无机材料学报 ›› 2023, Vol. 38 ›› Issue (11): 1245-1256.DOI: 10.15541/jim20230117 CSTR: 32189.14.10.15541/jim20230117
所属专题: 【能源环境】污染物催化去除(202506)
• 综述 • 下一篇
收稿日期:2023-01-27
修回日期:2023-04-25
出版日期:2023-11-20
网络出版日期:2023-05-04
通讯作者:
易志国, 研究员. E-mail: zhiguo@mail.sic.ac.cn;作者简介:孙 晨(1996-), 男, 硕士研究生. E-mail: 895029730@qq.com
基金资助:
SUN Chen1,2( ), ZHAO Kunfeng2(
), ZHAO Kunfeng2( ), YI Zhiguo1,2(
), YI Zhiguo1,2( )
)
Received:2023-01-27
Revised:2023-04-25
Published:2023-11-20
Online:2023-05-04
Contact:
YI Zhiguo, professor. E-mail: zhiguo@mail.sic.ac.cn;About author:SUN Chen (1996-), male, Master candidate. E-mail: 895029730@qq.com
Supported by:摘要:
甲烷是对全球温升贡献仅次于二氧化碳的温室气体, 且其全球增温潜势是CO2的80倍以上。在全球变暖和大气中甲烷含量不断增长的背景下, 完全催化氧化大气甲烷对于减缓温室效应和全球变暖具有重要价值。然而, 由于甲烷具有较高的结构稳定性, 在温和条件下将其催化氧化一直面临巨大的挑战。本文综述了近年来甲烷完全氧化在热催化、光催化以及光热协同催化三种反应条件下的研究进展, 热催化中高温增大了能耗并加速了催化剂的失活, 开发低温反应条件下的催化剂已经成为甲烷完全热催化的重点; 光催化提供了一种常温常压条件下利用光能氧化甲烷的方法, 但是相对热催化来说反应速率较低; 光热协同催化在光能和热能的协同作用下, 可实现温和条件下的甲烷高效完全催化氧化, 表现出潜在的应用前景。本文就三种反应催化剂的发展进行综述, 系统分析了不同反应的原理, 以及不同反应条件下甲烷完全催化氧化的优势与不足, 同时总结了催化氧化甲烷所面临的挑战, 并提供潜在的解决方案, 期望为今后的甲烷氧化研究提供借鉴。
中图分类号:
孙晨, 赵昆峰, 易志国. 甲烷完全催化氧化研究进展[J]. 无机材料学报, 2023, 38(11): 1245-1256.
