无机材料学报 ›› 2021, Vol. 36 ›› Issue (3): 225-244.DOI: 10.15541/jim20200224 CSTR: 32189.14.10.15541/jim20200224
• 特邀综述 • 下一篇
收稿日期:2020-04-26
修回日期:2020-07-01
出版日期:2021-03-20
网络出版日期:2020-10-10
通讯作者:
宋 礼, 教授. E-mail: song2012@ustc.edu.cn
作者简介:周煜筑(1998 -), 男, 硕士研究生. E-mail: zyz12345@mail.ustc.edu.cn
基金资助:
ZHOU Yuzhu1( ), ZHANG Youkui2, SONG Li1(
), ZHANG Youkui2, SONG Li1( )
)
Received:2020-04-26
Revised:2020-07-01
Published:2021-03-20
Online:2020-10-10
Contact:
SONG Li, professor. E-mail: song2012@ustc.edu.cn
About author:ZHOU Yuzhu(1998 -), male, Master candidate. E-mail: zyz12345@mail.ustc.edu.cn
Supported by:摘要:
电催化技术是可再生能源储存和转换领域中最有吸引力的技术之一, 其中贵金属纳米材料具有优异的电催化活性。贵金属在地球中的储量少且开发成本高, 如何在减少贵金属用量的同时提高催化剂活性和稳定性一直是电催化应用领域的研究焦点。贵金属磷化物作为新型电催化剂因其多功能活性位点、可调的结构和组分以及新颖的物理化学性质等优点, 受到了研究人员的广泛关注。与过渡金属磷化物相比, 贵金属磷化物具有更高的本征活性, 且在酸性条件下具有更好的稳定性。本综述介绍了近年来贵金属磷化物电催化剂的设计合成、结构调控、X射线吸收谱表征及其在电催化应用中的研究进展, 据此讨论当前所面临的机遇和挑战, 并展望原位同步辐射X射线表征技术在未来贵金属磷化物电催化剂研究中的应用前景。
中图分类号:
周煜筑, 张友魁, 宋礼. 贵金属磷化物催化剂及其同步辐射X射线吸收谱[J]. 无机材料学报, 2021, 36(3): 225-244.
ZHOU Yuzhu, ZHANG Youkui, SONG Li. Noble Metal Phosphide Electrocatalysts and Their Synchrotron-based X-ray Absorption Spectroscopy[J]. Journal of Inorganic Materials, 2021, 36(3): 225-244.

图1 铂族金属磷化物的结构示意图, 其中灰色为金属原子, 红色为磷原子[11]
Fig. 1 The structures of selected platinum group metal (PGM) phosphides. The metal atoms displayed in grey and the phosphorus atoms displayed in red[11] (a) Rh2P crystallizes in the anti-CaF2 structure; (b) Rh2P3; (c) Rh4P3; (d) RhP2 crystallizes in the CoSb2 structure forming P2 dimers; (e) RhP3 crystallizes in the CoAs3 structure forming Rh4-4 rings; (f) Co2P structure with chains of slightly distorted prisms
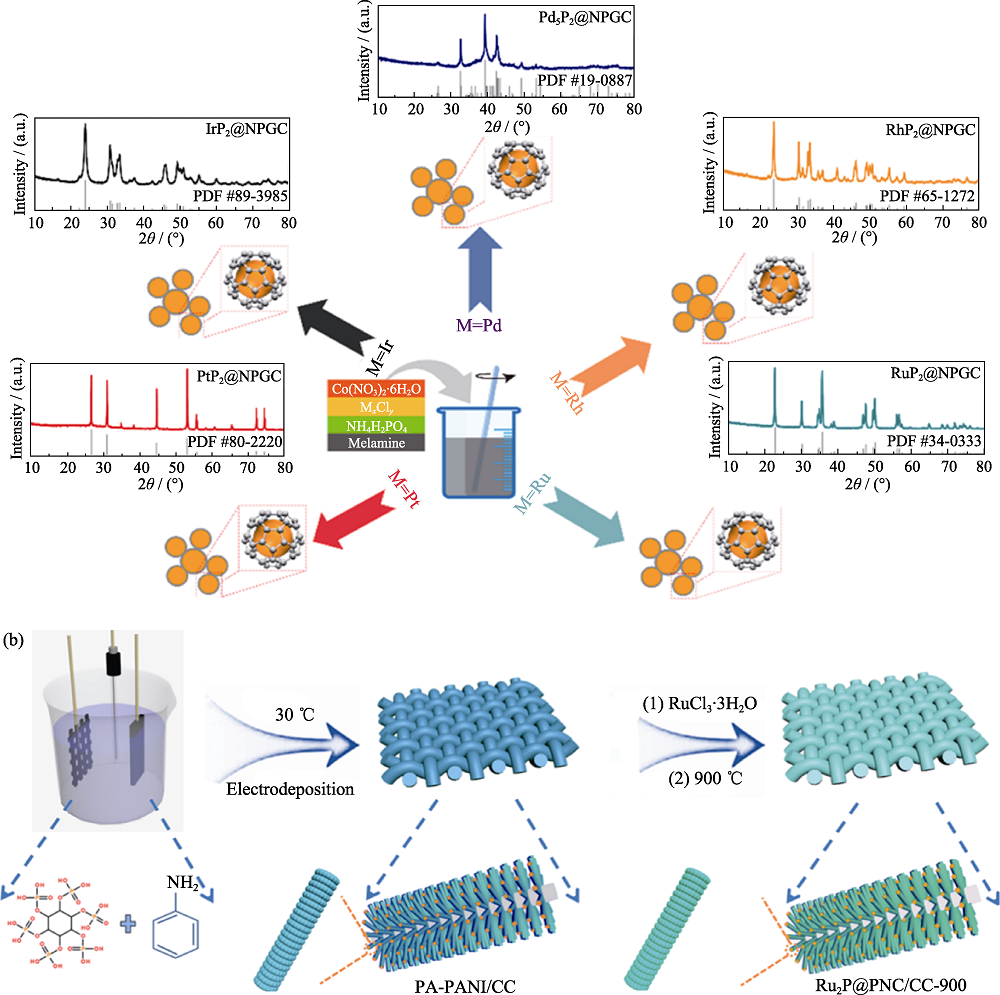
图3 MPx@NPGC的合成示意图及X射线衍射(XRD)图谱(a)[45]和Ru2P@PNC/CC-900的合成示意图(b)[53]
Fig. 3 General synthesis procedure of MPx@NPGC and corresponding XRD patterns (a)[45], schematic illustration of preparation for Ru2P@PNC/CC-900 (b)[53]

图4 Rh-P/CP中Rh-P颗粒的TEM(a)和HRTEM(b)照片, 单个Rh-P粒子Rh(c)和P(d)的STEM照片与元素分布图[56], Ru-Ru2PΦNPC和NPC@RuO2的合成示意图(e)[59];
Fig. 4 TEM (a) and HRTEM (b) images of single Rh-P particle of Rh-P/CP, STEM and elemental mapping images for single Rh-P particle Rh (c) and P (d)[56], synthesis diagram of Ru-Ru2PΦNPC and NPC@RuO2 (e)[59]
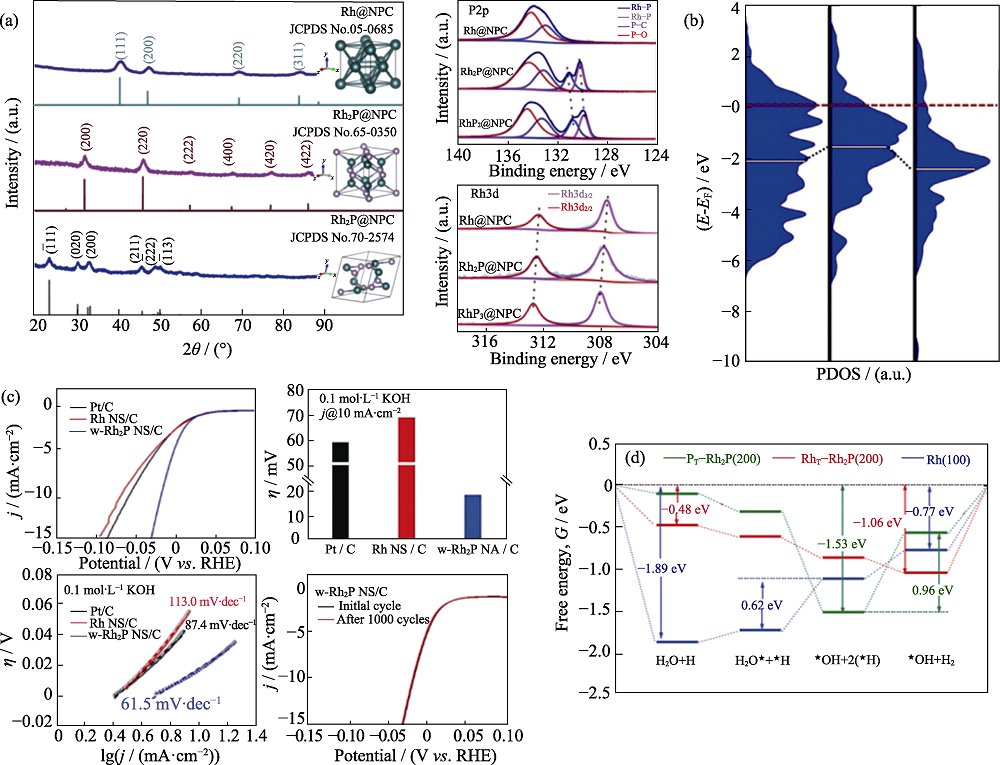
图5 RhPx@NPC的XRD图谱和Rh3d和P2p的XPS图谱(a), Rh(111)、Rh2P(200)和RhP2(ˉ111)表面Rh原子d轨道上的态密度(PDOS)(b)[65], 在0.1 mol/L KOH中Pt/C、Rh NS/c和w-Rh2P NS/C的电催化性能(c), 在碱性条件下P端(PT-Rh2P(200))、Rh端(RhT-Rh2P(200))和Rh(100)表面反应过程自由能变化(?G)(d)[71]
