无机材料学报 ›› 2021, Vol. 36 ›› Issue (3): 257-268.DOI: 10.15541/jim20200273 CSTR: 32189.14.10.15541/jim20200273
所属专题: 封面文章; 【虚拟专辑】抗菌材料(2020~2021)
收稿日期:2020-05-18
修回日期:2020-07-24
出版日期:2021-03-20
网络出版日期:2020-08-28
作者简介:傅佳骏(1980-), 男, 教授. E-mail: fujiajun668@njust.edu.cn
基金资助:
FU Jiajun1( ), SHEN Tao1, WU Jia2, WANG Chen1
), SHEN Tao1, WU Jia2, WANG Chen1
Received:2020-05-18
Revised:2020-07-24
Published:2021-03-20
Online:2020-08-28
About author:FU Jiajun(1980-), male, professor. E-mail: fujiajun668@njust.edu.cn
Supported by:摘要:
由细菌引发的相关疾病和环境污染等问题引起了人们的高度重视, 同时随着抗生素的使用, 细菌的耐药性逐渐增强, 人们急需开发新型抗菌剂。诸如溶菌酶、髓过氧化物酶等天然酶具有显著的抗菌能力, 但其作为抗菌剂存在保质期短、生产成本高等缺点, 很难大规模生产。因此, 人们正探索寻求天然酶的替代品。纳米酶是新一代人工模拟酶, 兼具纳米材料独特的理化性质和类酶催化活性, 因其结构稳定、生产成本低等优点受到广泛关注。本文综述了纳米酶的抗菌机制和近期抗菌纳米酶的主要研究进展, 并对未来该领域的研究进行展望。
中图分类号:
傅佳骏, 沈涛, 吴佳, 王成. 纳米酶: 对抗细菌的新策略[J]. 无机材料学报, 2021, 36(3): 257-268.
