无机材料学报 ›› 2024, Vol. 39 ›› Issue (7): 845-852.DOI: 10.15541/jim20230549 CSTR: 32189.14.10.15541/jim20230549
所属专题: 【材料计算】材料模拟计算(202506); 【能源环境】CO2绿色转换(202506)
• 研究快报 • 上一篇
收稿日期:2023-11-30
修回日期:2024-02-06
出版日期:2024-07-20
网络出版日期:2024-03-05
通讯作者:
朱永福, 教授. E-mail: yfzhu@jlu.edu.cn;作者简介:靳宇翔(1999-), 男, 硕士研究生. E-mail: jinyx21@mails.jlu.edu.cn
JIN Yuxiang1( ), SONG Erhong2(
), SONG Erhong2( ), ZHU Yongfu1(
), ZHU Yongfu1( )
)
Received:2023-11-30
Revised:2024-02-06
Published:2024-07-20
Online:2024-03-05
Contact:
ZHU Yongfu, professor. E-mail: yfzhu@jlu.edu.cn;About author:JIN Yuxiang (1999-), male, Master candidate. E-mail: jinyx21@mails.jlu.edu.cn
Supported by:摘要:
将CO2高效转化为有价值的化学品(如CO和HCOOH等)是缓解环境问题、实现碳中和的重要措施。然而CO2还原反应(CO2RR)有着产物多样和路径复杂的特点, 再加上目前难以确定影响CO2RR活性的真正因素, 使得设计对特定产物有高选择性和高活性的催化剂十分具有挑战性。本研究从第一性原理出发, 系统研究了3d过渡金属单原子掺杂石墨烯单个空位(TM@CSV)和双空位(TM@CDV)电催化还原CO2的潜力, 具体涵盖基底的稳定性、中间产物热力学吸附以及与之竞争的析氢反应(HER)。通过对Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu和Zn掺杂石墨烯缺陷后形成的20种催化剂进行筛选, 发现Sc原子掺杂石墨烯单个空位的Sc@CSV和Sc、Ti原子掺杂石墨烯双空位的Sc@CDV和Ti@CDV同时具备吸附CO2分子和抑制HER的能力。其中Sc@CDV对HCOOH表现出最佳的活性和选择性, 速率决定步骤的吉布斯自由能差仅为0.96 eV。最后, 通过电子结构分析进一步揭示了Sc@CDV优于其他催化剂的原因是Sc@CDV调整了费米能级附近的活性电子态, 从而实现对CO2的高效还原。
中图分类号:
靳宇翔, 宋二红, 朱永福. 3d过渡金属单原子掺杂石墨烯缺陷电催化还原CO2的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 845-852.
JIN Yuxiang, SONG Erhong, ZHU Yongfu. First-principles Investigation of Single 3d Transition Metals Doping Graphene Vacancies for CO2 Electroreduction[J]. Journal of Inorganic Materials, 2024, 39(7): 845-852.
| TM@CSV | Max length of TM-C/Å | Bader charge/|e| |
|---|---|---|
| Sc@CSV | 2.08 | +1.50 |
| Ti@CSV | 1.94 | +1.37 |
| V@CSV | 1.89 | +1.22 |
| Cr@CSV | 1.86 | +1.35 |
| Mn@CSV | 1.83 | +1.00 |
| Fe@CSV | 1.76 | +0.67 |
| Co@CSV | 1.76 | +0.72 |
| Ni@CSV | 1.79 | +0.63 |
| Cu@CSV | 1.89 | +0.02 |
| Zn@CSV | 1.96 | +0.76 |
Table S1 Max lengthes of TM-C and Bader charges of TM for TM@CSV
| TM@CSV | Max length of TM-C/Å | Bader charge/|e| |
|---|---|---|
| Sc@CSV | 2.08 | +1.50 |
| Ti@CSV | 1.94 | +1.37 |
| V@CSV | 1.89 | +1.22 |
| Cr@CSV | 1.86 | +1.35 |
| Mn@CSV | 1.83 | +1.00 |
| Fe@CSV | 1.76 | +0.67 |
| Co@CSV | 1.76 | +0.72 |
