PC和NaClO4/PC电解质, Cu3(HHTP)2薄膜在离子液体[EMIm]BF4中表现出更低的接触电阻和更高的离子扩散效率, 电极的着色/褪色速度得到了显著提高(着色时间由10.3 s缩短至8.0 s, 褪色时间由23.6 s缩短至5.2 s)。同时, Cu3(HHTP)2薄膜在[EMIm]BF4中也具有更高的光调制范围和着色效率。这项工作展现出MOFs/离子液体电化学体系在电致变色领域中的潜在应用价值。
中图分类号:
张笑宇, 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿, 王宏志. 基于Cu3(HHTP)2薄膜的离子液体电致变色电极[J]. 无机材料学报, 2022, 37(8): 883-890.
ZHANG Xiaoyu, LIU Yongsheng, LI Ran, LI Yaogang, ZHANG Qinghong, HOU Chengyi, LI Kerui, WANG Hongzhi. Cu3(HHTP)2 Film-based Ionic-liquid Electrochromic Electrode[J]. Journal of Inorganic Materials, 2022, 37(8): 883-890.
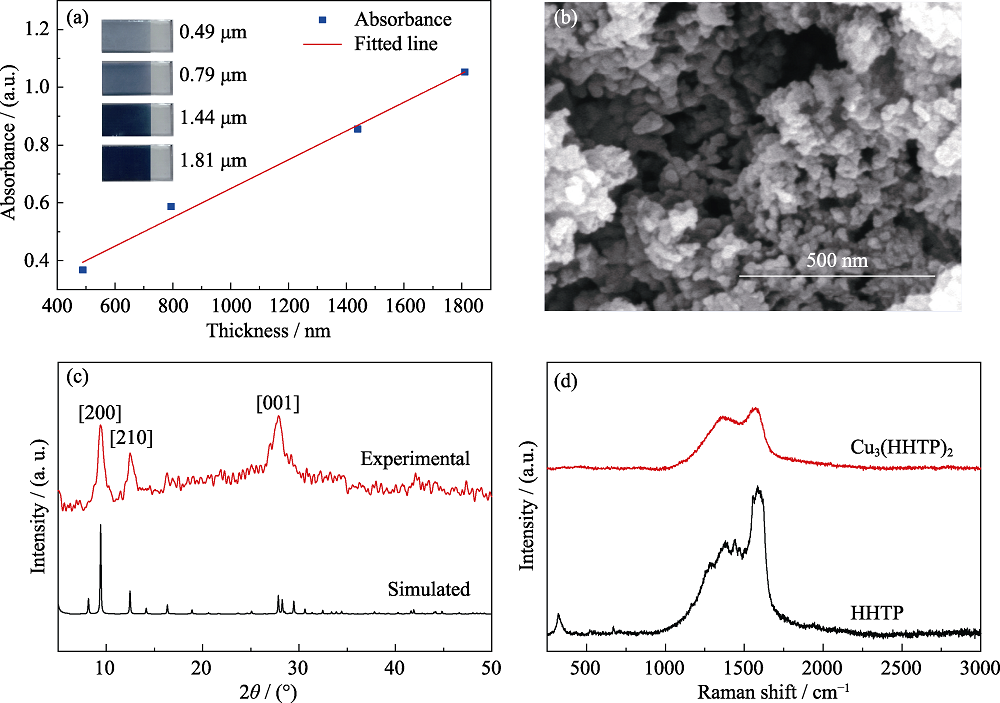
图1 (a) Cu3(HHTP)2薄膜的表征结果
Fig. 1 Characterization of Cu3(HHTP)2 films (a) Change of absorbance at 800 nm wavelength with film thickness, inset showing the pictures of Cu3(HHTP)2 films obtained in different growth-cycles; (b) Surface SEM image of the Cu3(HHTP)2 film obtained from 20 cycles; (c) XRD patterns of Cu3(HHTP)2; (d) Raman spectra of Cu3(HHTP)2 and HHTP ligand

图2 Cu3(HHTP)2的XPS谱图和孔径分布图