FU Jiajun, SHEN Tao, WU Jia, WANG Chen. Nanozyme: a New Strategy Combating Bacterial[J]. Journal of Inorganic Materials, 2021, 36(3): 257-268.

图3 (a)eDNA作为桥梁及(b)由DNase破坏EPS中eDNA成分[41]
Fig. 3 (a) eDNA acting as a bridge, and (b) disruption of EPS by DNase attacking the eDNA component of the EPS[41]

图4 (a, b)MSN-AuNPs在不同放大倍数下的TEM照片; 不同(c)pH和(d)温度条件下MSN-AuNPs的酶活性; 不同条件处理的(e)大肠杆菌和(f)金黄色葡萄球菌的生长密度[43]
Fig. 4 (a,b)TEM images of the MSN-AuNPs under different magnifications, enzyme activity of MSN-AuNPs under different (c) pH and (d) temperature conditions, and growth densities of (e) E. coli and (f) S. aureus under different conditions[43]
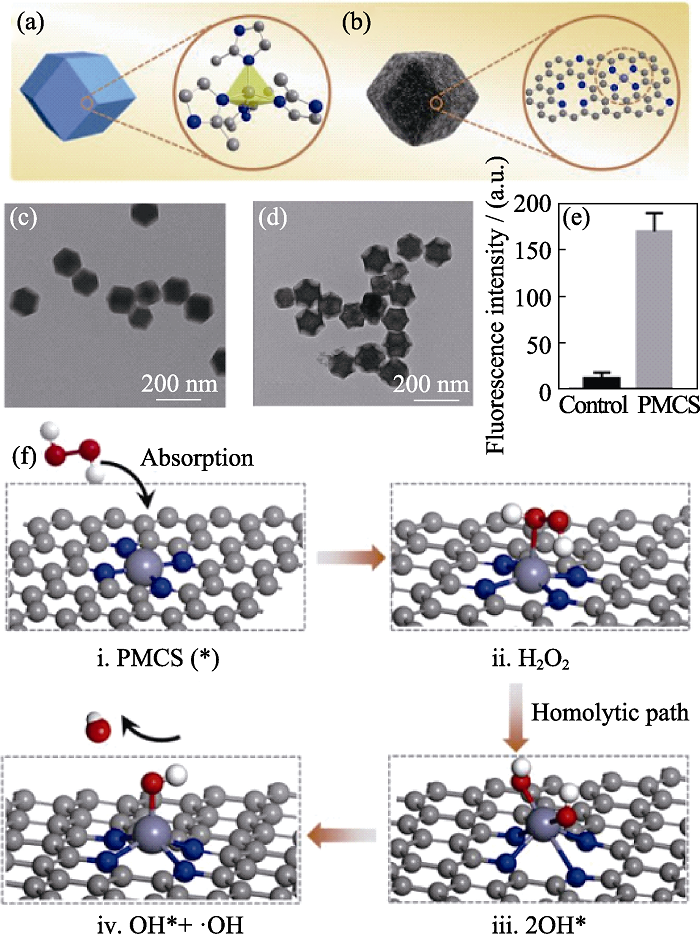
图5 (a)ZIF-8结构框架和(b)碳纳米球PMCS结构模型, (c)ZIF-8和(d)PMCS的TEM照片, (e)对照和PMCS处理的铜绿假单胞菌存活分析和(f)拟定的PMCS催化机理图[46]
Fig. 5 (a) ZIF-8 structural framework and (b) PMCS structural model, TEM images of (c) ZIF-8 and (d) PMCS, (e) apoptosis analysis of P. aeruginosa treated with PMCS, and (f) proposed catalytic mechanism of PMCS[46]