| Ni@CSV | 1.79 | +0.63 |
| Cu@CSV | 1.89 | +0.02 |
| Zn@CSV | 1.96 | +0.76 |
| TM@CDV | Max length of TM-C/Å | Bader charge/|e| |
|---|---|---|
| Sc@CDV | 2.24 | +1.32 |
| Ti@CDV | 2.07 | +0.94 |
| V@CDV | 2.02 | +1.22 |
| Cr@CDV | 2.01 | +0.93 |
| Mn@CDV | 1.99 | +0.64 |
| Fe@CDV | 1.97 | +0.61 |
| Co@CDV | 1.95 | +0.34 |
| Ni@CDV | 1.91 | +0.18 |
| Cu@CDV | 1.90 | +0.18 |
| Zn@CDV | 1.93 | +0.10 |
Table S2 Max lengthes of TM-C and Bader charges of TM for TM@CDV
| TM@CDV | Max length of TM-C/Å | Bader charge/|e| |
|---|---|---|
| Sc@CDV | 2.24 | +1.32 |
| Ti@CDV | 2.07 | +0.94 |
| V@CDV | 2.02 | +1.22 |
| Cr@CDV | 2.01 | +0.93 |
| Mn@CDV | 1.99 | +0.64 |
| Fe@CDV | 1.97 | +0.61 |
| Co@CDV | 1.95 | +0.34 |
| Ni@CDV | 1.91 | +0.18 |
| Cu@CDV | 1.90 | +0.18 |
| Zn@CDV | 1.93 | +0.10 |

Fig. 1 Analyses for Sc@CDV (a) Structure of Sc@CDV; (b) Charge density difference of Sc@CDV with an isovalue of 0.005 e/Å3 (positive and negative charges are shown in green and yellow); (c) PDOS diagram of Sc@CDV; (d) AIMD simulations of Sc@CDV (purple: Sc, brown: C); Colorful figures are available on website
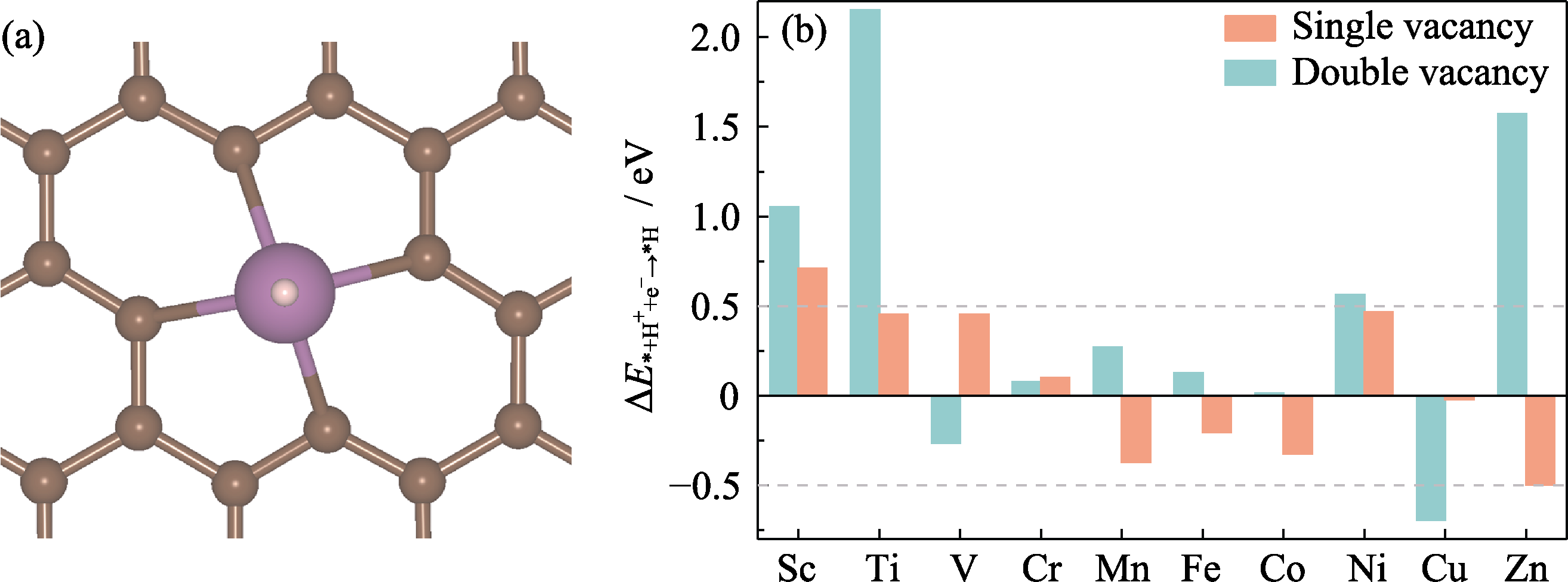
Fig. 2 Structure of H+ adsorption on Sc@CDV (a) and energy changes of H+ adsorption on TM@C (b) Green in (a): H; Colorful figures are available on website
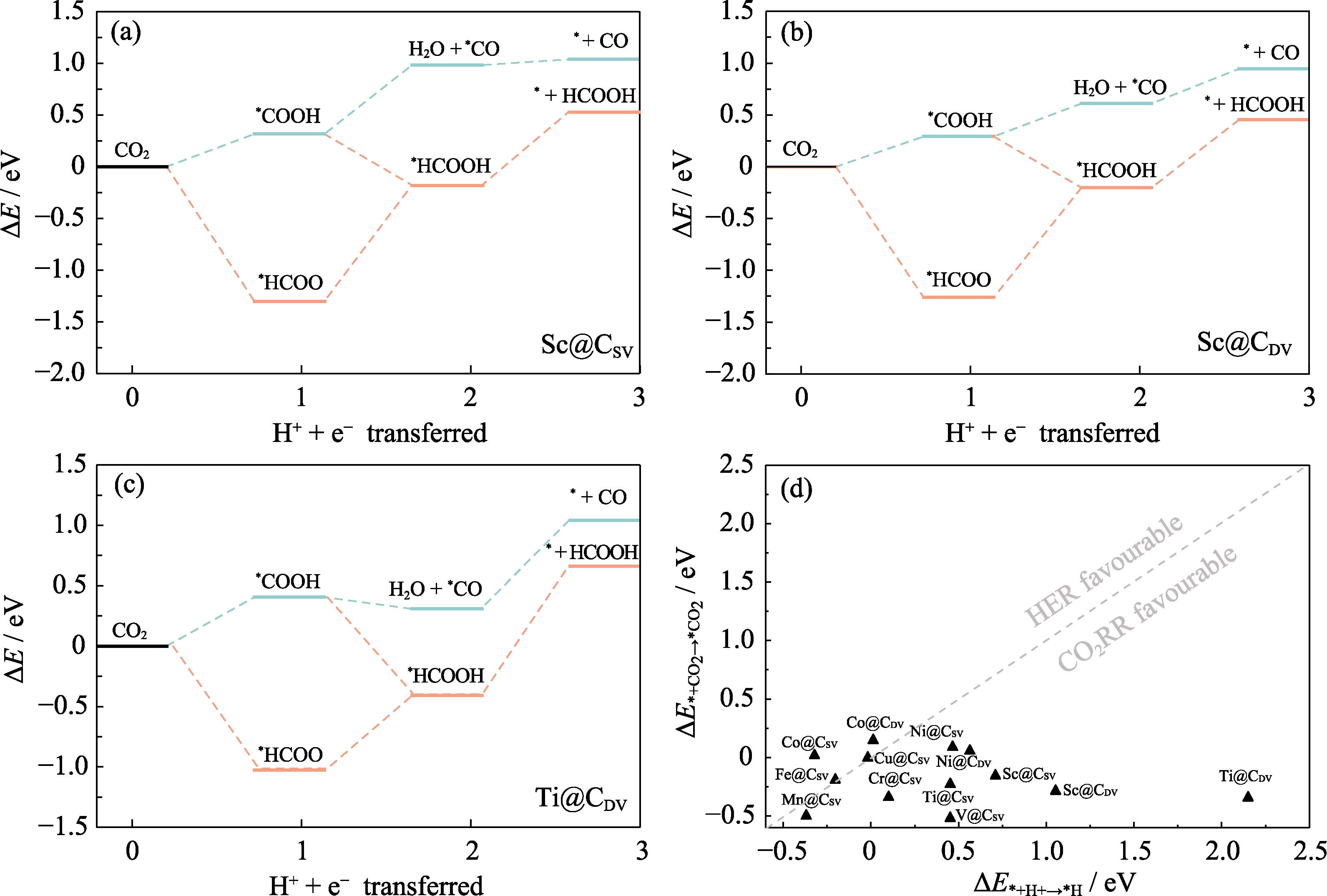
Fig. 3 Gibbs free energy profiles and the competitive reaction analysis (a-c) Free energies of Sc@CSV (a), Sc@CDV (b) and Ti@CDV (c); (d) CO2RR vs HER for TM@C; Colorful figures are available on website
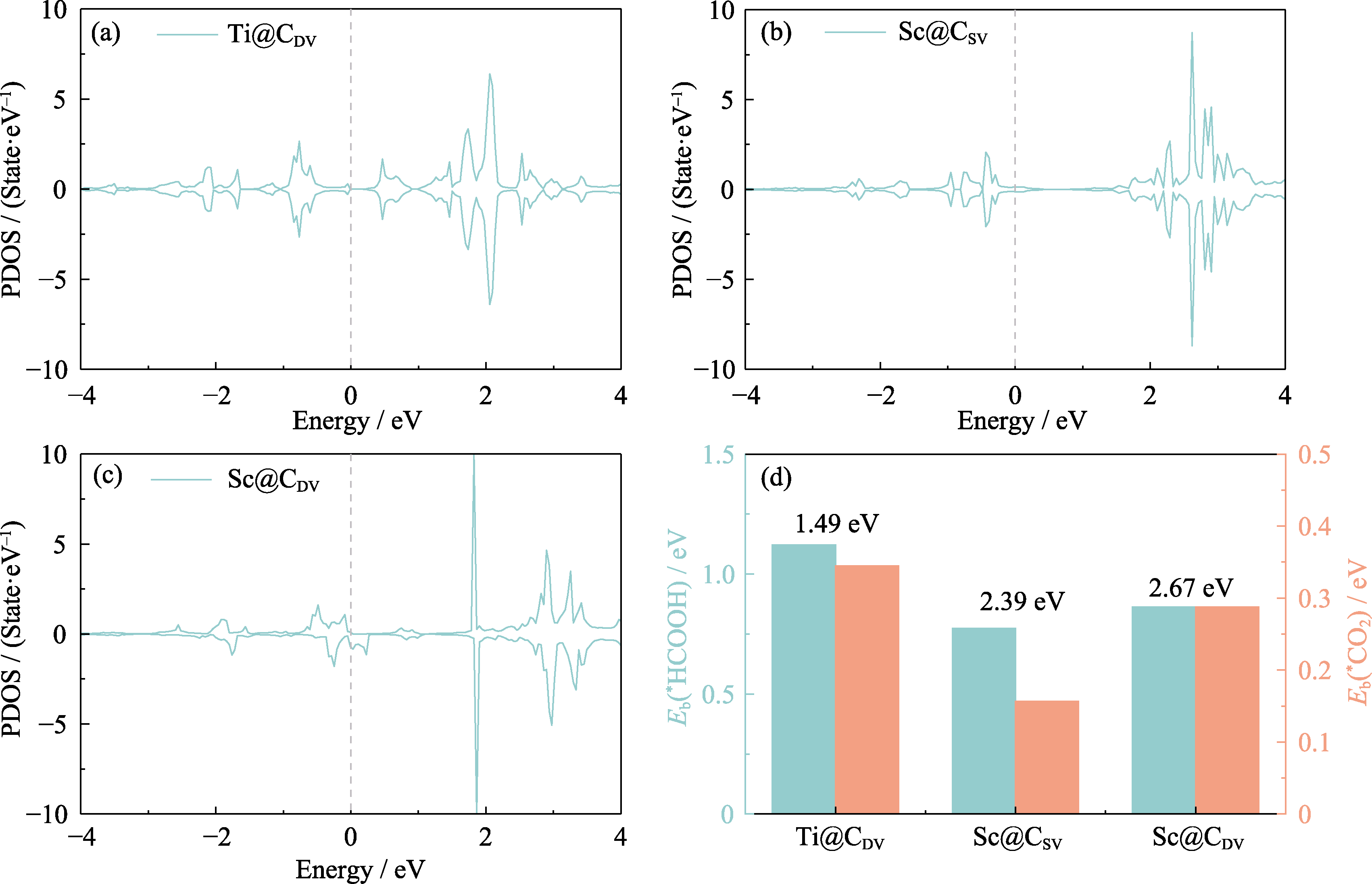
Fig. 4 Electron distributions of Ti@CDV, Sc@CSV and Sc@CDV (a-c) PDOS of Ti@CDV (a), Sc@CSV (b) and Sc@CDV (c) (Gray dashed lines mark the positions of the Fermi energy levels); (d) Eb(*HCOOH) and Eb(*CO2) of Ti@CDV, Sc@CSV and Sc@CDV; The d-band center as an average of the d-band energies; Colorful figures are available on website
| [1] | FENG Q, LIU D, ZHANG Y, et al. Thermodynamic and first-principles assessments of materials for solar-driven CO2 splitting using two-step thermochemical cycles. Journal of Inorganic Materials, 2022, 37(2): 223. |