SUN Chen, ZHAO Kunfeng, YI Zhiguo. Research Progress in Catalytic Total Oxidation of Methane[J]. Journal of Inorganic Materials, 2023, 38(11): 1245-1256.
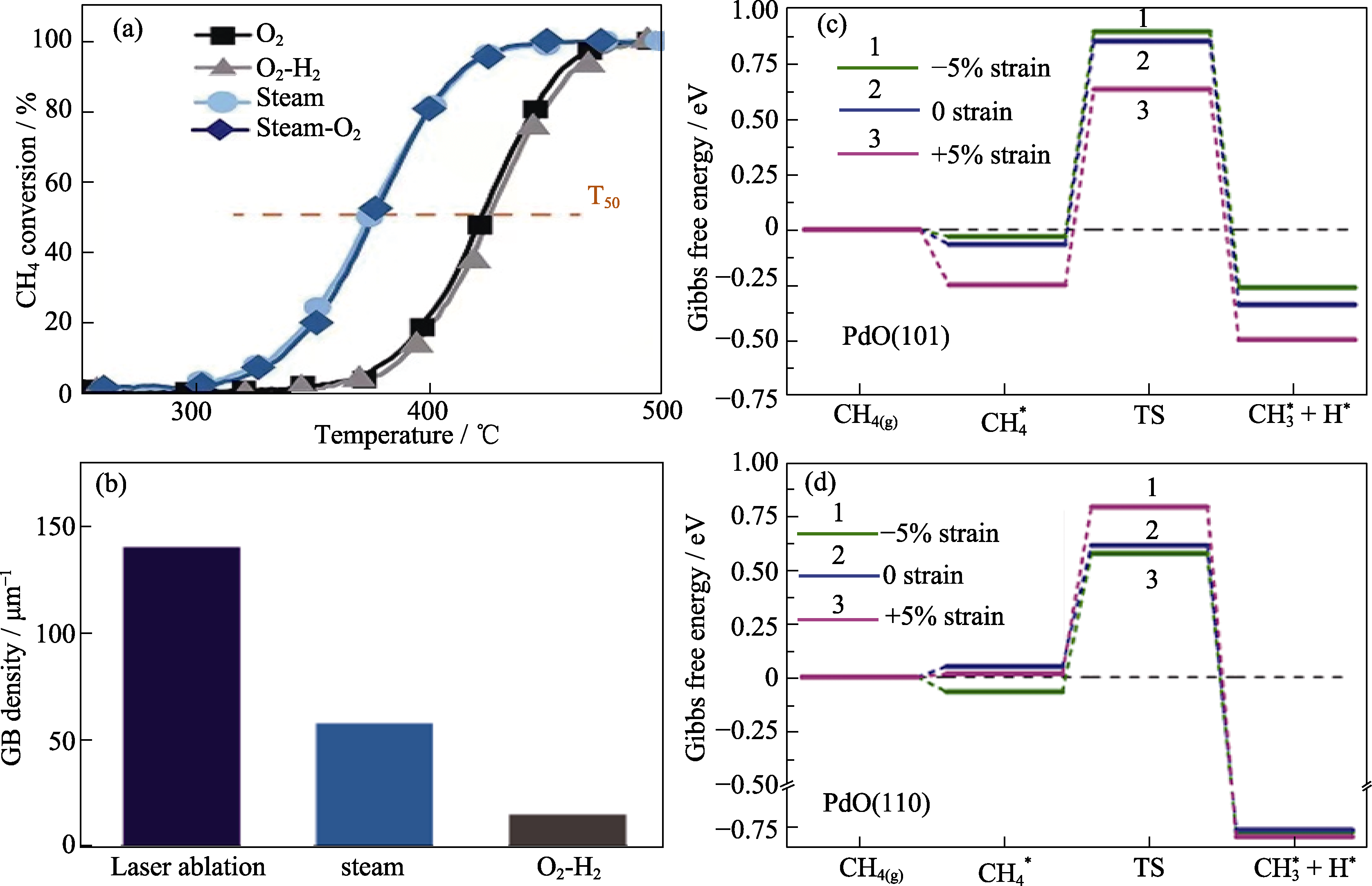
图2 催化剂的性能测试及理论计算[28]
Fig. 2 Performance tests and theoretical calculations of catalysts[28] (a) Light-off curves and T50 values of Pd/Al2O3 after O2 (600 ℃), O2-H2, steam (600 ℃), and steam-O2 pretreatments; (b) GB density statistical histogram of laser-generated Pd/Al2O3 and Pd/Al2O3 after steam (600 ℃) and O2-H2 pretreatments; (c, d) Calculated free energy diagrams for breaking the first C-H bond in CH4 on PdO(101) and PdO(110), respectively. Reprinted from Ref. [28] with permission, Copyright 2021 AAAS
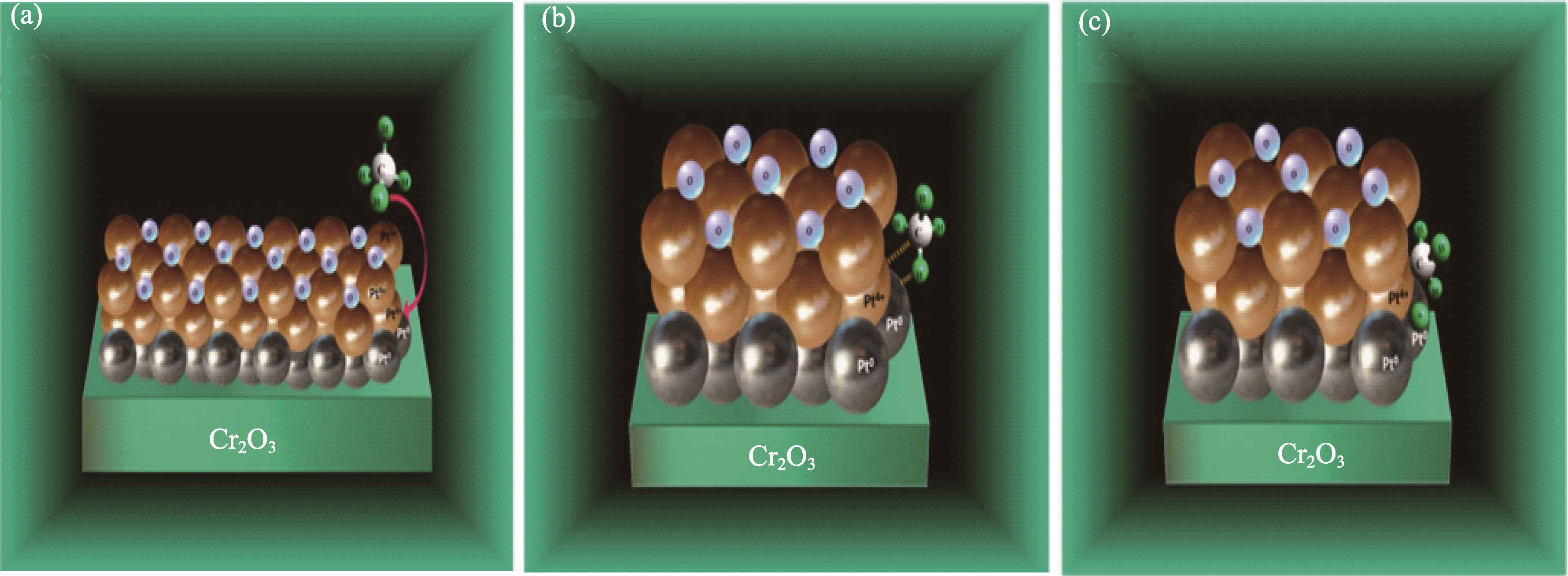
图3 化学吸附氧原子饱和的Pt0−Pt4+偶极子上的甲烷解离吸附模型[30]
Fig. 3 Proposed model for the CH4 dissociative adsorption over Pt0−Pt4+ dipoles saturated with chemisorbtion oxygen atoms[30] (a) Reactants: CH4 in the gas phase and 1% Pt/Cr2O3; (b) CH4 polarization by Pt0−Pt4+ site and formation of a transition state; (c) Abstraction of the first hydrogen on the adsorbed CH4 molecule
