无机材料学报 ›› 2020, Vol. 35 ›› Issue (10): 1117-1122.DOI: 10.15541/jim20190588 CSTR: 32189.14.10.15541/jim20190588
所属专题: 计算材料论文精选(2020)
林启民1( ),崔建功2,颜鑫1,袁学光1(
),崔建功2,颜鑫1,袁学光1( ),陈小瑜1,芦启超1,罗彦彬1,黄雪3,张霞1(
),陈小瑜1,芦启超1,罗彦彬1,黄雪3,张霞1( ),任晓敏1
),任晓敏1
收稿日期:2019-11-20
修回日期:2019-12-09
出版日期:2020-10-20
网络出版日期:2020-01-20
作者简介:林启民, 男, 博士研究生. E-mail:lqm@bupt.edu.cn.
基金资助:
LIN Qimin1( ),CUI Jiangong2,YAN Xin1,YUAN Xueguang1(
),CUI Jiangong2,YAN Xin1,YUAN Xueguang1( ),CHEN Xiaoyu1,LU Qichao1,LUO Yanbin1,HUANG Xue3,ZHANG Xia1(
),CHEN Xiaoyu1,LU Qichao1,LUO Yanbin1,HUANG Xue3,ZHANG Xia1( ),REN Xiaomin1
),REN Xiaomin1
Received:2019-11-20
Revised:2019-12-09
Published:2020-10-20
Online:2020-01-20
About author:LIN Qimin, male, PhD candidate. E-mail: lqm@bupt.edu.cn
Supported by:摘要:
本研究采用基于密度泛函理论的第一性原理方法, 在局域密度近似和广义梯度近似下, 研究了单点缺陷下不同结构氧化石墨烯的电子结构和光学特性。研究结果表明: 文中四种构型的氧化石墨烯为力学稳定结构, 其中包含不饱和氧原子的氧化石墨烯结构在水裂解及制氢中具有重要应用潜力。能带及分波态密度计算结果表明, 包含不饱和氧原子的构型为间接带隙半导体, 其余构型均为直接带隙半导体, 且掺杂类型和带隙值随结构不同而改变。氧化石墨烯的光学吸收表现为各向异性, 且在垂直于平面方向上的吸收边蓝移到近紫外可见光区。包含sp 3杂化形式的结构光学吸收系数比包含sp 2杂化的结构高, 说明碳氧双键和悬挂键的存在对吸收光谱有重要影响。
中图分类号:
林启民, 崔建功, 颜鑫, 袁学光, 陈小瑜, 芦启超, 罗彦彬, 黄雪, 张霞, 任晓敏. 单点缺陷氧化石墨烯电子结构与光学特性的第一性原理研究[J]. 无机材料学报, 2020, 35(10): 1117-1122.
LIN Qimin, CUI Jiangong, YAN Xin, YUAN Xueguang, CHEN Xiaoyu, LU Qichao, LUO Yanbin, HUANG Xue, ZHANG Xia, REN Xiaomin. First-principles Study on Electronic Structure and Optical Properties of Single Point Defect Graphene Oxide[J]. Journal of Inorganic Materials, 2020, 35(10): 1117-1122.