| [2] | TIAN J, MA X, WANG M, et al. Sn quantum dots for electrocatalytic reduction of CO2 to HCOOH. Journal of Inorganic Materials, 2021, 36(12): 1337. |
| [3] | CHEN S, LÜ G. CO2 methanation over Ru/TiO2 catalysts under UV irradiation and heating. Journal of Inorganic Materials, 2014, 29(12): 1287. |
| [4] | MIKKELSEN M, JØRGENSEN M, KREBS F C. The teraton challenge. a review of fixation and transformation of carbon dioxide. Energy & Environmental Science, 2010, 3(1): 43. |
| [5] | XU X, GOU X, ZHANG W, et al. A bibliometric analysis of carbon neutrality: research hotspots and future directions. Heliyon, 2023, 9(8): e18763. |
| [6] | HUA Y, WANG J, MIN T, et al. Electrochemical CO2 conversion towards syngas: recent catalysts and improving strategies for ratio-tunable syngas. Journal of Power Sources, 2022, 535: 231453. |
| [7] | HE R, XU N, HASAN I M U, et al. Advances in electrolyzer design and development for electrochemical CO2 reduction. EcoMat, 2023, 5(7): e12346. |
| [8] | KOUA K A J, PENG J, ZHANG P, et al. Unveiling the magnetic ordering effect in La-doped Ti3C2O2 MXenes on electrocatalytic CO2 reduction. Journal of Materials Chemistry A, 2024, 12(1): 303. |
| [9] | ROY S C, VARGHESE O K, PAULOSE M, et al. Toward solar fuels: photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano, 2010, 4(3): 1259. |
| [10] | ZHANG W, JIN Z, CHEN Z. Rational-designed principles for electrochemical and photoelectrochemical upgrading of CO2 to value-added chemicals. Advanced Science, 2022, 9(9): 2105204. |
| [11] | LI N, LIU J, DONG B X, et al. Polyoxometalate-based compounds for photo-and electrocatalytic applications. Angewandte Chemie International Edition, 2020, 59(47): 20779. |
| [12] | WU Q J, LIANG J, HUANG Y B, et al. Thermo-, electro-, and photocatalytic CO2 conversion to value-added products over porous metal/covalent organic frameworks. Accounts of Chemical Research, 2022, 55(20): 2978. |
| [13] | NIE X, ESOPI M R, JANIK M J, et al. Selectivity of CO2 reduction on copper electrodes: the role of the kinetics of elementary steps. Angewandte Chemie International Edition, 2013, 125(9): 2519. |