图6 (a)Cit-MoS2的合成路线; (b)光调节Cit-MoS2酶活性以实现革兰氏选择性抗菌性能; 不同(c) pH和(d)光照时间下细菌的存活率[49]
Fig. 6 (a) Synthesis route of Cit-MoS2, and (b) illustration of light regulating Cit-MoS2 enzyme activity to achieve Gram-selective antibacterial potential, and bacterial viabilities under different (c) pH and (d) irradiation time[49]
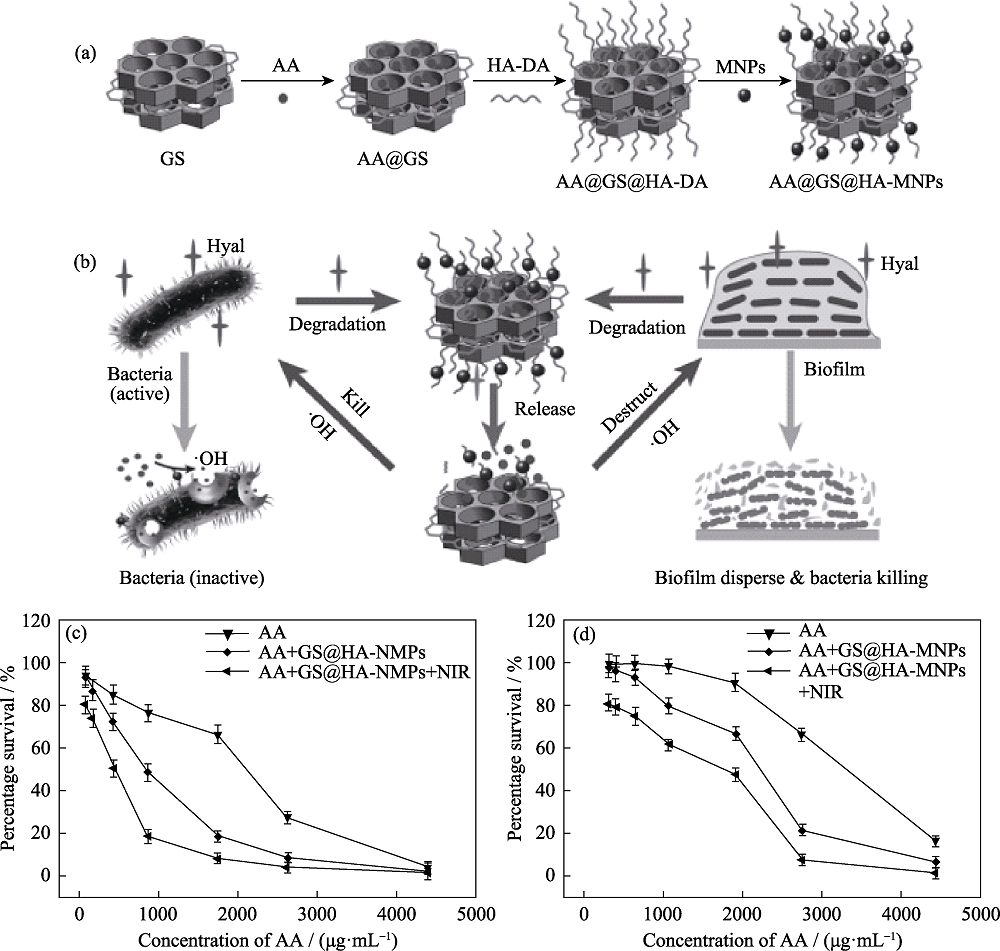
图7 (a)抗菌平台的制备及其(b)抗菌示意图, 不同浓度抗坏血酸处理的(c)金黄色葡萄球菌和(d)大肠杆菌的存活率[52]
Fig. 7 (a) Preparation of the antibacterial platform, and its (b) schematic diagram of antibacterial, and survival rates of (c) S. aureus and (d) E. coli treated with different concentrations of ascorbic acid[52]
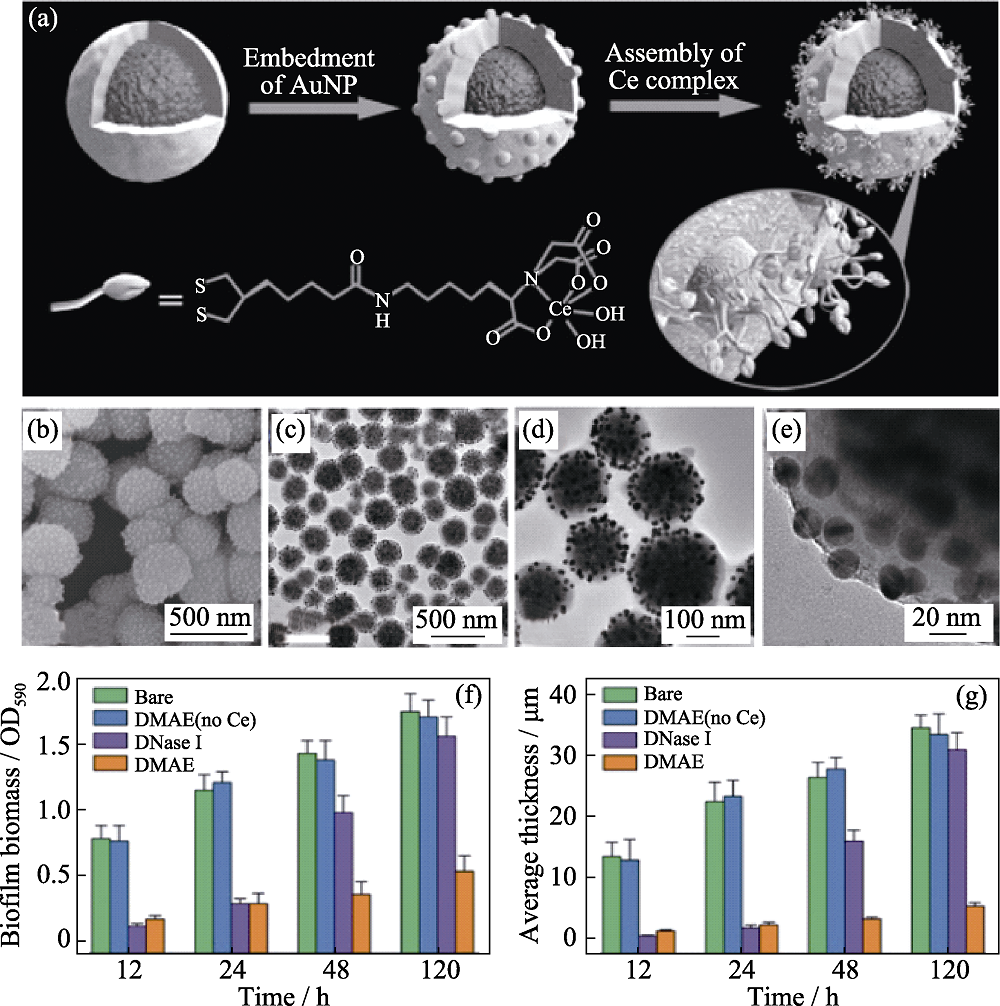
图8 (a)DMAE的制备; 组装在核/壳颗粒表面的金纳米颗粒的(b)SEM、(c)TEM和(d, e)放大的TEM照片; (f)生物膜生长情况和(g)形成生物膜的平均厚度[64]