Fig. 2 XPS spectrum and poresize distribution of Cu3(HHTP)2 (a) XPS full spectrum; (b) High resolution XPS spectrum of Cu2p3/2; (c) Pore size distribution diagram with inset showing N2 adsorption isotherm curves for Cu3(HHTP)2 powders measured at 77 K
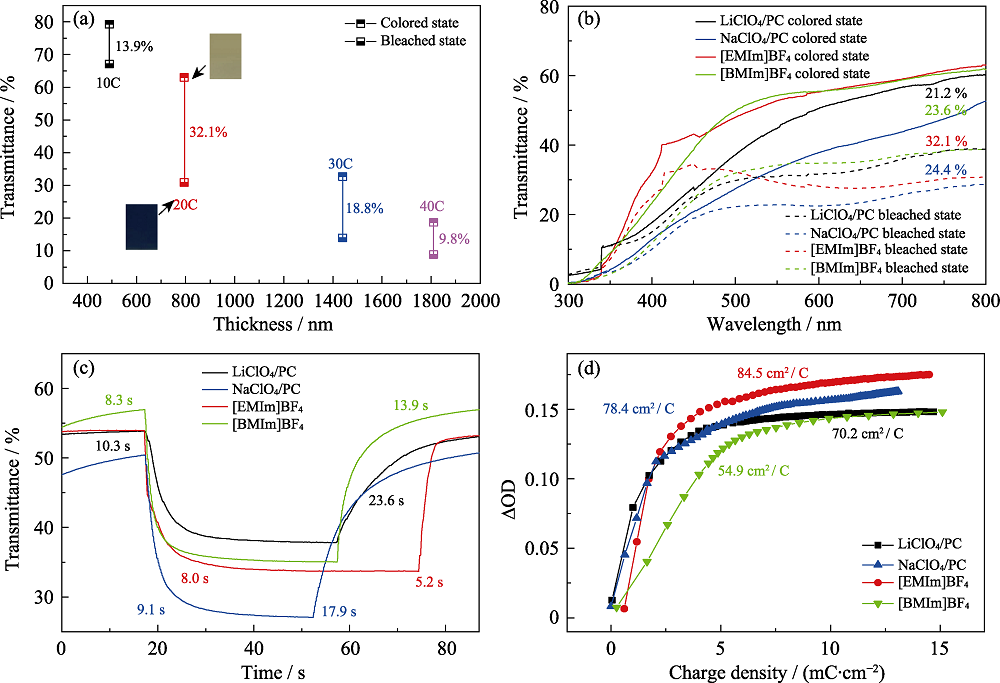
图3 (a)不同厚度的Cu3(HHTP)2薄膜在[EMIm]BF4中-0.9和0.4 V的恒定电压下, 在800 nm波长处的透过率变化图谱(插图为20C薄膜在-0.9 和0.4 V下的照片); (b) 20C薄膜在不同电解质中, -0.9和0.4 V下的紫外-可见透过光图谱(300~800 nm); (c) 20C薄膜在不同电解质中, 在波长800 nm处的透过光谱时间响应图; (d) 20C薄膜分别在LiClO4/PC、NaClO4/PC、[EMIm]BF4和[BMIm]BF4溶液中800 nm波长处的着色效率
Fig. 3 (a) Transmittance at 800 nm wavelength for Cu3(HHTP)2 films with different thicknesses at constant voltages of -0.9 and 0.4 V in [EMIm]BF4 with inset photos showing 20C film at -0.9 V and 0.4 V ; (b) UV-Vis transmission spectra of 20C films measured in various electrolytes at wavelength from 300 to 800 nm; (c) Temporal response of the transmittance of 20C films measured in various electrolytes; (d) Coloring efficiencies of 20C films in various electrolytes, respectively
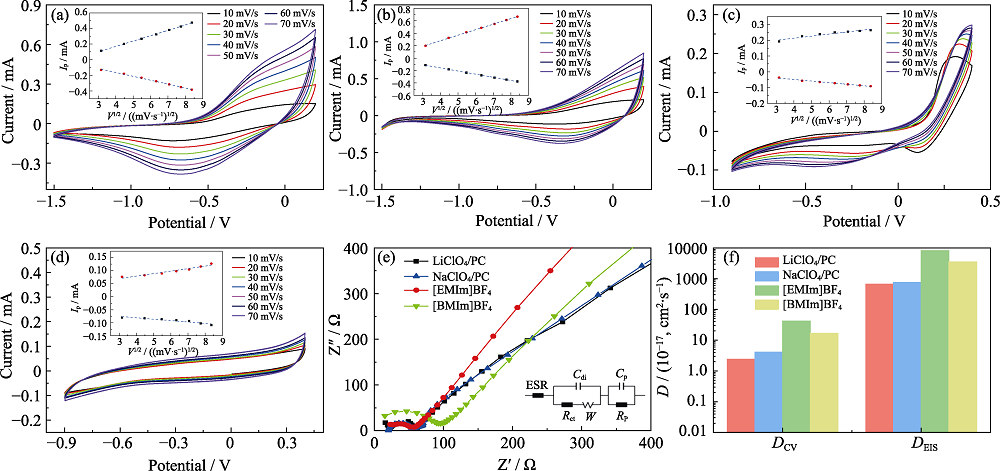
图4 20C薄膜在LiClO4/PC(a)、NaClO4/PC(b)、[EMIm]BF4(c)和[BMIm]BF4(d)中10~70 mV∙s-1扫描速率下的循环伏安曲线(插图为不同扫速下峰值电流(ip)与扫描速率平方根(V1/2)的函数; (e) 20C薄膜分别在不同电解质中的Nyquist阻抗数据(点)和相应拟合结果(线)(插图为对应的等效电路); (f)从电化学阻抗谱和循环伏安测试中计算得出20C薄膜在不同电解质中的扩散系数