| [14] | JIA Y, ZHANG L, DU A, et al. Defect graphene as a trifunctional catalyst for electrochemical reactions. Advanced Materials, 2016, 28(43): 9532. |
| [15] | XUE Z H, ZHANG S N, LIN Y X, et al. Electrochemical reduction of N2 into NH3 by donor-acceptor couples of Ni and Au nanoparticles with a 67.8% Faradaic efficiency. Journal of the American Chemical Society, 2019, 141(38): 14976. |
| [16] | JONES J P, PRAKASH G K S, OLAH G A. Electrochemical CO2 reduction: recent advances and current trends. Israel Journal of Chemistry, 2014, 54(10): 1451. |
| [17] | ZHAO K, QUAN X. Carbon-based materials for electrochemical reduction of CO2 to C2+ oxygenates: recent progress and remaining challenges. ACS Catalysis, 2021, 11(4): 2076. |
| [18] | DENG J, IÑIGUEZ J A, LIU C. Electrocatalytic nitrogen reduction at low temperature. Joule, 2018, 2(5): 846. |
| [19] | AZOFRA L M, LI N, MACFARLANE D R, et al. Promising prospects for 2D d2-d4 M3C2 transition metal carbides (MXenes) in N2 capture and conversion into ammonia. Energy & Environmental Science, 2016, 9(8): 2545. |
| [20] | LI B, WU Y, LI N, et al. Single-metal atoms supported on MBenes for robust electrochemical hydrogen evolution. ACS Applied Materials & Interfaces, 2020, 12(8): 9261. |
| [21] | FU Y, BI M, LI C, et al. Research progress on non-noble metal/nitrogen-doped carbon composite materials in electrocatalytic oxygen evolution reaction. Journal of Inorganic Materials, 2022, 37(2): 163. |
| [22] | LI Z, SUN Q, CHEN S, et al. Hydrothermal synthesized nickel copper composite phosphides as bifunctional electrocatalysts for hydrogen evolution and hydrazine oxidation. Journal of Inorganic Materials, 2020, 35(10): 1149. |
| [23] | FRANCKE R, SCHILLE B, ROEMELT M. Homogeneously catalyzed electroreduction of carbon dioxide-methods, mechanisms, and catalysts. Chemical Reviews, 2018, 118(9): 4631. |
| [24] | TODOROVA T K, SCHREIBER M W, FONTECAVE M. Mechanistic understanding of CO2 reduction reaction (CO2RR) toward multicarbon products by heterogeneous copper-based catalysts. ACS Catalysis, 2020, 10(3): 1754. |