| Catalyst | Tc* /℃ | Ea/(kJ·mol-1) | Feed gas | GHSV/(mL·g-1·h-1) | Stability | Ref. |
|---|---|---|---|---|---|---|
| Pd-Ce@SiO2 | T100=350 | 100.4 | 1% CH4, 21% O2, bal. N2 | 36000 | 25 h | [ |
| Pd/TiO2 | T99=370 | 83.1 | 1% CH4, 10% O2, bal. N2 | 30000 | 4 cycles | [ |
| Pd/Na-MOR | T50=335 | 75 | 1% CH4, 4% O2, bal. N2 | 70000 | 90 h | [ |
| Pd-Pt/CeO2 | T50=325 | 74 | 680 μg/mL CH4, 14% O2, 5% CO2, bal. N2 | 300000 | 12 h# | [ |
| Au/Al2O3 | T50=480 | 73 | 0.8% CH4, 3.2% O2, bal. He, | 15000 | / | [ |
| Rh/ZrO2 | T50=400 | / | 1% CH4, 2% O2, bal. He | 15000 | / | [ |
| Ir/TiO2-H | T50=267 | 55.5 | 1% CH4, 20% O2, bal. N2 | 30000 | 50 h | [ |
| Ag/MnLaO3 | T50=580 | 74 | 2% CH4, 98% air | 12000 | / | [ |
| Pt/Cr2O3 | T50=350 | / | 0.2% CH4, 10% O2, bal. N2 | 30000 | / | [ |
| MgO | T50=225 | / | 1% CH4, 99% air | 6000 | 70 h | [ |
| LaCoO3 | T50=470 | / | 0.8% CH4, 5% O2, bal. N2 | 60000 | / | [ |
| NiCo2O4 | T100=350 | / | 5% CH4, 25% O2, bal. Ar | 24000 | 48 h# | [ |
| La0.6Sr0.4MnO3 | T50=566 | 56.6 | 2% CH4, 20% O2, bal. N2 | 30000 | / | [ |
| CoAlOx/CeO2 | T50=415 | 92.2 | 10% CH4, 25% O2, bal. Ar | 24000 | 50 h | [ |
表1 甲烷完全热催化氧化催化剂的性能比较