Fig. 5 XRD patterns for RhPx@NPC and XPS spectra for the Rh3d and P2p regions of RhPx@NPC catalysts (a)[65], Partial density of states (PDOS) projected on the d orbitals of Rh atoms at the surfaces of Rh(111), Rh2P(200) and RhP2(ˉ111) (b) (The red dash and yellow solid lines indicate the Fermi level (EF) and the location of d-bands center, respectively), electrocatalytic activities of Pt/C, Rh NS/C, and w-Rh2P NS/C in 0.1 mol/L KOH (c), free energy pathways (?G) for HER of P-terminated (PT-Rh2P (200)), Rh-terminated (RhT-Rh2P (200)), and Rh (100) surfaces, respectively, under alkaline condition (d)[71]

图6 OER/HER测试前后IrP2/NPC的Ir4f XPS光谱图(a, b)[32], RuxPNFs在碱性环境下的HER原理图(c)[75], 在1 mol/L KOH电解液中进行耐久性测试前(d)试验后(e)PdP2@CB的TEM、HR-TEM照片, 以及PdP2@CB在0.5 mol/L H2SO4的HER活性(f)[69]
Fig. 6 XPS results (a, b): Ir4f for IrP2/NPC before and after the OER/HER tests[32], schematic of the HER processes by RuxPNFs under alkaline conditions (c)[75], TEM, HR-TEM images of PdP2@CB before durability test in 1 mol/L KOH electrolyte (d) after durability test in 1 mol/L KOH electrolyte (e), and HER performance (f) of PdP2@CB in 0.5 mol/L H2SO4[69]

图7 RuPx/NPG催化剂的合成和结构示意图(a)[77], NC、NPC、Rh@NC、RhPx@NPC纳米壳的TEM照片(b)[78], Rh2P@NPC和RhP2@NPC的合成和结构示意图(c)[65], RhP2@NPC的TEM照片和元素分布图(d)[65], PtNiP-MNs的SEM照片(e)[73]
Fig. 7 Schematic illustration of the synthesis and structure of the RuPx/NPG electrocatalyst (a)[77], TEM images of NC, NPC, Rh@NC, and RhPx@NPC nanoshells (b)[78], schematic illustration of the synthesis and structure of Rh2P@NPC and RhP2@NPC (c)[65], TEM image and EDS elemental mapping of Rh, P, C and N for RhP2@NPC (d), and SEM image of the PtNiP MNs (e)[73]
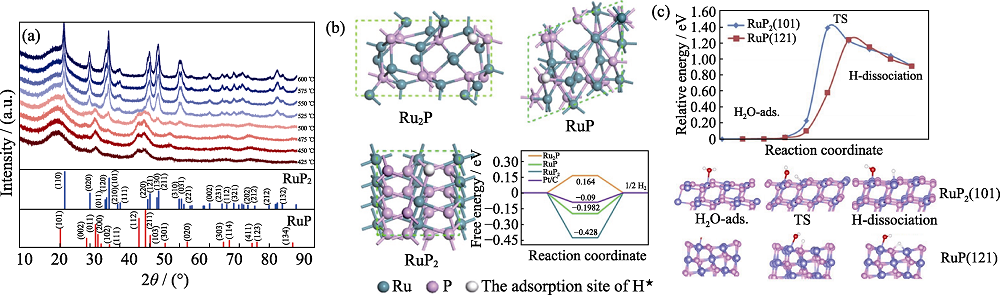
图8 不同温度合成RuPx的XRD图谱(a)[44], DFT计算中使用的理论模型以及在这些模型的表面H*的吸附位置, Ru2P、RuP和RuP2在平衡势下的HER自由能(b)[46], RuP(121)和RuP2(101)晶面水离解途径和势垒(c)[54]
Fig. 8 XRD patterns of RuPx synthesized in different temperatures (a)[44], theoretical models used in DFT calculations and adopted adsorption sites of H* on the surface of these models, calculated free-energy diagram of HER at equilibrium potential for Ru2P, RuP, and RuP2 (b)[46], the calculated water dissociation barrier and water dissociation pathway for RuP (121) and RuP2 (101) surfaces (c)[54]

图9 Ru-Ru2PΦNPC的HRTEM照片(a)和FFT图像(b), Ru-Ru2PΦNPC、Ru/C、Ru2P/C、NPC和20wt%Pt/C在0.5 mol/L H2SO4中的性能(c)[59], Ru-Ru2P/PC的HAADF-STEM(d)和HR-TEM(e)照片, Ru-Ru2P/P C和Pt/C催化剂Tafel斜率与pH关系及循环稳定性(f)[40], 空心Ru-RuPx-CoxP多面体的合成过程示意图(g), Co2P和Ru-RuPx-CoxP模型表面不同电位下OER过程Gibbs自由能(h)[85]。