| [25] | WANG G, CHEN J, DING Y, et al. Electrocatalysis for CO2 conversion: from fundamentals to value-added products. Chemical Society Reviews, 2021, 50(8): 4993. |
| [26] | MA W, HE X, WANG W, et al. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chemical Society Reviews, 2021, 50(23): 12897. |
| [27] | PENG J, SHI Z, JIANG J, et al. Charge-orbital synergistic engineering of TM@Ti3C2O1-xBx for highly selective CO2 electrochemical reduction. Materials Horizons, 2023, 10(10): 4278. |
| [28] | QIAO B, LIU J, ALLARD L, et al. Single-atom catalysis: Pt1/FeOx for CO oxidation and preferential oxidation of CO in H2. Microscopy and Microanalysis, 2012, 18(S2): 350. |
| [29] | WANG X, ZHANG Y, WU J, et al. Single-atom engineering to ignite 2D transition metal dichalcogenide based catalysis: fundamentals, progress, and beyond. Chemical Reviews, 2022, 122(1): 1273. |
| [30] | CHENG N, ZHANG L, DOYLE-DAVIS K, et al. Single-atom catalysts: from design to application. Electrochemical Energy Reviews, 2019, 2(4): 539. |
| [31] | LI M, WANG H, LUO W, et al. Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction. Advanced Materials, 2020, 32(34): 2001848. |
| [32] | QU G, WEI K, PAN K, et al. Emerging materials for electrochemical CO2 reduction: progress and optimization strategies of carbon-based single-atom catalysts. Nanoscale, 2023, 15(8): 3666. |
| [33] | WANG Y, LIU Y, LIU W, et al. Regulating the coordination structure of metal single atoms for efficient electrocatalytic CO2 reduction. Energy & Environmental Science, 2020, 13(12): 4609. |
| [34] | CHEN X, ZHAO X, KONG Z, et al. Unravelling the electrochemical mechanisms for nitrogen fixation on single transition metal atoms embedded in defective graphitic carbon nitride. Journal of Materials Chemistry A, 2018, 6(44): 21941. |
| [35] | HUANG B, LI N, ONG W J, et al. Single atom-supported MXene: how single-atomic-site catalysts tune the high activity and selectivity of electrochemical nitrogen fixation. Journal of Materials Chemistry A, 2019, 7(48): 27620. |
| [36] | YAO M, SHI Z, ZHANG P, et al. Density functional theory study of single metal atoms embedded into MBene for electrocatalytic conversion of N2 to NH3. ACS Applied Nano Materials, 2020, 3(10): 9870. |