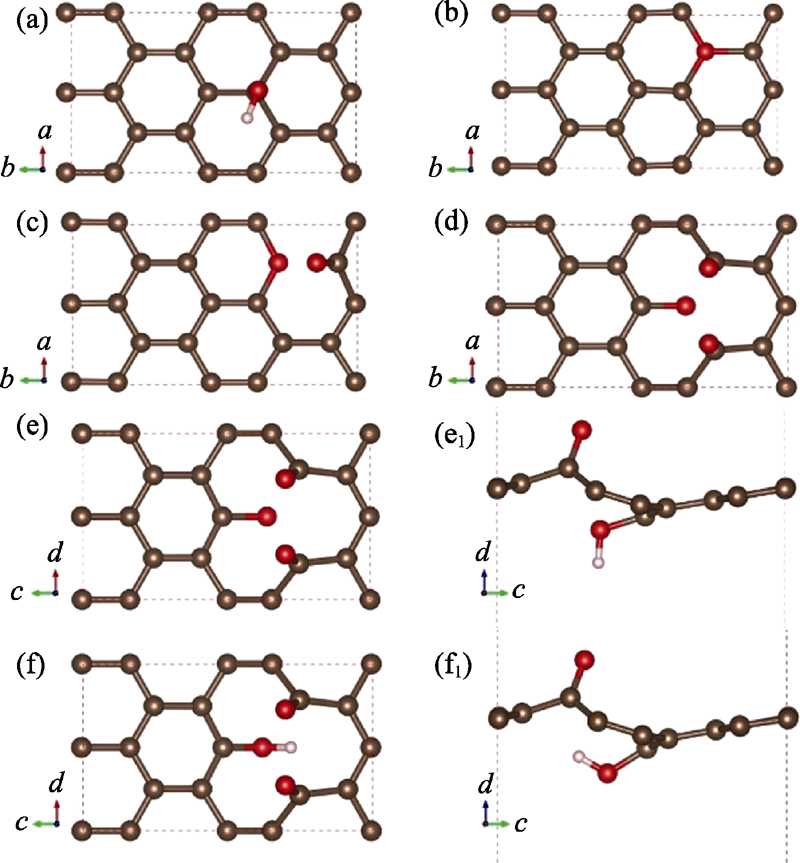
图1 不同构型氧化石墨烯结构
Fig. 1 Different types of graphene oxide structures (a) Graphene oxide adsorbed with hydroxyl; (b) Graphene oxide with single substitution epoxy bond; (c) Graphene oxide with carbon oxygen double bond and sp3 hybrid epoxy bond; (d) Graphene oxide with two carbon-oxygen double bonds and one carbon-oxygen single bond; (e, f) and (e1, f1) Top and side views of the structure in (d) adsorbed with hydrogen on the upper and lower side of the suspended oxygen atom, respectively
| * | O1-C2 | O2-C6 | O3-C12 | |
|---|---|---|---|---|
| LDA | d1 | 0.128 | 0.128 | 0.137 |
| d2 | 0.128 | 0.128 | 0.136 | |
| e | 0.124 | 0.124 | 0.138 | |
| f | 0.124 | 0.124 | 0.137 | |
| GGA | d | 0.124 | 0.124 | 0.133 |
| e | 0.122 | 0.122 | 0.139 | |
| f | 0.122 | 0.122 | 0.138 |
表1 不同泛函计算的不同结构中的键长(nm)
Table 1 Bond length in different structures calculated by different pseudopotential functions (nm)
| * | O1-C2 | O2-C6 | O3-C12 | |
|---|---|---|---|---|
| LDA | d1 | 0.128 | 0.128 | 0.137 |
| d2 | 0.128 | 0.128 | 0.136 | |
| e | 0.124 | 0.124 | 0.138 | |
| f | 0.124 | 0.124 | 0.137 | |
| GGA | d | 0.124 | 0.124 | 0.133 |
| e | 0.122 | 0.122 | 0.139 | |
| f | 0.122 | 0.122 | 0.138 |
| * | (a) | (b) | (c) | (d) | (e) | (f) |
|---|---|---|---|---|---|---|
| LDA | -12.7 | -6.1 | -6.5 | -13.1 | -19.6 | -18.6 |
表2 各氧化石墨烯结构形成能(eV)
Table 2 Formation energy with different structures (eV)
| * | (a) | (b) | (c) | (d) | (e) | (f) |
|---|---|---|---|---|---|---|
| LDA | -12.7 | -6.1 | -6.5 | -13.1 | -19.6 | -18.6 |
| * | C11 | C22 | C12 | C66 |
|---|---|---|---|---|