Fig. 8 (a) Preparation of DMAE and its (b) TEM and (c) SEM images, as well as magnified (d, e) TEM images of Au NPs confined on the surface of core/shell particles, and (f) growth of biofilms and (g) average thickness of the biofilms[64]

图9 CeO2-x纳米棒的(a)TEM和(b)HRTEM照片, (c)海洋挂板实验, (d)拟定的CeO2-x纳米棒催化溴化机理[72]
Fig. 9 (a) TEM and (b) HRTEM images of CeO2-x NPs, (c) ocean hanging board experiment, and (d) proposed catalytic bromination mechanism of the CeO2-x NPs[72]
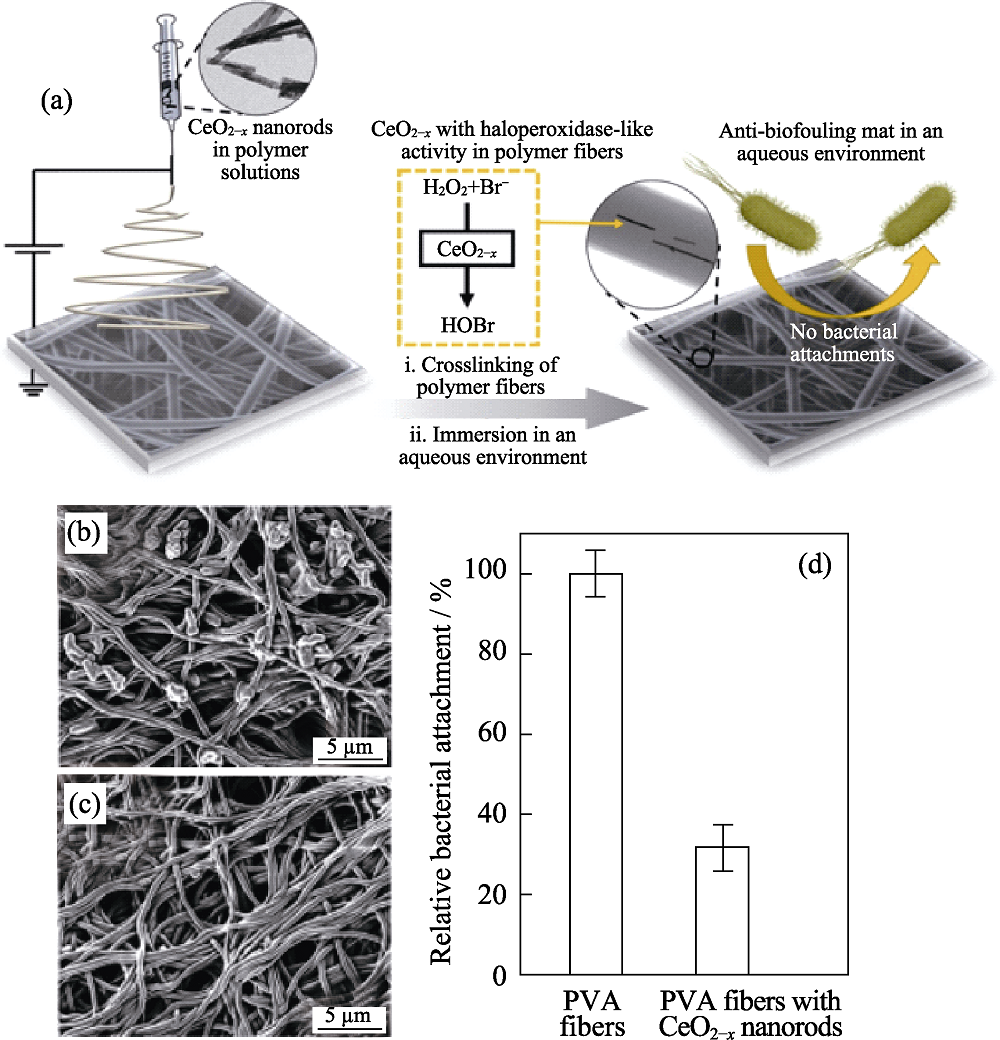
图10 (a)纳米纤维垫的制备及防污示意图, (b)未填充和(c)填充CeO2-x纳米棒的PVA垫抑菌SEM照片, (d)22wt%填充PVA垫的细菌附着对比[73]
Fig. 10 (a) Schematic diagram of preparation and anti-fouling of nanofibrous, SEM images of PVA (b) without and (c) with CeO2-x NRs after incubation, and (d) comparison of bacterial adhesion between 22wt% filled and unfilled PVA nanofibrous[73]
| [1] |
RIZZELLO L, POMPA P P. Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chemical Society Reviews, 2014,43(5):1501-1518.