| [37] | DU Z, CHEN X, HU W, et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. Journal of the American Chemical Society, 2019, 141(9): 3977. |
| [38] | GU J, HSU C S, BAI L, et al. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science, 2019, 364(6445): 1091. |
| [39] | ZHANG Z, ZHU J, CHEN S, et al. Liquid fluxional Ga single atom catalysts for efficient electrochemical CO2 reduction. Angewandte Chemie International Edition, 2023, 62(3): e202215136. |
| [40] | HAN S S, LIU L Y, SHAN Y K, et al. Research of graphene/antireflection nanostructure composite transparent conducting films. Journal of Inorganic Materials, 2017, 32(2): 197. |
| [41] | WANG Y, LI S, YANG H, et al. Progress in the functional modification of graphene/graphene oxide: a review. RSC Advances, 2020, 10(26): 15328. |
| [42] | WANG J, JIN X, LI C, et al. Graphene and graphene derivatives toughening polymers: toward high toughness and strength. Chemical Engineering Journal, 2019, 370: 831. |
| [43] | HE J L, SONG E H, WANG L J, et al. DFT Calculation of NO adsorption on Cr doped graphene. Journal of Inorganic Materials, 2021, 36(10): 1047. |
| [44] | XING W W, ZHANG C X, FAN S C, et al. Research progress on resonant characteristics of graphene. Journal of Inorganic Materials, 2016, 31(7): 673. |
| [45] | NAN H, WANG W L, HAN J H, et al. Low-cost preparation of graphene papers from chemical reduction with FeI2/Ni2+ for conductivity and catalytic property. Journal of Inorganic Materials, 2017, 32(9): 997. |
| [46] | CHOI J H, LEE J, BYEON M, et al. Graphene-based gas sensors with high sensitivity and minimal sensor-to-sensor variation. ACS Applied Nano Materials, 2020, 3(3): 2257. |
| [47] | GATTRELL M, GUPTA N, CO A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. Journal of Electroanalytical Chemistry, 2006, 594(1): 1. |
| [48] | LI C W, KANAN M W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. Journal of the American Chemical Society, 2012, 134(17): 7231. |
| [49] | SCHOUTEN K J P, PÉREZ GALLENT E, KOPER M T. Structure sensitivity of the electrochemical reduction of carbon monoxide on copper single crystals. ACS Catalysis, 2013, 3(6): 1292. |