| a | 1738.99 | 1618.45 | 282.75 | 1.03 |
| b | 1789.57 | 1761.01 | 309.15 | 1.63 |
| c | 1607.47 | 958.73 | 163.94 | 0.99 |
| d | 2015.11 | 1405.41 | 147.00 | 1.39 |
| e | 1968.65 | 825.43 | 148.48 | -11.90 |
| f | 1846.90 | 710.38 | 240.54 | -8.83 |
表3 不同结构形式氧化石墨烯的弹性系数
Table 3 Elastic coefficients of graphene oxide with different structures
| * | C11 | C22 | C12 | C66 |
|---|---|---|---|---|
| a | 1738.99 | 1618.45 | 282.75 | 1.03 |
| b | 1789.57 | 1761.01 | 309.15 | 1.63 |
| c | 1607.47 | 958.73 | 163.94 | 0.99 |
| d | 2015.11 | 1405.41 | 147.00 | 1.39 |
| e | 1968.65 | 825.43 | 148.48 | -11.90 |
| f | 1846.90 | 710.38 | 240.54 | -8.83 |
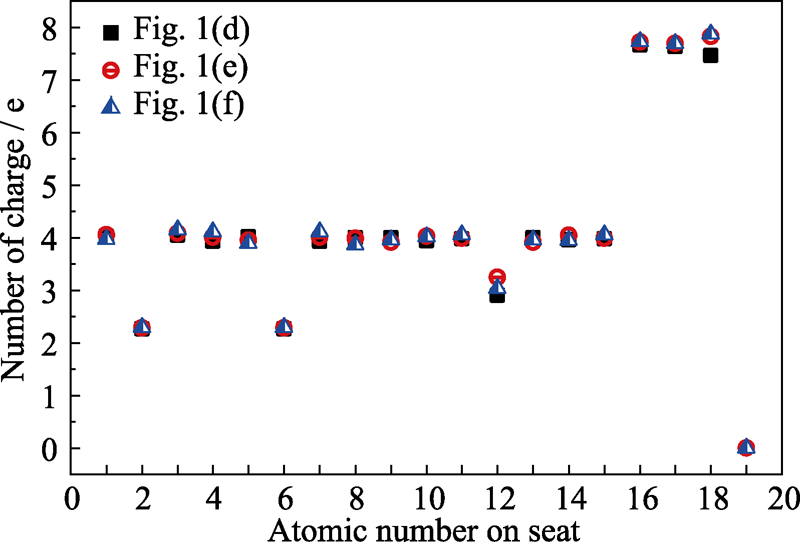
图2 三种结构中不同原子的电荷数
Fig. 2 The charge number of three kinds of different atoms in three structures showing in Fig. 1(d), (e) and (f) (d) Graphene oxide with two carbon-oxygen double bonds and one carbon-oxygen single bond; (e, f) Structure (d) adsorbed with hydrogen on the upper and lower side of the suspended oxygen atom, respectively
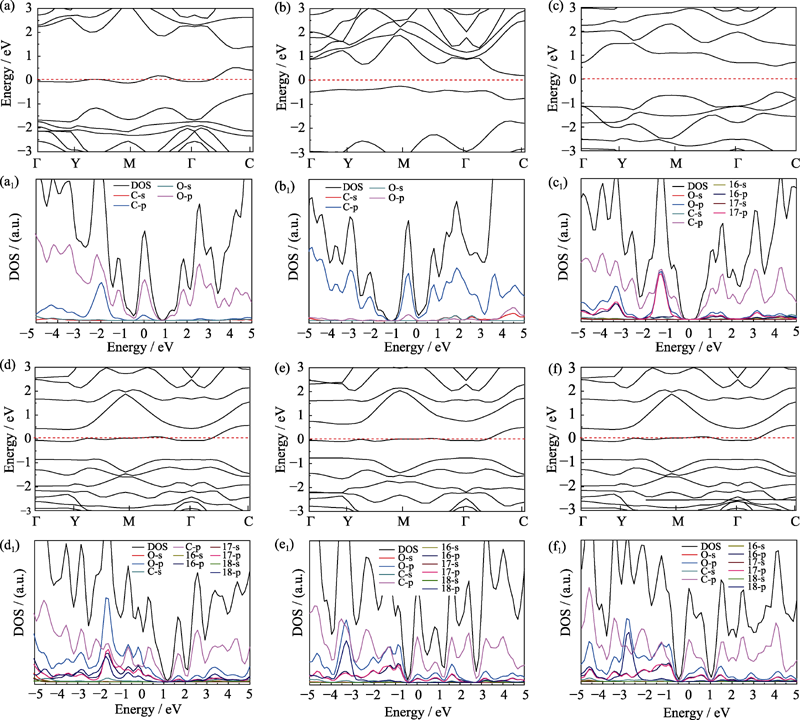
图3 各结构能带结构及态密度图
Fig. 3 Band structures and density of states (DOS) of different structure models (a) Graphene oxide adsorbed with hydroxyl; (b) Graphene oxide with single substitution epoxy bond; (c) Graphene oxide with carbon oxygen double bond and sp3 hybrid epoxy bond; (d) Graphene oxide with two carbon-oxygen double bonds and one carbon-oxygen single bond; (e,f) Structure (d) adsorbed with hydrogen on the upper and lower side of the suspended oxygen atom, respectively
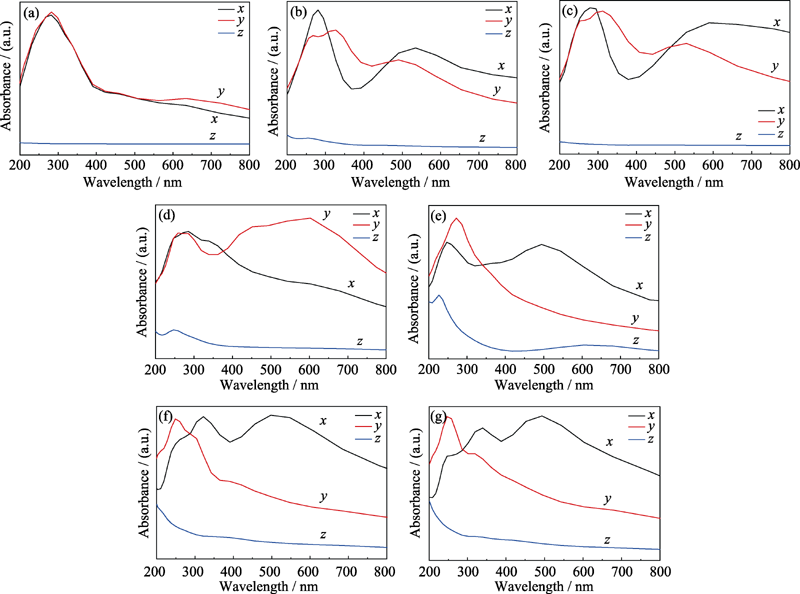
图4 各结构光学吸收系数