Fig. 4 Cyclic voltammetry curves of 20C films at scan rates from 10 to 70 mV∙s-1 in (a) LiClO4/PC, (b) NaClO4/PC solution, (c) [EMIm]BF4, and (d) [BMIm]BF4 with inset showing peak current at different scan rates (ip) as a function of square root of the scan rate (V1/2)); (e) Nyquist impedance data (dots) and corresponding fitting results (lines) of 20C films in various electrolytes, respectively with inset showing corresponding equivalent circuit; (f) Calculated diffusion coefficients of 20C films in various electrolytes from electrochemical impedance spectroscopy and cyclic voltammetry, respectively

图5 Cu3(HHTP)2基电致变色器件在(a)初始态和(b)透明态的照片; (c)器件在+3和-3 V电压下的紫外-可见光透射图谱
Fig. 5 Photos of (a) bleaching state and (b) coloring state of Cu3(HHTP)2 EC device, and (c) UV-Vis transmission spectra of Cu3(HHTP)2 EC devices at voltages of +3 and -3 V
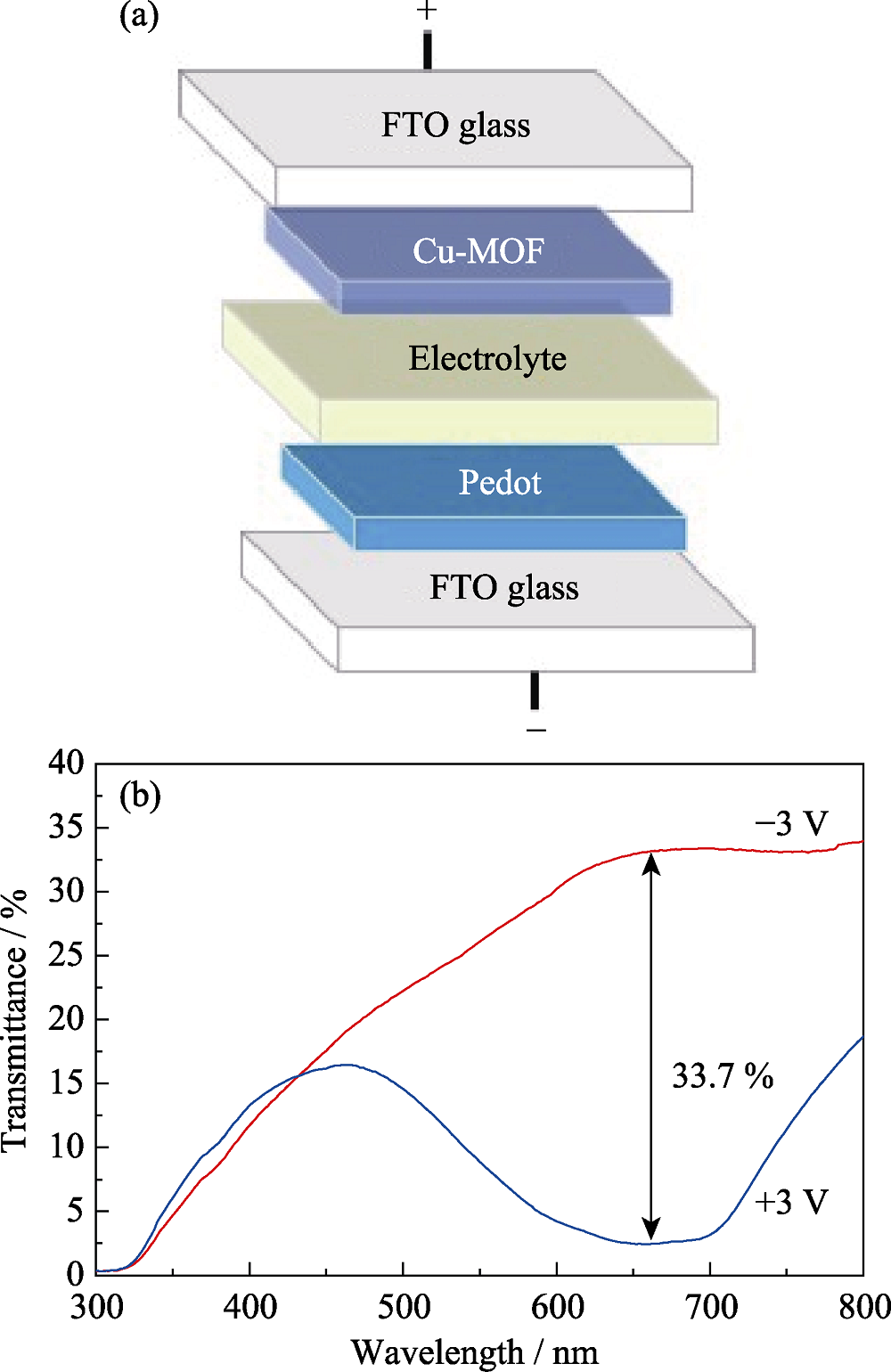
图6 (a)基于Cu3(HHTP)2和PEDOT电致变色全器件结构示意图和(b)电致变色全器件在+3和-3 V电压下的紫外-可见光透射图谱