| [50] | REN D, DENG Y, HANDOKO A D, et al. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper (I) oxide catalysts. ACS Catalysis, 2015, 5(5): 2814. |
| [51] | RESKE R, MISTRY H, BEHAFARID F, et al. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. Journal of the American Chemical Society, 2014, 136(19): 6978. |
| [52] | JIAO L, YANG W, WAN G, et al. Single-atom electrocatalysts from multivariate metal-organic frameworks for highly selective reduction of CO2 at low pressures. Angewandte Chemie International Edition, 2020, 59(46): 20589. |
| [53] | KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science, 1996, 6(1): 15. |
| [54] | KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B, 1996, 54(16): 11169. |
| [55] | PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple. Physical Review Letters, 1996, 77(18): 3865. |
| [56] | KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B, 1999, 59(3): 1758. |
| [57] | BLÖCHL P E. Projector augmented-wave method. Physical Review B, 1994, 50(24): 17953. |
| [58] | GRIMME S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. Journal of Computational Chemistry, 2006, 27(15): 1787. |
| [59] | SKÚLASON E, BLIGAARD T, GUDMUNDSDÓTTIR S, et al. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Physical Chemistry Chemical Physics, 2012, 14(3): 1235. |
| [60] | LI J, XU J, ZHAO J, et al. Modulation of oxygen-etching for generating nickel single atoms for efficient electroreduction of CO2 to syngas (CO/H2). Journal of Catalysis, 2023, 421: 332. |
| [61] | LI N, WANG X, LU X, et al. Comprehensive mechanism of CO2 electroreduction on non-noble metal single-atom catalysts of Mo2CS2-MXene. Chemistry-A European Journal, 2021, 27(71): 17900. |
| [62] | LI N, CHEN X, ONG W J, et al. Understanding of electrochemical mechanisms for CO2 capture and conversion into hydrocarbon fuels in transition-metal carbides (MXenes). ACS Nano, 2017, 11(11): 10825. |
| [63] | JIAO S, FU X, HUANG H. Descriptors for the evaluation of electrocatalytic reactions: d-band theory and beyond. Advanced Functional Materials, 2022, 32(4): 2107651. |