Fig. 9 HRTEM (a) and FFT (b) images of Ru-Ru2PΦNPC, HER performance of Ru-Ru2PΦNPC, Ru/C, Ru2P/C, NPC, and 20wt% Pt/C, ?GH* calculated at the equilibrium potential of different models (c)[59], high-resolution HAADF-STEM(d) and HR-TEM (f) images of a Ru-Ru2P nanoparticle, pH dependences of the Tafel slopes of the Ru-Ru2P/PC and Pt/C catalysts, cyclic stability of the Ru-Ru2P/PC (f)[40], schematic illustration of a formation process for the hollow Ru-RuxP-CoxP polyhedra (g), Gibbs free energy diagram for the OER at different potentials on the surface of Co2P and Ru-RuPx-CoxP models (h)[85]
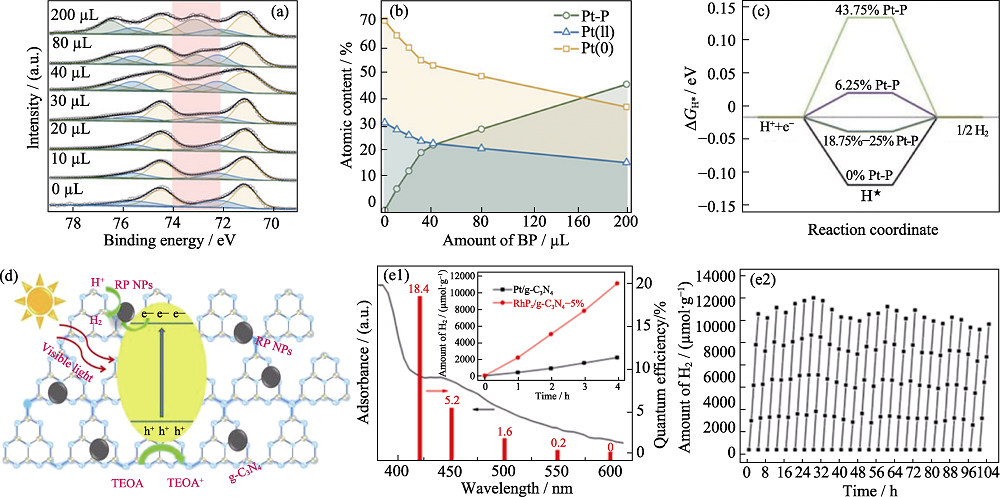
图10 不同BP含量的BPed-Pt/GR XPSβPt4f光谱(a), PtNP的化学态随BP含量变化(b), 不同Pt-P含量体系中的ΔGH*(c)[88], 0.1%-RP/g-CN的光催化析氢机理(d)[89], Pt/g-C3N4和RhPx/g-C3N4-5%产氢曲线(e1~e2)[90]
Fig. 10 Pt4f core level of BPed Pt/GR with different amounts of BP (a), chemical state contents of the PtNPs as a function of BP adding amount (b), free energy diagram for HER with different Pt-P contents with ΔGH* in each system (c)[88], proposed photocatalytic mechanism of 0.1%-RP/g-CN for H2 evolution (d)[89], kinetics curves of H2 production over Pt/g-C3N4 and RhPx/g-C3N4-5%, cyclic running kinetics curves of H2 production over RhPx/g-C3N4-5% (e1-e2)[90]

图11 Rh2P@NC的TEM(a, b)和HRTEM(c)照片, Rh2P@NC的HER稳定性测试(d)[51], Ru2P/RGO-20、Ru2P和Pt/C在1.0 mol/L KOH中的HER性能(e), RGO、Ru2P、Pt和Ru2P/RGO-20的HER自由能图(f)[81], RuP2和RuP2@NPC的能带结构(g)[93], 纯金属磷化物和不同金属磷化物与石墨烯的物理混合物在0.5 mol/L H2SO4和1 mol/L KOH中的极化曲线(h)[45]
Fig. 11 TEM (a,b), and HRTEM (c) images of Rh2P@NC, polarization curves for Rh2P@NC (initial and after 1000 CV scanning) and time-dependent current density curve for Rh2P@NC under static overpotential of 20 mV for 10 h (d)[51], electrocatalytic properties for the HER in 1.0 mol/L KOH of Ru2P/RGO-20, Ru2P and Pt/C (e), free-energy diagram of the HER for RGO, Ru2P, Pt and Ru2P/RGO-20 (f)[81], band structure of pure RuP2 (left) and RuP2@NPC hybrid (right) (g)[93], HER polarization curves of the pure metal phosphides and the physical mixture of different metal phosphides and graphene in 0.5 mol/L H2SO4 and 1 mol/L KOH (h)[45]
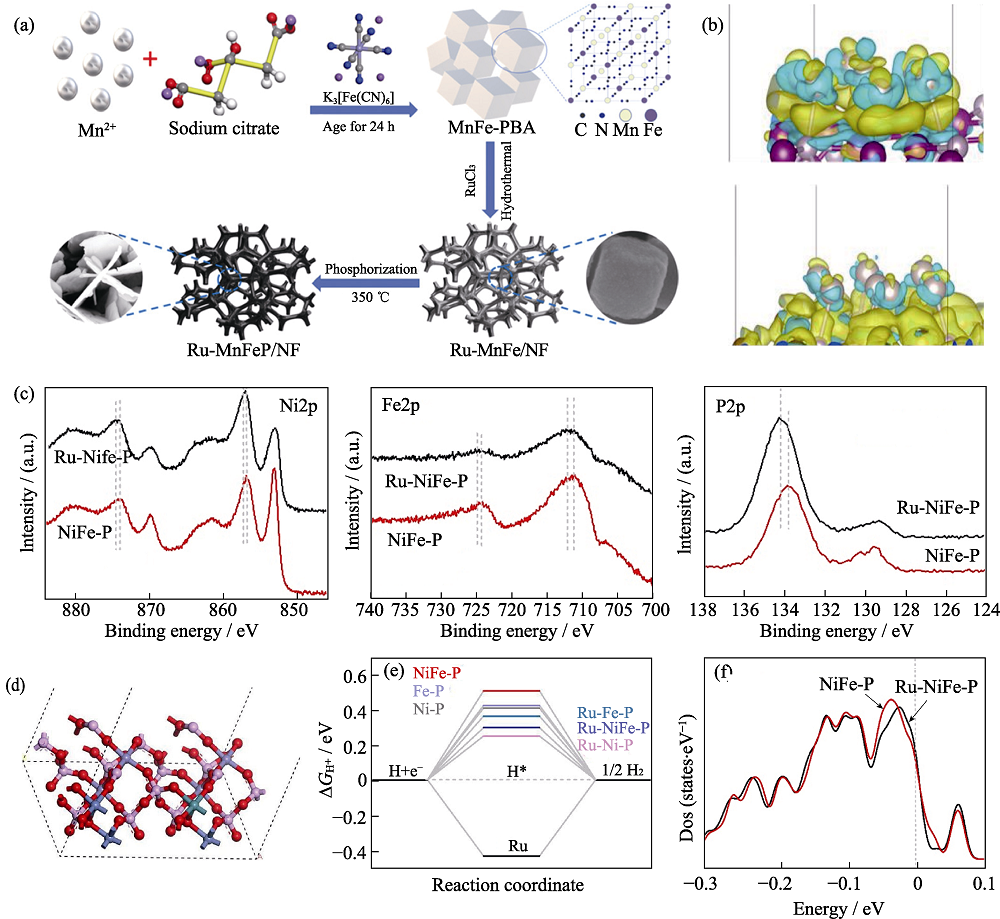
图12 Ru-MnFeP/NF催化剂制备示意图(a), Fe2P-Ru h和Mn2P-Ru结构的电荷分布(b)[98], Ru-NiFe-P和NiFe-P的XPS谱图(c), Ru-NiFe-P的吸收模型表面(d), 平衡电势下计算的?GH*(e), Ru-NiFe-P和NiFe-P的总态密度(f)[99]
Fig. 12 Schematic illustration of the fabrication of Ru-MnFeP/NF catalysts (a), calculated charge density differences of Fe2P-Ru and Mn2P-Ru structures (b)[98], high-resolution XPS spectra of Ni2p, Fe2p and P2p in the Ru-NiFe-P and the NiFe-P (c), the absorption modeled surfaces of Ru-NiFe-P (d), calculated ΔGH* for Ru-NiFe-P, NiFe-P, Ru-Ni-P, Ni-P, Ru-Fe-P and Fe-P (e), total density of states of Ru-NiFe-P and NiFe-P (f)[99]