Table 1 Comparison of properties of catalysts for total oxidation of methane by thermal catalysis
| Catalyst | Tc* /℃ | Ea/(kJ·mol-1) | Feed gas | GHSV/(mL·g-1·h-1) | Stability | Ref. |
|---|---|---|---|---|---|---|
| Pd-Ce@SiO2 | T100=350 | 100.4 | 1% CH4, 21% O2, bal. N2 | 36000 | 25 h | [ |
| Pd/TiO2 | T99=370 | 83.1 | 1% CH4, 10% O2, bal. N2 | 30000 | 4 cycles | [ |
| Pd/Na-MOR | T50=335 | 75 | 1% CH4, 4% O2, bal. N2 | 70000 | 90 h | [ |
| Pd-Pt/CeO2 | T50=325 | 74 | 680 μg/mL CH4, 14% O2, 5% CO2, bal. N2 | 300000 | 12 h# | [ |
| Au/Al2O3 | T50=480 | 73 | 0.8% CH4, 3.2% O2, bal. He, | 15000 | / | [ |
| Rh/ZrO2 | T50=400 | / | 1% CH4, 2% O2, bal. He | 15000 | / | [ |
| Ir/TiO2-H | T50=267 | 55.5 | 1% CH4, 20% O2, bal. N2 | 30000 | 50 h | [ |
| Ag/MnLaO3 | T50=580 | 74 | 2% CH4, 98% air | 12000 | / | [ |
| Pt/Cr2O3 | T50=350 | / | 0.2% CH4, 10% O2, bal. N2 | 30000 | / | [ |
| MgO | T50=225 | / | 1% CH4, 99% air | 6000 | 70 h | [ |
| LaCoO3 | T50=470 | / | 0.8% CH4, 5% O2, bal. N2 | 60000 | / | [ |
| NiCo2O4 | T100=350 | / | 5% CH4, 25% O2, bal. Ar | 24000 | 48 h# | [ |
| La0.6Sr0.4MnO3 | T50=566 | 56.6 | 2% CH4, 20% O2, bal. N2 | 30000 | / | [ |