Fig. 6 (a) Structure diagram of Cu3(HHTP)2 and poly (3,4-ethylene dioxythiophene) (PEDOT) electrochromic multiple device, and (b) UV-Vis transmission spectra of multiple devices at voltages of +3 and -3 V
| [1] |
XU C, LIU L, LEGENSKI S, et al. Switchable window based on electrochromic polymers. Journal of Materials Research, 2004, 19(7): 2072-2080.
DOI URL |
| [2] |
WU X, ZHENG J, XU C. A newly-designed self-powered electrochromic window. Science China Chemistry, 2016, 60(1): 84-89.
DOI URL |
| [3] |
WADE C R, LI M, DINCĂ M. Facile deposition of multicolored electrochromic metal-organic framework thin films. Angewandte Chemie International Edition, 2013, 52: 13377-13381.
DOI URL |
| [4] |
WADE C R, LI M, DINCĂ M. Transparent-to-dark electrochromic behavior in naphthalene-diimide-based mesoporous MOF-74 analogs. Chem, 2016, 1(11): 264-272.
DOI URL |
| [5] |
RIERA M, LAMBROS E, NGUYEN T, et al. Low-order many-body interactions determine the local structure of liquid water. Chemical Science, 2019, 10(35): 8211-8218.
DOI URL |
| [6] |
LI R, LI K, WANG G, et al. Ion-transport design for high- performance Na+-based electrochromics. ACS Nano, 2018, 12: 3759-3768.