图13 PdNP-CN的HRTEM照片(a), PdPNP-CN(b)和PdPSA-CN(c)的HAADF-STEM照片, PdNP-CN(d)、PdPNP-CN(e)和PdPSA-CN(f)的几何结构, Pd K边XANES光谱图(g), EXAFS在R(h)和k(i)空间对应的k3加权傅里叶变换谱图[103], RuCl3@HPN和Ru SAs@PN的EXAFS光谱图(j), Ru SAs@PN、Ru箔和RuCl3@HPN的小波变换谱(k), N的K边(l)、P的L边(m)NEXAFS光谱图, PN和Ru SAs@PN的质子去耦31P固态MAS NMR谱图(n)[104]
Fig. 13 HRTEM image of PdNP-CN (a), HAADF-STEM images of PdPNP-CN (b) and PdPSA-CN (c), the geometry structures of PdNP-CN (d), PdPNP-CN (e) and PdPSA-CN (f), Pd K-edge XANES spectra (g) and the corresponding k3-weighted FT spectra at R(h) and k(i) space[ 103], EXAFS spectra (j) of RuCl3@HPN and Ru SAs@PN, Wavelet transform (k) of Ru SAs@PN, Ru foil and RuCl3@HPN samples, N K edge (l) and P L edge (m) NEXAFS spectra, 31P solid state MAS NMR spectra at room temperature using a direct acquisition with proton decoupling of PN and RuβSAs@PN (n)[104] (1 ?=10 nm)

图14 AgP2 NCs、AgO、Ag2O和Ag箔的原位K边XANES(a)和EXAFS光谱(b), AgP2和Ag中CO、CO:H2、法拉第效率与Agδ+的关系(c), AgP2 NCs的Ag K边与拟合结果(d), 在RHE=-0.8 V恒电位下CRR, AgP2-NCs的Ag K边XANES谱(e), RHE=-0.8 V, AgO和Ag2O在测试前与CRR 50 s后的Ag-K边光谱(f)[38]
Fig. 14 In situ silver K-edge XANES (a) and EXAFS spectra (b) of AgP2 NCs, AgO, Ag2O, and Ag foil, Faradaic efficiency of the CO and CO:H2 ratio as a function of Agδ+ in AgP2 and Ag (c), linear combination of AgO, Ag2O, and Ag spectra (solid line) compared to the raw Ag K-edge (d), Ag K-edge XANES spectra of AgP2 NCs with respect to CRR time under a constant applied potential of -0.8 V (vs. RHE) (e), Ag K-edge spectra of AgO and Ag2O references before and after CRR at -0.8 V (vs. RHE) for 50 s (f)[38]

图15 Ni@Ni2P-Ru HNRs合成示意图(a), Ni@Ni2P-Ru、Ni@Ni2P和Ni箔的Ni K边XANES光谱图(b)和EXAFS光谱图(c), Ni@Ni2P-Ru和Ru箔的Ru K边XANES光谱图(d), Ni@Ni2P-Ru HNRs的Ru(0)和Ru(IV)分布(e)[108], Ni5P4-Ru和Ni5P4的Ni2p XPS谱图(f)和Ni L边XANES(g), Ni5P4-Ru、Ni5P4、Ni箔和NiO在Ni K边的XANES(h)和EXAFS光谱图(i), Ru箔、Ni5P4-Ru和RuO2的Ru K边XAFS光谱图(j)和EXAFS谱图(k)[97]
Fig. 15 Schematic illustration of the formation of Ni@Ni2P-Ru HNRs (a), Ni K-edge XANES spectra (b) and Fourier transformed k3-weighted EXAFS spectra of Ni@Ni2P-Ru, Ni@Ni2P, and reference Ni foil (c), Ru K-edge XANES spectra of Ni@Ni2P-Ru and reference Ru foil (d), dependence of the Ru(0) and Ru(IV) atomic fractions of the Ni@Ni2P-Ru HNRs as a function of photon energy (e)[108], Ni2p XPS comparison (f) and Ni L edge XANES comparison between Ni5P4-Ru and Ni5P4 (g), XANES (h) and EXAFS (i) results of Ni5P4-Ru, Ni5P4, Ni foil, and NiO at Ni K edge, respectively, Ru K-edge XAFS spectra (j) and the Ru K-edge k2-weighted EXAFS profiles of Ru foil, Ni5P4-Ru, and RuO2 (k)[97]
| [1] |
SHI Q, ZHU C, DU D, et al. Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem. Soc. Rev., 2019,48(12):3181-3192.