DOI URL PMID |
| [2] | WANG LI-SHENG, GUPTA A, ROTELLO V M. Nanomaterials for the treatment of bacterial biofilms. ACS Infections Dieases, 2015,2(1):3-4. |
| [3] |
YANG LIANG, LIU YANG, WU HONG. Combating biofilms. Fems Immunology & Medical Microbiology, 2012,65(2):146-157.
DOI URL PMID |
| [4] |
SIMOES MANUEL. Antimicrobial strategies effective against infectious bacterial biofilms. Current Medicinal Chemistry, 2011,18(14):2129-2145.
DOI URL PMID |
| [5] |
GAO LI-ZENG, ZHUANG JIE, LENG NIE, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology, 2007,2(9):577-583.
URL PMID |
| [6] | YAN XI-YUN. Nanozyme: a new type of artificial enzyme. Progress in Biochemistry and Biophysics, 2018,45(2):101-104. |
| [7] |
WEI HUI, WANG ERKANG. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chemical Society Reviews, 2013,42(14):6060-6093.
DOI URL PMID |
| [8] | TANG YAN, QIU ZHI YUE, XU ZHUO BING. Antibacterial mechanism and applications of nanozymes. Progress in Biochemistry and Biophysics, 2018,45(2):118-128. |
| [9] |
JIANG DA-WEI, NI DA-LONG, ROSENKRANS Z T. Nanozyme: new horizons for responsive biomedical applications. Chemical Society Reviews, 2019,48(14):3683-3704.
DOI URL PMID |
| [10] |
LIANG MIN-MIN, YAN XI-YUN. Nanozyme: from new concepts, mechanisms, and standards to applications. Accounts of Chemical Research, 2019,52(8):2190-2200.
DOI URL PMID |
| [11] |
LAURENTS D V, BALDWIN R L. Characterization of the unfolding pathway of hen egg with white lysozyme. Biochemistry, 1997,36(6):1496-1504.
DOI URL PMID |
| [12] |
FATMA V, DE M W C M A, PINAR A, et al. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiology Reviews, 2013,37(6):955-989.
DOI URL PMID |
| [13] | HOU YA-XIN, ZHANG RUO-FEI, YAN XI-YUN, et al. Nanozymes: a new choice for disease treatment. Science Sinica(Vitae), 2020,50(3):311-328. |
| [14] | NELSON D, ROSA M A, ALESSANDRO D, et al. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme & Microbial Technology, 2002,31(7):907-931. |
| [15] | ZUO PENG, YU SHAO-MING, YANG JIE-RU, et al. Research progress in support material for immobilization of horseradish peroxidase. Materials Review, 2007,21(11):46-49. |
| [16] | VEITHC N C. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry (Amsterdam), 2004,65(3):249-259. |
| [17] |
WU JIANG-JIEXING, WANG XIAO-YU, WANG QUAN, et al. Nanomaterials with enzyme-like characteristics (nanozymes): next- generation artificial enzymes (Ⅱ). Chemical Society Reviews, 2019,48(4):1004-1076.
URL PMID |
| [18] |
JIANG BING, FANG LONG, WU KONG-MING, et al. Ferritins as natural and artificial nanozymes for theranostic. Theranostics, 2019,10(2):687-706.
DOI URL PMID |
| [19] |
CHEN ZHAO-WEI, WANG ZHEN-ZHEN, REN JINSONG, et al. Enzyme mimicry for combating bacterial and biofilms. Accounts of Chemical Research, 2018,51(3):789-799.