DOI URL |
| [7] |
MUKHIYA T, OJHA G, DAHAL B, et al. Designed assembly of porous cobalt oxide/carbon nanotentacles on electrospun hollow carbon nanofibers network for supercapacitor. ACS Applied Energy Materials, 2020, 3(4): 3435-3444.
DOI URL |
| [8] |
QIU X, WANG N, DONG X, et al. A high-voltage Zn-organic battery using a nonflammable organic electrolyte. Angewandte Chemie International Edition, 2021, 60: 21025-21032.
DOI URL |
| [9] |
KIM J, LEE J, YOU J, et al. Conductive polymers for next- generation energy storage systems: recent progress and new functions. Materials Horizons, 2016, 3(6): 517-535.
DOI URL |
| [10] |
CHEN J, NAVEED A, NULI Y, et al. Designing an intrinsically safe organic electrolyte for rechargeable batteries. Energy Storage Materials, 2020, 31: 382-400.
DOI URL |
| [11] |
DANG L, WICK C. Anion effects on interfacial absorption of gases in ionic liquids: a molecular dynamics study. Journal of Physical Chemistry B, 2011, 115(21): 6964-6970.
DOI URL |
| [12] | WILKES J, ZAWOROTKO M. Air and water stable 1-ethyl-3- methylimidazolium based ionic liquids. Chemical Society Chemical Communications, 1992, 13: 965-967. |
| [13] |
CHEN Y, ZHANG X, ZHANG D, et al. High performance supercapacitors based on reducedgraphene oxide in aqueous and ionic liquid electrolytes. Carbon, 2011, 49: 573-580.
DOI URL |
| [14] |
BALDUCCI A, DUGAS R, TABERNA P, et al. High temperature carbon-carbon supercapacitor using ionic liquid as electrolyte. Journal of Power Sources, 2007, 165: 922-927.
DOI URL |
| [15] |
JIN J, WEN Z, LIANG X, et al. Gel polymer electrolyte with ionic liquid for high performance lithium sulfur battery. Solid State Ionics, 2012, 225: 604-607.
DOI URL |
| [16] | GELMAN D, SHVARTSEV B, EIN-ELI Y. Aluminum-air battery based on an ionic liquidelectrolyte. Journal of Physical Chemistry A, 2014, 2: 20237-20242. |
| [17] |
KAZEMIABNAVI S, ZHANG Z, THORNTON K, et al. Electrochemical stability window of imidazolium-based ionic liquids as electrolytes for lithium batteries. Journal of Physical Chemistry B, 2016, 120: 5691-5702.
DOI URL |
| [18] |
LI K, SHAO Y, YAN H, et al. Lattice-contraction triggered synchronous electrochromic actuator. Nature Communications, 2018, 9: 1-11.
DOI URL |
| [19] |
SUN Z, PENG Y, WANG M, et al. Electrochemical deposition of Cu metal-organic framework films for the dual analysis of pathogens. Analytical Chemistry, 2021, 93(25): 8994-9001.
DOI URL |
| [20] | WU F, FANG W, YANG X, et al. Two-dimensional-conjugated metal-organic framework with high electrical conductivity for electrochemical sensing. Journal of the Chinese Society, 2019, 66(5): 522-528. |
| [21] |
LIU J, YANG D, ZHOU Y, et al. Tricycloquinazoline-based 2D conductive metal-organic frameworks as promising electrocatalysts for CO2 reduction. Angewandte Chemie International Edition, 2021, 60(26): 14473-14479.
DOI URL |
| [22] | LI R, LI S, ZHANG Q, et al. Layer-by-layer assembled triphenylene-based MOFs films for electrochromic electrode. Inorganic Chemistry Communications, 2021, 123: 108354. |
| [23] | GUARR T, ANSON C. Electropolymerization of ruthenium (bis (1, 10-phenanthroline) (4-methyl-4’-vinyl2, 2’-bipyridine) complexes through direct attack on the ligand ring system. Journal of Physical Chemistry, 1987, 91: 4037-4043. |
| [24] |
LIANG H, LI R, LI C, et al. Regulation of carbon content in MOF-derived hierarchical-porous NiO@C films for high-performance electrochromism. Materials Horizons, 2019, 6(3): 571-579.