DOI URL PMID |
| [2] | LIU D, LI X, CHEN S, et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy, 2019,4(6):512-518. |
| [3] |
ZHANG E, WANG T, YU K, et al. Bismuth single atoms resulting from transformation of metal-organic frameworks and their use as electrocatalysts for CO2 reduction. J. Am. Chem. Soc., 2019,141(42):16569-16573.
DOI URL PMID |
| [4] | ZHAO S, LU X, WANG L, et al. Carbon based metal free catalysts for electrocatalytic reduction of nitrogen for synthesis of ammonia at ambient conditions. Adv. Mater., 2019,31(13):1805367. |
| [5] |
YE W, CHEN S, LIN Y, et al. Precisely tuning the number of Fe atoms in clusters on N-doped carbon toward acidic oxygen reduction reaction. Chem, 2019,5(11):2865-2878.
DOI URL |
| [6] |
WANG C, XIE H, CHEN S, et al. Atomic cobalt covalently engineered interlayers for superior lithium ion storage. Adv. Mater., 2018,30(32):1802525.
DOI URL |
| [7] |
SEH Z W, KIBSGAARD J, DICKENS C F, et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science, 2017, 355(6321): eaad4998.
DOI URL PMID |
| [8] | BAE S Y, MAHMOOD J, JEON I Y, et al. Recent advances in ruthenium-based electrocatalysts for the hydrogen evolution reaction. Nanoscale Horiz., 2020,5(1):43-56. |
| [9] | RUNDQVIST S. Phosphides of the platinum metals. Nature, 1960,185(4705):31-32. |
| [10] | CHAKRABARTTY S, BARMAN B K, RAJ C R. Nitrogen and phosphorous co-doped graphitic carbon encapsulated ultrafine OsP2 nanoparticles: a pH universal highly durable catalyst for hydrogen evolution reaction. Chem. Commun., 2019,55(30):4399-4402. |
| [11] | ALVARADO R L, BOSCAGLI C, SCHUNK S A. Platinum group metal phosphides as efficient catalysts in hydroprocessing and syngas-related catalysis. Catalysts, 2018,8(3):122. |
| [12] | CARENCO S, FLOREA I, ERSEN O, et al. Towards nanoscaled gold phosphides: surface passivation and growth of composite nanostructures. New J. Chem., 2013,37(4):1231-1237. |
| [13] | KANDA Y, KAWANISHI K, TSUJINO T, et al. Catalytic activities of noble metal phosphides for hydrogenation and hydrodesulfurization reactions. Catalysts, 2018,8(4):160. |
| [14] |
BELYKH L B, SKRIPOV N I, STERENCHUK T P, et al. Pd-P nanoparticles as active catalyst for the hydrogenation of acetylenic compounds. J. Nanopart. Res., 2019,21(9):198.
DOI URL |
| [15] |
DU X, CAI P, LUO W, et al. Facile synthesis of P-doped Rh nanoparticles with superior catalytic activity toward dehydrogenation of hydrous hydrazine. Int. J. Hydrog. Energy, 2017,42(9):6137-6143.
DOI URL |
| [16] |
BOWKER R H, SMITH M C, PEASE M L, et al. Synthesis and hydrodeoxygenation properties of ruthenium phosphide catalysts. ACS Catal., 2011,1(8):917-922.
DOI URL |
| [17] | HABAS S E, BADDOUR F G, RUDDY D A, et al. A facile molecular precursor route to metal phosphide nanoparticles and their evaluation as hydrodeoxygenation catalysts. Chem. Mat., 2015,27(22):7580-7592. |
| [18] |
SAMPATH A, CHANG S W A, FLAHERTY D W. Catalytic hydrogen transfer and decarbonylation of aromatic aldehydes on Ru and Ru phosphide model catalysts. J. Phys. Chem. C, 2018,122(41):23600-23609.
DOI URL |
| [19] | GUAN Q, SUN C, LI R, et al. The synthesis and investigation of ruthenium phosphide catalysts. Catal. Commun., 2011,14(1):114-117. |
| [20] |
LIU Y, MCCUE A J, MIAO C, et al. Palladium phosphide nanoparticles as highly selective catalysts for the selective hydrogenation of acetylene. J. Catal., 2018,364:406-414.
DOI URL |
| [21] | LI J S, LI J Y, HUANG M J, et al. Anchoring RuxP on 3D hollow graphene nanospheres as efficient and pH-universal electrocatalysts for the hydrogen evolution reaction. Carbon, 2020,161:44-50. |
| [22] |
YIN L, YANG T, DING X, et al. Synthesis of phosphorus-iridium nanocrystals and their superior electrocatalytic activity for oxygen evolution reaction. Electrochem. Commun., 2018,94:59-63.
DOI URL |
| [23] | DENG K, XU Y, YANG D, et al. Pt-Ni-P nanocages with surface porosity as efficient bifunctional electrocatalysts for oxygen reduction and methanol oxidation. J. Mater. Chem. A, 2018,7(16):9791-9797. |
| [24] |
YANG F, BAO X, GONG D, et al. Rhodium phosphide: a new type of hydrogen oxidation reaction catalyst with non linear correlated catalytic response to pH. ChemElectroChem, 2019,6(7):1990-1995.
DOI URL |
| [25] | XIE H, GENG Q, ZHU X, et al. PdP2 nanoparticles-reduced graphene oxide for electrocatalytic N2 conversion to NH3 under ambient conditions. J. Mater. Chem. A, 2019,7(43):24760-24764. |
| [26] |
ZHAO R, LIU C, ZHANG X, et al. An ultrasmall Ru2P nanoparticles-reduced graphene oxide hybrid: an efficient electrocatalyst for NH3 synthesis under ambient conditions. J. Mater. Chem. A, 2020,8(1):77-81.