DOI URL PMID |
| [20] |
MURRAY P J, WYNN T A. Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology, 2011,11(11):723-737.
DOI URL PMID |
| [21] | LOO A E K, WANG Y T, HO R J, et al. Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLOS One, 2012,7(11):49215. |
| [22] | RAGG R, TAHIR M N, TREMEL W. Solids go bio: inorganic nanoparticles as enzyme mimics. European Journal of Inorganic Chemistry, 2016(13/14):1906-1915. |
| [23] |
ASATI ATUL SANTRA, SANTIMUKUL KAITTANIS, CHARALAMBOS NATH, et al. Oxidase-like activity of polymer- coated cerium oxide nanoparticles. Angewandte Chemie International Edition, 2009,48(13):2308-2312.
URL PMID |
| [24] |
ASATI A, KAITTANIS C, SANTRA S, et al. pH-tunable oxidase- like activity of cerium oxide nanoparticles achieving sensitive fluorigenic detection of cancer biomarkers at neutral pH. Analytical Chemistry, 2011,83(7):2547-2553.
DOI URL PMID |
| [25] | FATMA V, DE M W C M A, PINAR A, et al. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiology Reviews, 2013,6(6):955-989. |
| [26] | HOFLER G T, BUT A, HOLLMANN. , Haloperoxidase as catalysts in organic synthesis. Orgainc & Biomolecular Chemistry, 2019,17(42):9267-9274. |
| [27] | SHAW P D, HAGER L P. Choroperoxidase: a component of the β-ketoadipate chlorinase system. Journal of Biological Chemistry, 1961,236(6):1626-1630. |
| [28] |
TIMMINS A, VISSER S P D. Enzymatic halogenases and haloperoxidases: computantional studies on mechanism and function. Advances in Protein Chemistry & Structural Biology, 2015,100:113-151.
DOI URL PMID |
| [29] | KAROLINE H, HAJO F, FELIX P, et al. Functional enzyme mimics for oxidative halogenation reactions that combat biofilm formation. Advanced Materials, 2018,30(36):1701703. |
| [30] |
THOMAS E L. Myeloperoxidase-hydrogen peroxide-chloride antimicrobial system: effect of exogenous amines on antibacterial action against Escherichia Coli. Infection & Immunity, 1979,25(1):110-116.
DOI URL PMID |
| [31] |
REIS P A, MARTINS M L, ARAUJO E F, et al. AiiA quorum- sensing quenching controls proteolytic activity and biofilm formation by enterobacter cloacae. Current Microbiology, 2012,65(6):758-763.
DOI URL PMID |
| [32] | ZHANG JING-JING, FENG TAO, WANG JIA-YI, et al. The mechanisms and applications of quorum sensing (QS) and quorum quenching (QQ). Journal of Ocean University of China, 2019,18(6):1427-1442. |
| [33] | BEZEK K, KURINI M, KNAUDER E, et al. Attenuation of adhesion, biofilm formation and quorum sensing of campylobacter jejuni by euodia ruticarpa. Phytotherapy, 2016,30(9):1527-1532. |
| [34] |
TAUNK A, CHEN R, ISKANDER G, et al. Dual-action biomaterial surfaces with quorum sensing inhibitor and nitric oxide to reduce bacterial colonization. ACS Biomaterials Science & Engineering, 2018,4(12):4174-4182.
URL PMID |
| [35] |
CORDONNIER C, BERNARDI G. A comparative study of acid deoxyribonucleases extracted from different tissues and species. Canadian Journal of Biochemistry, 1968,46(8):989-995.
DOI URL PMID |
| [36] |
MEHTA H J, BISWAS A, PENLEY A M, et al. Management of intrapleural sepsis with once daily use of tissue plasminogen activator and deoxyribonuclease. Respiration, 2016,91(2):101-106.