Fig. 4 Absorption coefficient of different structure models in which (b-g) the absorption coefficient of graphene and structures in Fig. 1(a-f) (a) Graphene; (b) Graphene oxide adsorbed with hydroxyl; (c) Graphene oxide with single substitution epoxy bond; (d) Graphene oxide with carbon oxygen double bond and sp3 hybrid epoxy bond; (e) Graphene oxide with two carbon-oxygen double bonds and one carbon-oxygen single bond; (f, g) Structure (d) adsorbed with hydrogen on the upper and lower side of the suspended oxygen atom, respectively
| [1] | YANG K, SHUAI X R, YANG H C , et al. Electrochemical performance of activated graphene powder supercapacitors using a room temperature ionic lLiquid electrolyte. Acta Phys. -Chim. Sin., 2019,35(7):755-765. |
| [2] |
PERES N M R . The Transport properties of graphene. J. Phys. Condens. Matter., 2009,21(32):323201-323210.
DOI URL PMID |
| [3] | CHEN D M . Variation of graphene Raman G peak splitting with strain. Acta. Phys. Sin., 2010,59(9):6399-6404. |
| [4] | WANG Y F, LI X W . First-principle calculation on electronic structures and optical properties of hybrid graphene and BiOI nanosheets. Acta. Phys. Sin., 2018,67(11):168-175. |
| [5] | WANG J J, WANG F, YUAN P F , et al. First-principles study of nanoscale friction between graphenes. Acta Phys. Sin., 2012,61(10):337-343. |
| [6] | JOSHI R K, ALWARAPPAN S, YOSHIMURA M , et al. Graphene oxide: the new membrane material. Applied Materials Today, 2015,1:1-12. |
| [7] |
GAO W, SINGH N, SONG L , et al. Direct laser writing of micro- supercapacitors on hydrated graphite oxide films. Nature Nanotechnology, 6:496-500.