DOI URL |
| [27] |
ZHANG K, WANG C, BIN D, et al. Fabrication of Pd/P nanoparticle networks with high activity for methanol oxidation. Catal. Sci. Technol., 2016,6(16):6441-6447.
DOI URL |
| [28] | LI T, FU G, SU J, et al. Carbon supported ultrafine gold phosphorus nanoparticles as highly efficient electrocatalyst for alkaline ethanol oxidation reaction. Electrochim. Acta, 2017,231:13-19. |
| [29] |
SONG H, CHENG Y, LI B, et al. Carbon dots and RuP2 nanohybrid as an efficient bifunctional catalyst for electrochemical hydrogen evolution reaction and hydrolysis of ammonia borane. ACS Sustain. Chem. Eng., 2020,8(9):3995-4002.
DOI URL |
| [30] | ZHAO Y, JIA N, WU X R, et al. Rhodium phosphide ultrathin nanosheets for hydrazine oxidation boosted electrochemical water splitting. Appl. Catal. B-Environ., 2020,270:118880. |
| [31] |
QIN Q, JANG H, CHEN L, et al . Low loading of RhxP and RuP on N, P codoped carbon as two trifunctional electrocatalysts for the oxygen and hydrogen electrode reactions. Adv. Energy Mater., 2018,8(29):1801478.
DOI URL |
| [32] |
QIN Q, JANG H, CHEN L, et al. Coupling a low loading of IrP2, PtP2, or Pd3P with heteroatom-doped nanocarbon for-overall water- splitting cells and zinc-air batteries. ACS Appl. Mater. Interfaces, 2019,11(18):16461-16473.
DOI URL PMID |
| [33] |
ZHANG M, HU R, LIU J, et al. AgP2/C as an anode for high rate performance lithium-ion batteries. J. Alloys Compd., 2018,762:246-253.
DOI URL |
| [34] |
GUO Z, LI J, QI H, et al. A highly reversible long life Li-CO2 battery with a RuP2 based catalytic cathode. Small, 2019,15(29):1803246.
DOI URL |
| [35] | SU J, ZHOU J, WANG L, et al. Synthesis and application of transition metal phosphides as electrocatalyst for water splitting. Sci. Bull., 2017,62(9):633-644. |
| [36] | CALLEJAS J F, READ C G, ROSKE C W, et al. Synthesis, characterization, and properties of metal phosphide catalysts for the hydrogen-evolution reaction. Chem. Mat., 2016,28(17):6017-6044. |
| [37] | YANG F, ZHAO Y, DU Y, et al. A monodisperse Rh2P-based electrocatalyst for highly efficient and pH universal hydrogen evolution reaction. Adv. Energy Mater., 2018,8(18):1703489. |
| [38] |
LI H, WEN P, ITANZE D S, et al. Colloidal silver diphosphide (AgP2) nanocrystals as low overpotential catalysts for CO2 reduction to tunable syngas. Nat. Commun., 2019,10(1):1-10.
DOI URL PMID |
| [39] |
KIM C, JEON H S, EOM T, et al. Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J. Am. Chem. Soc., 2015,137(43):13844-13850.
DOI URL PMID |
| [40] | LIU Z, LI Z, LI J, et al. Engineering of Ru/Ru2P interfaces superior to Pt active sites for catalysis of the alkaline hydrogen evolution reaction. J. Mater. Chem. A, 2019,7(10):5621-5625. |
| [41] | GUO R, BI W, ZHANG K, et al. Phosphorization treatment improves the catalytic activity and durability of platinum catalysts toward oxygen reduction reaction. Chem. Mat., 2019,31(19):8205-8211. |
| [42] |
TIANOU H, WANG W, YANG X, et al. Inflating hollow nanocrystals through a repeated Kirkendall cavitation process. Nat. Commun., 2017,8(1):1-9.
DOI URL PMID |
| [43] | SU W, SUN R, REN F, et al. Graphene supported palladium- phosphorus nanoparticles as a promising catalyst for ethylene glycol oxidation. Appl. Surf. Sci., 2019,491:735-741. |
| [44] | CHANG Q, MA J, ZHU Y, et al. Controllable synthesis of ruthenium phosphides (RuP and RuP2) for pH-universal hydrogen evolution reaction. ACS Sustain. Chem. Eng., 2018,6(5):6388-6394. |
| [45] | YU J, WU X, ZHANG H, et al. Core effect on the performance of N/P codoped carbon encapsulating noble-metal phosphide nanostructures for hydrogen evolution reaction. ACS Appl. Energy Mater., 2019,2(4):2645-2653. |
| [46] |
LIU T, WANG J, ZHONG C, et al. Benchmarking three ruthenium phosphide phases for electrocatalysis of the hydrogen evolution reaction: experimental and theoretical insights. Chem. -Eur. J., 2019,25(33):7826-7830.
URL PMID |
| [47] |
PU Z, LIU T, ZHAO W, et al. Versatile route to fabricate precious-metal phosphide electrocatalyst for acid-stable hydrogen oxidation and evolution reactions. ACS Appl. Mater. Interfaces, 2020,12(10):11737-11744.
DOI URL PMID |
| [48] | LUO Q, XU C, CHEN Q, et al. Synthesis of ultrafine ruthenium phosphide nanoparticles and nitrogen/phosphorus dual-doped carbon hybrids as advanced electrocatalysts for all-pH hydrogen evolution reaction. Int. J. Hydrog. Energy, 2019,44(47):25632-25641. |
| [49] |
JIN L, ZHANG X, ZHAO W, et al. General method for synthesizing transition-metal phosphide/N-doped carbon nanomaterials for hydrogen evolution. Langmuir, 2019,35(28):9161-9168.
DOI URL PMID |
| [50] | PU Z, ZHAO J, AMIINU I S, et al. A universal synthesis strategy for P-rich noble metal diphosphide-based electrocatalysts for the hydrogen evolution reaction. Energy Environ. Sci., 2019,12(3):952-957. |
| [51] |
PU Z, AMIINU I S, HE D, et al. Activating rhodium phosphide-based catalysts for the pH-universal hydrogen evolution reaction. Nanoscale, 2018,10(26):12407-12412.
DOI URL PMID |
| [52] | LI L, WANG K, HUANG Z, et al. Highly ordered graphene architectures by duplicating melamine sponges as a three-dimensional deformation-tolerant electrode. Nano Res., 2016,9(10):2938-2949. |
| [53] | LIU T, FENG B, WU X, et al. Ru2P nanoparticle decorated P/N-doped carbon nanofibers on carbon cloth as a robust hierarchical electrocatalyst with platinum-comparable activity toward hydrogen evolution. ACS Appl. Energy Mater., 2018,1(7):3143-3150. |
| [54] |
GE R, WANG S, SU J, et al. Phase-selective synthesis of self- supported RuP films for efficient hydrogen evolution electrocatalysis in alkaline media. Nanoscale, 2018,10(29):13930-13935.