DOI URL PMID |
| [37] | GRUBER B, KATAEV E, ASCHENBRENNER J, et al. Vesicles and micelles from amphilic zinc(Ⅱ)-cyclen complexes as highly potent promoters of hydrolytic DNA cleavage. Journal of American Chemical Society, 2011,133(5):20704-20707. |
| [38] | BRANUM M E, TIPTON A K, ZHU SHOU-RONG, et al. Double-strand hydrolysis of plasmid DNA by dicerium complexes at 37 ℃. Journal of American Chemical Society, 2001,123(9):1898-1904. |
| [39] | FABRIZIO M, LEONARD P, PAOLO P, et al. Hydrolytic metallo- nanozymes: from micelles and vesicles to gold nanoparticles. Molecules, 2016,21(8):1014. |
| [40] | JENNINGS L K, STOREK K M, LEDVINA H E, et al. Pel is a cationic exopolysaccharide that cross-link extracellular DNA in the pseudomonas aeruginosa biofilm matrix. Proceeding of the National Academy of Science, 2015,112(36):11353-11358. |
| [41] | SWARTIES J, DAS T, SHARIFI S, et al. A functional DNase I coating to prevent adhesion of bacteria and the formation of biofilm. Advanced Functional Materials, 2013,23(22):2843-2849. |
| [42] | HU WEN-CHAO, YOUNIS M R, ZHOU YUE, et al. In situ fabrication of ultrasmall gold nanoparticles/2D MOFs hybrid as nanozyme for antibacterial therapy. Small, 2020,16(23):200553. |
| [43] | XI JU-QUN, WEO GEN, AN LAN-FANG, et al. Copper/carbon hybrid nanozyme: tuning catalytic activity by the copper state for antibacterial therapy. Nano Letter, 2019,19(11):7645-7654. |
| [44] |
TAO YU, JU EN-GUO, REN JIN-SONG, et al. Bifunctionalized mesoporous silicasupported gold nanoparticles: intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Advanced Materials, 2015,27(6):1097-1104.
DOI URL PMID |
| [45] |
SHAN JING-YANG, LI XIAO, YANG KAI-LI, et al. Efficient bacteria killing by Cu2WS4 nanocrystals with enzyme-like properties and bacteria-binding ability. ACS Nano, 2019,13(12):13797-13808.
DOI URL PMID |
| [46] |
XU BO-LONG, WANG HUI, WANG WEI-WEI, et al. A single-atom nanozyme for wound disinfection applications. Angewandte Chemie International Edition, 2019,58(15):4911-4916.
URL PMID |
| [47] |
HUANG LIANG, CHEN JIN-XING, GAN LIN-FENG, et al. Single-atom nanozymes. Science Advances, 2019, 5(5): eaav5490.
DOI URL PMID |
| [48] |
FANG GE, LI WEI-FENG, SHEN XIAO-MEI, et al. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against gram-positive and gram-negative bacteria. Nature Communications, 2018,9:129.
DOI URL PMID |
| [49] | NIU JING-SHENG, SUN YU-HUAN, WANG FA-MING, et al. Photomodulated nanozyme used for a Gram-selective antimicrobial. Chemistry of Materials, 2018,30(20):7027-7033. |
| [50] | NATAN M, EDIN F, PERKAS N, et al. Two are better than one: combating ZnO and MgF2 nanoparticles reduces streptococcus penumoniae and staphylococcus aures biofilm formation on cochlear implants. Advanced Functional Materials, 2016,26(15):2473-2481. |
| [51] |
YIN WEN-YAN, YU JIE, LV FENG-TING, et al. Functionalized nano-MoS2 with peroxidase catalytic and near-infrared photothermal activities for safe and synergetic wound antibacterial applications. ACS Nano, 2016,10(12):11000-11011.
DOI URL PMID |
| [52] |
JI HAI-WEI, DONG KAI, YAN ZHENG-QIN, et al. Bacterial hyaluronidase self-triggered prodrug release for chemo-photothermal synergistic treatment of bacterial infection. Small, 2016,12(45):6200-6206.