DOI URL PMID |
| [8] | WANG G X, PEI Z B, YE C H , et al. Inkjet-printing and performance investigation of self-powered flexible graphene oxide humidity sensors. Journal of Inorganic Materials , 2019,34(1):114-120. |
| [9] | HUANG J R, WANG L Y, SHI C C , et al. Selective detection of picric acid using functionalized reduced graphene oxide sensor device. Sensors & Actuators B Chemical, 196:567-573. |
| [10] | LI C, CAI L, LI W W , et al. Adsorption of NO2 by hydrazine hydrate-reduced graphene oxide. Acta Phys. Sin., 2019,68(11):257-262. |
| [11] | PENG P, LIU H T, WU B , et al. Nitrogen doped graphene with a p-type field-effect and its fine modulation. Acta Phys. Chim. Sin., 2019,35(11):1282-1290. |
| [12] | CHU C, ZHANG J, BEI Z , et al. Hydrogen adsorption of Mg- doped Graphene oxide: afirst-principles study. Journal of Physical Chemistry C , 2013,117:4337-4344. |
| [13] |
ROGERS G W, LIU J Z . High-performance graphene oxide electromechanical actuators. Journal of the American Chemical Society, 2012,134:1250-1255.
DOI URL PMID |
| [14] | ZHU Y, MURALI S, CAI W , et al. Graphene and graphene oxide: synthesis, properties, and applications. Cheminform, 2010,22:3906-3924. |
| [15] |
KIM S, ZHOU S, HU, Y, et al. Room-temperature metastability of multilayer graphene oxide films. Nature Materials , 2012,11(6):544-549.
DOI URL PMID |
| [16] | ZHAO H, ZHOU L, WEI D , et al. Effects of external electric field on hydrogen storage performance of Li-decorated graphene oxide. Chemical Journal of Chinese Universities, 37(1):100-107. |
| [17] |
LOH K P, BAO Q, EDA G , et al. Graphene oxide as a chemically tunable platform for optical applications. Nature Chemistry, 2:1015-1024.
DOI URL PMID |
| [18] | ZHANG Q, ZHANG H, CHENG X L . Highly stable two-dimensional graphene oxide: electronic properties of its periodic structure and optical properties of its nanostructures. Chinese Physics B, 27(2): 027301-1-7. |
| [19] |
BAE S, KIM H, LEE Y , et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol, 2010,5:574-578.
DOI URL PMID |
| [20] | COMPTON, O C, NGUYEN, S B T. Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small, 2010,6:711-723. |
| [21] | HONG F, ZHOU L Q, HUANG Y , et al. Synthesis and characterization of graphene by improved hummers method. Chemistry & Bioengineering, 2012,29:31-33. |
| [22] |
CAI W W, PINER R D, STADERMANN F J , et al. Synthesis and solid-state NMR structural characterization of 13c-labeled graphite oxide. Science, 2008,321:1815-1817
DOI URL PMID |
| [23] | MO J W, QIU Y W, YI R B , et al. Temperature-dependent properties of metastable graphene oxide. Acta Phys. Sin. , 2019,68(15):284-292. |
| [24] |
YAN J A, XIAN L, CHOU M Y. Structural and electronic properties of oxidized graphene. Phys. Rev. Lett., 2009,103: 086802-1-4.