DOI URL PMID |
| [55] | JIANG N, YOU B, SHENG M, et al. Electrodeposited cobalt phosphorous derived films as competent bifunctional catalysts for overall water splitting. Angew. Chem. Int. Ed., 2015,54(21):6251-6254. |
| [56] | KIM J, KIM H, AHN S H. Electrodeposited rhodium phosphide with high activity for hydrogen evolution reaction in acidic medium. ACS Sustain. Chem. Eng., 2019,7(16):14041-14050. |
| [57] | WANG Q, MING M, NIU S, et al. Scalable solid state synthesis of highly dispersed uncapped metal (Rh, Ru, Ir) nanoparticles for efficient hydrogen evolution. Adv. Energy Mater., 2018,8(31):1801698. |
| [58] |
ZHANG T Q, LIU J, HUANG L B, et al. Microbialphosphorus- enabled synthesis of phosphide nanocomposites for efficient electrocatalysts. J. Am. Chem. Soc., 2017,139(32):11248-11253.
DOI URL PMID |
| [59] | YU J, LI G, LIU H, et al. Ru-Ru2PΦNPC and NPC@RuO2 synthesized via environment friendly and solid phase phosphating process by saccharomycetes as n/p sources and carbon template for overall water splitting in acid electrolyte. Adv. Funct. Mater., 2019,29(22):1901154. |
| [60] | LI Y, DONG Z, JIAO L. Multifunctional transition metal based phosphides in energy related electrocatalysis. Adv. Energy Mater., 2020,10(11):1902104. |
| [61] | KIM J S, KIM B, KIM H, et al. Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv. Energy Mater, 2018,8(11):1702774. |
| [62] | LI Y, CHU F, BU Y, et al. Controllable fabrication of uniform ruthenium phosphide nanocrystals for the hydrogen evolution reaction. Chem. Commun., 2019,55(54):7828-7831. |
| [63] | HU C, MA Q, HUNG S F, et al. In situ electrochemical production of ultrathin nickel nanosheets for hydrogen evolution electrocatalysis. Chem, 2017,3(1):122-133. |
| [64] |
CHENG M, GENG H, YANG Y, et al. Optimization of the hydrogen adsorption free energy of Ru based catalysts towards high efficiency hydrogen evolution reaction at all pH. Chem. -Eur. J., 2019,25(36):8579-8584.
URL PMID |
| [65] | SU J, ZHAO H, FU W, et al. Fine rhodium phosphides nanoparticles embedded in N, P dual-doped carbon film: new efficient electrocatalysts for ambient nitrogen fixation. Appl. Catal. B-Environ., 2020,265:118589. |
| [66] |
DUAN H, LI D, TANG Y, et al. High-performance Rh2P electrocatalyst for efficient water splitting. J. Am. Chem. Soc., 2017,139(15):5494-5502.
DOI URL PMID |
| [67] |
WANG Y, LIU Z, LIU H X, et al. Electrochemical hydrogen evolution reaction efficiently catalyzed by Ru2P nanoparticles. ChemSusChem, 2018,11(16):2724-2729.
DOI URL PMID |
| [68] | SUN F, WANG Y, FANG L, et al. New vesicular carbon-based rhenium phosphides with all-pH range electrocatalytic hydrogen evolution activity. Appl. Catal. B-Environ., 2019,256:117851. |
| [69] | LUO F, ZHANG Q, YU X, et al. Palladium phosphide as a stable and efficient electrocatalyst for overall water splitting. Angew. Chem. Int. Ed., 2018,57(45):14862-14867. |
| [70] | JI X, LIU B, REN X, et al. P-doped Ag nanoparticles embedded in N-doped carbon nanoflake: an efficient electrocatalyst for the hydrogen evolution reaction. ACS Sustain. Chem. Eng., 2018,6(4):4499-4503. |
| [71] | WANG K, HUANG B, LIN F, et al. Wrinkled Rh2P nanosheets as superior pH universal electrocatalysts for hydrogen evolution catalysis. Adv. Energy Mater., 2018,8(27):1801891. |
| [72] | ZHAO J, PU Z, JIN H, et al. Phosphorous-doped carbon coordinated iridium diphosphide bifunctional catalyst with ultralow iridium amount for efficient all-pH-value hydrogen evolution and oxygen reduction reactions. J. Catal., 2020,383:244-253. |
| [73] | LI C, XU Y, YANG D, et al. Boosting electrocatalytic activities of pt-based mesoporous nanoparticles for overall water splitting by a facile Ni, P co-incorporation strategy. ACS Sustain. Chem. Eng., 2019,7(10):9709-9716. |
| [74] | OH S, KIM H, KWON Y, et al. Porous Co-P foam as an efficient bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater. Chem. A, 2016,4(47):18272-18277. |
| [75] | KIM J, LEE C W, KIM D, et al. Dynamic evolution of a hydroxylated layer in ruthenium phosphide electrocatalysts for an alkaline hydrogen evolution reaction. J. Mater. Chem. A, 2020,8(11):5655-5662. |
| [76] | ZHU J, CHEN Z, XIE M, et al. Iridium based cubic nanocage with 1.1 nm thick walls: a highly efficient and durable electrocatalyst for water oxidation in an acidic medium. Angew. Chem. Int. Ed., 2019,58(22):7244-7248. |
| [77] | ZHAI T, ZHU Y, LI Y, et al. Ultra-small RuPx nanoparticles on graphene supported schiff-based networks for all pH hydrogen evolution. Int. J. Hydrog. Energy, 2019,44(12):5717-5724. |
| [78] | CHI J Q, ZENG X J, SHANG X, et al. Embedding RhPx in N, P Co doped carbon nanoshells through synergetic phosphorization and pyrolysis for efficient hydrogenevolution. Adv. Funct. Mater., 2019,29(33):1901790. |
| [79] | CHEN W, PEI J, HE C T, et al. Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction. Angew. Chem. Int. Ed., 2017,56(50):16086-16090. |
| [80] | PAN Y, LIU Y, ZHAO J, et al. Monodispersed nickel phosphide nanocrystals with different phases: synthesis, characterization and electrocatalytic properties for hydrogen evolution. J. Mater. Chem. A, 2015,3(4):1656-1665. |
| [81] | LIU T, WANG S, ZHANG Q, et al. Ultrasmall Ru2P nanoparticles on graphene: a highly efficient hydrogen evolution reaction electrocatalyst in both acidic and alkaline media. Chem. Commun., 2018,54(27):3343-3346. |
| [82] | YANG Y, LUO M, ZHANG W, et al. Metal surface and interface energy electrocatalysis: fundamentals, performance engineering, and opportunities. Chem, 2018,4(9):2054-2083. |
| [83] | ZHANG J, ZHANG Q, FENG X. Support and interface effects in water splitting electrocatalysts. Adv. Mater., 2019,31(31):1808167. |
| [84] | WANG J, WEI Z, MAO S, et al. Highly uniform Ru nanoparticles over N-doped carbon: pH and temperature-universal hydrogen release from water reduction. Energy Environ. Sci., 2018,11(4):800-806. |
| [85] | WANG L, ZHOU Q, PU Z, et al. Surface reconstruction engineering of cobalt phosphides by Ru inducement to form hollow Ru-RuPx-CoxP pre-electrocatalysts with accelerated oxygen evolution reaction. Nano Energy, 2018,53:270-276. |
| [86] |
LI J, GUAN Q, WU H, et al. Highly active and stable metal single-atom catalysts achieved by strong electronic metal-support interactions. J. Am. Chem. Soc., 2019,141(37):14515-14519.