DOI URL |
| [25] |
SONG X, WANG X, LI Y, et al. 2D semiconducting metal-organic framework thin films for organic spin valves. Angewandte Chemie International Edition, 2020, 59(3): 1118-1123.
DOI URL |
| [26] |
NINAWE P, GUPTA K, BALLAV N. Chemically integrating a 2D metal-organic framework with 2D functionalized graphene. Inorganic Chemistry, 2021, 60(24): 19079-19085.
DOI URL |
| [27] |
AMMAR F, SAVEANT J. Convolution potential sweep voltammetry: Part IV. Homogenrous follow-up chemical-reactions. Journal of Electroanalytical Chemistry, 1975, 61: 251-263.
DOI URL |
| [1] | 杨光, 张楠, 陈舒锦, 王义, 谢安, 严育杰. 基于多孔ITO电极的WO3薄膜的制备及其电致变色性能[J]. 无机材料学报, 2025, 40(7): 781-789. |
| [2] | 董晨雨, 郑维杰, 马一帆, 郑春艳, 温峥. 压电力显微镜表征Pb(Mg,Nb)O3-PbTiO3超薄膜弛豫特性[J]. 无机材料学报, 2025, 40(6): 675-682. |
| [3] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| [4] | 赵志翰, 郭鹏, 魏菁, 崔丽, 刘山泽, 张文龙, 陈仁德, 汪爱英. Ti-DLC薄膜压阻性能及载流子输运行为研究[J]. 无机材料学报, 2024, 39(8): 879-886. |
| [5] | 张慧, 许志鹏, 朱从潭, 郭学益, 杨英. 大面积有机-无机杂化钙钛矿薄膜及其光伏应用研究进展[J]. 无机材料学报, 2024, 39(5): 457-466. |
| [6] | 甄明硕, 刘晓然, 范向前, 张文平, 严东东, 刘磊, 李晨. 电致变色型智能可视化湿度系统[J]. 无机材料学报, 2024, 39(4): 432-440. |
| [7] | 鲍可, 李西军. 化学气相沉积法制备智能窗用热致变色VO2薄膜的研究进展[J]. 无机材料学报, 2024, 39(3): 233-258. |
| [8] | 杨志亮, 杨鏊, 刘鹏, 陈良贤, 安康, 魏俊俊, 刘金龙, 吴立枢, 李成明. 热管理用3英寸硅衬底金刚石薄膜的制备[J]. 无机材料学报, 2024, 39(3): 283-290. |
| [9] | 刘松, 张发强, 罗进, 刘志甫. 0.9BaTiO3-0.1Bi(Mg1/2Ti1/2)O3铁电薄膜制备及储能特性[J]. 无机材料学报, 2024, 39(3): 291-298. |
| [10] | 徐向明, Husam N ALSHAREEF. MXetronics—MXene电子学[J]. 无机材料学报, 2024, 39(2): 171-178. |
| [11] | 冯星哲, 马董云, 王金敏. 多孔NiMn-LDH纳米片薄膜的溶剂热生长及其电致变色性能[J]. 无机材料学报, 2024, 39(12): 1391-1396. |
| [12] | 张波涛, 孙婷婷, 王连军, 江莞. 喷墨打印制备AgCuTe热电薄膜[J]. 无机材料学报, 2024, 39(12): 1325-1330. |
| [13] | 刘锁兰, 栾福园, 吴子华, 寿春晖, 谢华清, 杨松旺. 原位生长钙钛矿太阳能电池共形氧化锡薄膜[J]. 无机材料学报, 2024, 39(12): 1397-1403. |
| [14] | 任冠源, 李宜冠, 丁冬海, 梁瑞虹, 周志勇. CaBi2Nb2O9铁电薄膜的生长取向调控和性能研究[J]. 无机材料学报, 2024, 39(11): 1228-1234. |
| [15] | 张哲, 孙婷婷, 王连军, 江莞. 不同维度Ag2Se构筑柔性热电薄膜的性能优化与器件集成研究[J]. 无机材料学报, 2024, 39(11): 1221-1227. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||