DOI URL PMID |
| [25] | WANG L, SUN Y Y, LEE K , et al. Stability of graphene oxide phases from firstp calculations. Physical Review B 2010, 82: 161406-1-4. |
| [26] | PENG Y, LI J . Ammonia adsorption on graphene and graphene oxide: a first-principles study. Frontiers of Environmental Science & Engineering, 2013,7:403-411. |
| [27] | ZHANG Y, SHI Y M, BAO Y Z , et al. Effect of surface passivation on the electronic properties of GaAs nanowire: A first-principle study. Acta Phys. Sin. , 2017,66(19):295-301. |
| [28] | YI W C, HU T, SU T , et al. A CNH monolayer: a direct gap 2d semiconductor with anisotropic electronic and optical properties. Journal of Materials Chemistry C , 2017,5:8498-8503. |
| [29] | LIN Q M, ZHANG X, LU Q C , , et al. First-principles study on structural stability of graphene oxide. First-principles study on structural stability of graphene oxide and catalytic activity of nitric acid. Acta Phys. Sin., 2019, 68(24): 247302-1-6. |
| [1] | 王悦, 王欣, 于显利. 室温铁磁性还原氧化石墨烯基全碳膜[J]. 无机材料学报, 2025, 40(3): 305-313. |
| [2] | 吴玉豪, 彭仁赐, 程春玉, 杨丽, 周益春. HfxTa1-xC体系力学性能及熔化曲线的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 761-768. |
| [3] | 靳宇翔, 宋二红, 朱永福. 3d过渡金属单原子掺杂石墨烯缺陷电催化还原CO2的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 845-852. |
| [4] | 王伟华, 张磊宁, 丁峰, 代兵, 韩杰才, 朱嘉琦, 贾怡, 杨宇. 铱衬底上金刚石外延形核与生长: 第一性原理计算[J]. 无机材料学报, 2024, 39(4): 416-422. |
| [5] | 陈浩, 樊文浩, 安德成, 陈少平. 能带优化和载流子调控改善SnTe的热电性能[J]. 无机材料学报, 2024, 39(3): 306-312. |
| [6] | 张宇晨, 陆知遥, 赫晓东, 宋广平, 朱春城, 郑永挺, 柏跃磊. 硫族MAX相硼化物的物相稳定性和性能预测[J]. 无机材料学报, 2024, 39(2): 225-232. |
| [7] | 周靖渝, 李兴宇, 赵晓琳, 王有伟, 宋二红, 刘建军. Ti和Cu掺杂β-NaMnO2正极材料:钠离子电池的倍率和循环性能[J]. 无机材料学报, 2024, 39(12): 1404-1412. |
| [8] | 陈梦杰, 王倩倩, 吴成铁, 黄健. 基于DFT的描述符预测生物陶瓷的降解性[J]. 无机材料学报, 2024, 39(10): 1175-1181. |
| [9] | 周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158. |
| [10] | 吴晓维, 张涵, 曾彪, 明辰, 孙宜阳. 杂化泛函HSE和PBE0计算CsPbI3缺陷性质的比较研究[J]. 无机材料学报, 2023, 38(9): 1110-1116. |
| [11] | 董怡曼, 谭占鳌. 宽带隙钙钛矿基二端叠层太阳电池复合层的研究进展[J]. 无机材料学报, 2023, 38(9): 1031-1043. |
| [12] | 张守超, 陈洪雨, 刘洪飞, 杨羽, 李欣, 刘德峰. 6H-SiC中子辐照肿胀高温回复及光学特性研究[J]. 无机材料学报, 2023, 38(6): 678-686. |
| [13] | 杨颖康, 邵怡晴, 李柏良, 吕志伟, 王路路, 王亮君, 曹逊, 吴宇宁, 黄荣, 杨长. Cl掺杂对CuI薄膜发光性能增强研究[J]. 无机材料学报, 2023, 38(6): 687-692. |
| [14] | 邓陶丽, 陈河莘, 黑玲丽, 李淑星, 解荣军. 第二相引入荧光转换材料实现激光驱动高均匀性白光光源[J]. 无机材料学报, 2022, 37(8): 891-896. |
| [15] | 文志勤, 黄彬荣, 卢涛仪, 邹正光. 压力对PbTiO3结构和热物性质影响的第一性原理研究[J]. 无机材料学报, 2022, 37(7): 787-794. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||