| [64] | NØRSKOV J K, BLIGAARD T, LOGADOTTIR A, et al. Trends in the exchange current for hydrogen evolution. Journal of The Electrochemical Society, 2005, 152(3): J23. |
| [65] | NØRSKOV J K, ABILD-PEDERSEN F, STUDT F, et al. Density functional theory in surface chemistry and catalysis. Proceedings of the National Academy of Sciences, 2011, 108(3): 937. |
| [66] | KITCHIN J R, NØRSKOV J K, BARTEAU M A, et al. Role of strain and ligand effects in the modification of the electronic and chemical properties of bimetallic surfaces. Physical Review Letters, 2004, 93(15): 156801. |
| [1] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [2] | 吴玉豪, 彭仁赐, 程春玉, 杨丽, 周益春. HfxTa1-xC体系力学性能及熔化曲线的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 761-768. |
| [3] | 王伟华, 张磊宁, 丁峰, 代兵, 韩杰才, 朱嘉琦, 贾怡, 杨宇. 铱衬底上金刚石外延形核与生长: 第一性原理计算[J]. 无机材料学报, 2024, 39(4): 416-422. |
| [4] | 张宇晨, 陆知遥, 赫晓东, 宋广平, 朱春城, 郑永挺, 柏跃磊. 硫族MAX相硼化物的物相稳定性和性能预测[J]. 无机材料学报, 2024, 39(2): 225-232. |
| [5] | 周靖渝, 李兴宇, 赵晓琳, 王有伟, 宋二红, 刘建军. Ti和Cu掺杂β-NaMnO2正极材料:钠离子电池的倍率和循环性能[J]. 无机材料学报, 2024, 39(12): 1404-1412. |
| [6] | 陈梦杰, 王倩倩, 吴成铁, 黄健. 基于DFT的描述符预测生物陶瓷的降解性[J]. 无机材料学报, 2024, 39(10): 1175-1181. |
| [7] | 周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158. |
| [8] | 吴晓维, 张涵, 曾彪, 明辰, 孙宜阳. 杂化泛函HSE和PBE0计算CsPbI3缺陷性质的比较研究[J]. 无机材料学报, 2023, 38(9): 1110-1116. |
| [9] | 张守超, 陈洪雨, 刘洪飞, 杨羽, 李欣, 刘德峰. 6H-SiC中子辐照肿胀高温回复及光学特性研究[J]. 无机材料学报, 2023, 38(6): 678-686. |
| [10] | 杨颖康, 邵怡晴, 李柏良, 吕志伟, 王路路, 王亮君, 曹逊, 吴宇宁, 黄荣, 杨长. Cl掺杂对CuI薄膜发光性能增强研究[J]. 无机材料学报, 2023, 38(6): 687-692. |
| [11] | 文志勤, 黄彬荣, 卢涛仪, 邹正光. 压力对PbTiO3结构和热物性质影响的第一性原理研究[J]. 无机材料学报, 2022, 37(7): 787-794. |
| [12] | 孙铭, 邵溥真, 孙凯, 黄建华, 张强, 修子扬, 肖海英, 武高辉. RGO/Al复合材料界面性质第一性原理研究[J]. 无机材料学报, 2022, 37(6): 651-659. |
| [13] | 肖美霞, 李苗苗, 宋二红, 宋海洋, 李钊, 毕佳颖. 表面端基卤化Ti3C2 MXene应用于锂离子电池高容量电极材料的研究[J]. 无机材料学报, 2022, 37(6): 660-668. |
| [14] | 袁罡, 马新国, 贺华, 邓水全, 段汪洋, 程正旺, 邹维. 平面应变对二维单层MoSi2N4能带结构和光电性质的影响[J]. 无机材料学报, 2022, 37(5): 527-533. |
| [15] | 冯清影, 刘东, 张莹, 冯浩, 李强. 太阳能驱动的两步热化学循环二氧化碳裂解反应活性材料的热力学与第一性原理评价[J]. 无机材料学报, 2022, 37(2): 223-229. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||