DOI URL PMID |
| [53] | ZOU XUE-FENG, ZHANG LI, WANG ZHAO-JUN, et al. Mechanisms of the antimicrobial activities of graphene material. J. Am. Chem. Soc., 2016,138(7):2064-2077. |
| [54] | PERELSHTEIN I, LIPOVSKY A, PERKAS N, et al. The influence of the crystalline nature of nano-metal oxides on their antibacterial and toxicity properties. Nano Res., 2015,8(2):695-707. |
| [55] | DICKINSON B C, CHANG CJ J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol., 2011,7(8):504-511. |
| [56] | WANG WAN-SHUN, LI BING-LIN, YANG HUI-LI, et al. Efficient elimination of multidrug-resistant bacteria using copper sulfide nanozymes anchored to graphene oxide nanosheets. Nano Res., 2020,13(8):2156-2164. |
| [57] | LEVY S. Antibacterial resistance worldwide: causes, challenges and responses. Nature Medicine, 2004,10(S12):S122-129. |
| [58] | WU HONG, MOSER C, WANG HENG-ZHUANG, et al. Strategies for combating bacterial biofilm infections. International Journal of Oral Science, 2015,1:1-7. |
| [59] |
ARCIOLA C R, CAMPOCCIA D, SPEZIALE P, et al. Biofilm formation in staphylococcus implant infections: a review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials, 2012,33(26):5967-5982.
DOI URL PMID |
| [60] |
LEBEAUX D, GHIGO J M, BELOIN C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiology & Molecular Biology Reviews, 2014,78(3):510-543.
DOI URL PMID |
| [61] |
GAO LI-ZENG, GIGLIO K M, NELSON J L, et al. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale, 2014,6(5):2588.
DOI URL PMID |
| [62] |
GAO LI-ZENG, LIU YUAN, KIM D, et al. Nanocatalysts promote streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials, 2016,101:272-284.
DOI URL PMID |
| [63] |
NAHA P C, LIU YUAN, HWANG G, et al. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano, 2019,13(5):4960-4971.
DOI URL PMID |
| [64] |
CHEN ZHAO-WEI, JI HAI-WEI, LIU CHAO-QUN, et al. A multinuclear metal complex based DNase-mimetic artificial enzyme: matrix cleavage for combating bacterial biofilms. Angewandte Chemie International Edition, 2016,55(36):10732-10736.
DOI URL PMID |
| [65] |
LIU ZHENG-WEI, WANG FA-MING, REN JIN-SONG, et al. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials, 2019,208:21-31.
DOI URL PMID |
| [66] |
LEJARS M, MARGAILLAN A, BRESSY C. Fouling release coatings: a nontoxic alternative to biocidal antifouling coatings. Chemical Reviews, 2012,112(8):4347-4390.
DOI URL PMID |
| [67] | GAO ZHI-GIANG, JIANG SHEN-MING, ZHANG QI-FU, et al. Advances in research of marine antifouling fluorine resin coatings with low surface energy. Electroplating & Finishing, 2017,36(6):273-279. |
| [68] | 陈吉 . 船舶纳米防污涂料的研究与应用. 武汉: 武汉大学硕士学位论文, 2015. |
| [69] |
NATALIO F, ANDRE R, HARTOG A F, et al. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nature Nanotechnology, 2012,7(8):530-535.
DOI URL PMID |
| [70] | ANDRE R, NATALIO F, HUMANES M, et al. V2O5 nanowires with an intrinsic peroxidase-like activity. Advanced Functional Materials, 2011,21(3):501-509. |
| [71] | ASSENM F L, LEVY S. A review of current toxicological concerns on vanadium pentoxide and other vnadium compounds: gaps in knowledge and directions for future research. Journal of Toxicology & Environment, 2009,12(4):289-306. |
| [72] | HERGET K, HUBACH P, PUSH S, et al. Haloperoxidase mimicry by CeO2-x nanorods combats biofouling. Advanced Materials, 2017,29(4):1603823. |
| [73] | HU MING-HAN, KORSCHELT K, VIEL MELANIE, et al. Nanozymes in nanofibrous mats with haloperoxidase-like activity to combat biofouling. ACS Applied Materia HOUUls & Interfaces, 2018,10(51):44722-44730. |
| [74] |
ZHAO YU-YUN, TIAN YUE, CUI YAN, et al. Small molecule-capped gold nanoparticles as potent antibacterial agents that target Gram- negative bacterial. J. Am. Chem. Soc, 2010,132(35):12349-12356.
DOI URL PMID |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [8] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [9] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [10] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [11] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [12] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [13] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [14] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| [15] | 王月月, 黄佳慧, 孔红星, 李怀珠, 姚晓红. 载银放射状介孔二氧化硅的制备及其在牙科树脂中的应用[J]. 无机材料学报, 2025, 40(1): 77-83. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||