DOI URL PMID |
| [87] | LIU H, HU K, YAN D, et al. Recent advances on black phosphorus for energy storage, catalysis, and sensor applications. Adv. Mater., 2018,30(32):1800295. |
| [88] | WANG X, BAI L, LU J, et al. Rapid activation of platinum with black phosphorus for efficient hydrogen evolution. Angew. Chem. Int. Ed., 2019,58(52):19060-19066. |
| [89] | WANG J, CHEN J, WANG P, et al. Robust photocatalytic hydrogen evolution over amorphous ruthenium phosphide quantum dots modified g-C3N4 nanosheet. Appl. Catal. B-Environ., 2018,239:578-585. |
| [90] | DONG H, XIAO M, YU S, et al. Insight into the activity and stability of RhxP nano-species supported on g-C3N4 for photocatalytic H2 production. ACS Catal., 2019,10(1):458-462. |
| [91] | LIU D, NI K, YE J, et al. Tailoring the structure of carbon nanomaterials toward high end energy applications. Adv. Mater., 2018,30(48):1802104. |
| [92] | YU J, GUO Y, SHE S, et al. Bigger is surprisingly better: agglomerates of larger RuP nanoparticles outperform benchmark Pt nanocatalysts for the hydrogen evolution reaction. Adv. Mater., 2018,30(39):1800047. |
| [93] | PU Z, AMIINU I S, KOU Z, et al. RuP2-based catalysts with platinum-like activity and higher durability for the hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed., 2017,56(38):11559-11564. |
| [94] | FANG L, WANG Y, YANG X, et al. Uniform OsP2 nanoparticles anchored on N, P-doped carbon: a new electrocatalyst with enhanced activity for hydrogen generation at all pH values. J. Catal., 2019,370:404-411. |
| [95] | ZHAO Y, HUANG S, XIA M, et al. NPO co-doped high performance 3D graphene prepared through red phosphorous- assisted “cutting-thin” technique: a universal synthesis and multifunctional applications. Nano Energy, 2016,28:346-355. |
| [96] |
SI C D, WU Z X, WANG J, et al. Enhanced the hydrogen evolution performance by ruthenium nanoparticles doped into cobalt phosphide nanocages. ACS Sustain. Chem. Eng., 2019,7(11):9737-9742.
DOI URL |
| [97] | HE Q, TIAN D, JIANG H. Achieving efficient alkaline hydrogen evolution reaction over a Ni5P4 catalyst incorporating single-atomic Ru sites. Adv. Mater., 2020,32(11):1906972. |
| [98] | CHEN D, PU Z, LU R, J, et al. Ultralow Ru loading transition metal phosphides as high-efficient bifunctional electrocatalyst for a solar to hydrogen generation system. Adv. Energy Mater., 2020,10:2000814. |
| [99] | QU M, JIANG Y, YANG M, et al. Regulating electron density of NiFe-P nanosheets electrocatalysts by a trifle of Ru for high- efficient overall water splitting. Appl. Catal. B-Environ., 2020,263:118324. |
| [100] |
VELASCO-VELEZ J J, WU C H, PASCAL T A, et al. The structure of interfacial water on gold electrodes studied by X-ray absorption spectroscopy. Science, 2014,346(6211):831-834.
DOI URL PMID |
| [101] | BENFATTO M, CONGIU-CASTELLANO A, DANIELE A, et al. MXAN: a new software procedure to perform geometrical fitting of experimental XANES spectra. J. Synchrot. Radiat., 2001,8(2):267-269. |
| [102] | ZHANG H, LI X, JIANG Z. Probe active sites of heterogeneous electrocatalysts by X-ray absorption spectroscopy: from single atom to complex multi-element composites. Curr. Opin. Electrochem., 2019,14:7-15. |
| [103] | ZHOU P, LI N, CHAO Y, et al. Thermolysis of noble metal nanoparticles into electron rich phosphorus coordinated noble metal single atoms at low temperature. Angew. Chem. Int. Ed., 2019,131(40):14322-14326. |
| [104] | YANG J, CHEN B, LIU X, et al. Efficient and robust hydrogen evolution: phosphorus nitride imide nanotubes as supports for anchoring single ruthenium sites. Angew. Chem. Int. Ed., 2018,57(30):9495-9500. |
| [105] | JIANG H, HE Q, ZHANG Y, et al. Structural self reconstruction of catalysts in electrocatalysis. Accounts Chem. Res., 2018,51(11):2968-2977. |
| [106] |
GORLIN Y, LASSALLE-KAISER B, BENCK J D, et al. In situ X-ray absorption spectroscopy investigation of a bifunctional manganese oxide catalyst with high activity for electrochemical water oxidation and oxygen reduction. J. Am. Chem. Soc., 2013,135(23):8525-8534.
DOI URL PMID |
| [107] | JIANG H, HE Q, LI X, et al. Tracking structural self reconstruction and identifying true active sites toward cobalt oxychloride precatalyst of oxygen evolution reaction. Adv. Mater., 2019,31(8):1805127. |
| [108] |
LIU Y, LIU S, WANG Y, et al. Ru modulation effects in the synthesis of unique rod-like Ni@Ni2P-Ru heterostructures and their remarkable electrocatalytic hydrogen evolution performance. J. Am. Chem. Soc., 2018,140(8):2731-2734.
DOI URL PMID |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [8] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [9] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [10] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [11] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [12] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [13] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [14] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| [15] | 周帆, 田志林, 李斌. 热防护系统用碳化物超高温陶瓷抗烧蚀涂层研究进展[J]. 无机材料学报, 2025, 40(1): 1-16. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||