| CoAlOx/CeO2 | T50=415 | 92.2 | 10% CH4, 25% O2, bal. Ar | 24000 | 50 h | [ |

图4 (a)半导体基光催化剂的甲烷活化示意图[1]和(b)常用半导体的能带结构和不同反应物的氧化还原电位示意图[55]
Fig. 4 (a) Schematic diagram of methane activation over semiconductor-based photocatalysts[1]; (b) Schematic diagram of band structures of commonly used semiconductors and redox potentials of different reactants[55]
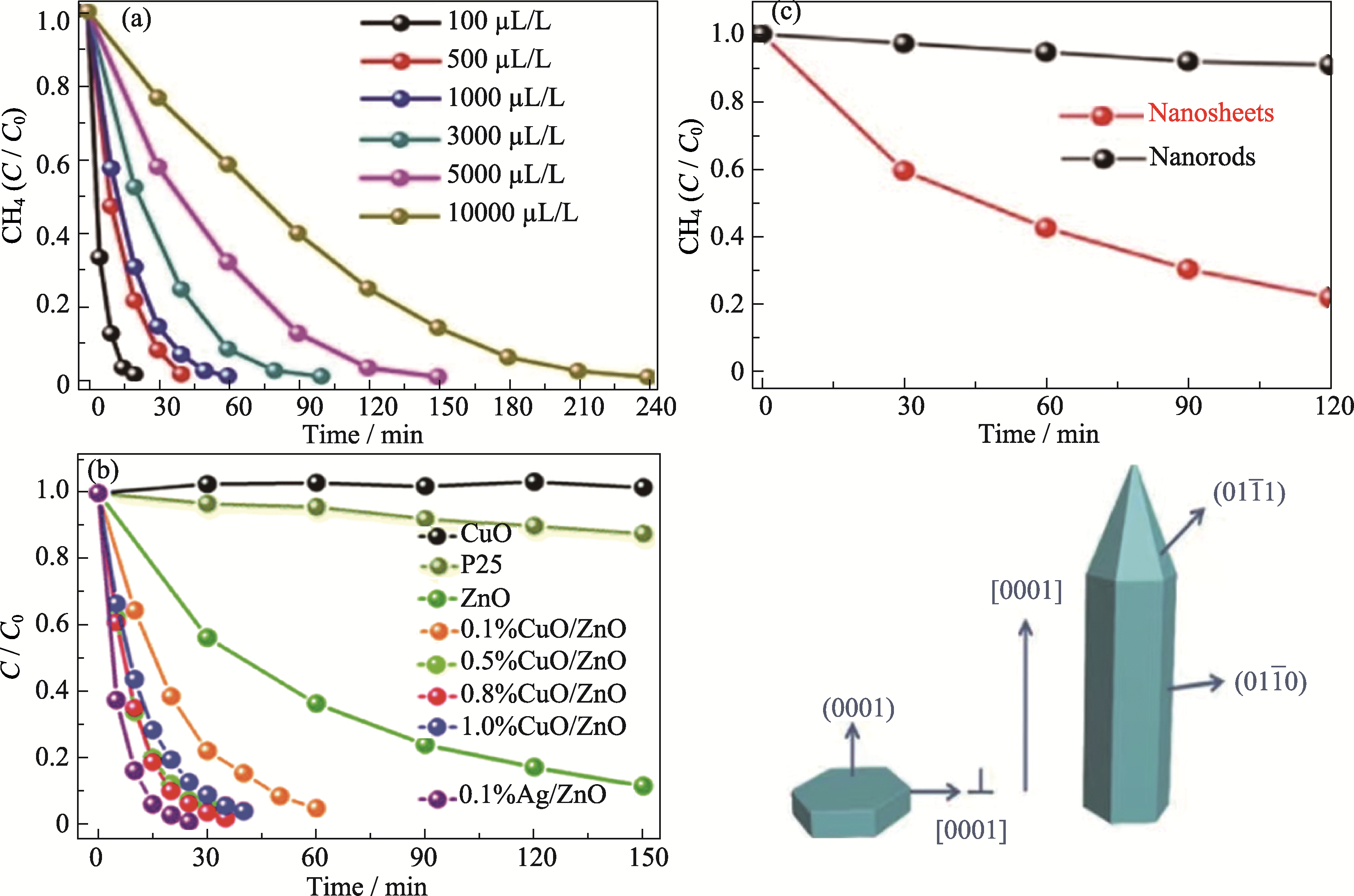
图5 ZnO基半导体在甲烷完全光催化氧化中的应用
Fig. 5 Application of ZnO-based semiconductor in photocatalytic total oxidation of methane (a) Time evolution of photocatalytic total oxidation of methane over 0.1% Ag-decorated ZnO nanocatalysts at different CH4 concentrations[56] (Reprinted from Ref. [56] with permission, Copyright 2016 Springer Nature); (b) Time evolution of photocatalytic total oxidation of methaneover various catalysts with a CH4 input of 100 μL/L[58] (Reprinted from Ref. [58] with permission, Copyright 2019 Royal Society of Chemistry); (c) Catalytic activity of total oxidation of methane (top) and the crystal morphology (bottom) of a ZnO nanosheet and nanorod[59] (Reprinted from Ref. [59] with permission, Copyright 2019 American Chemical Society)
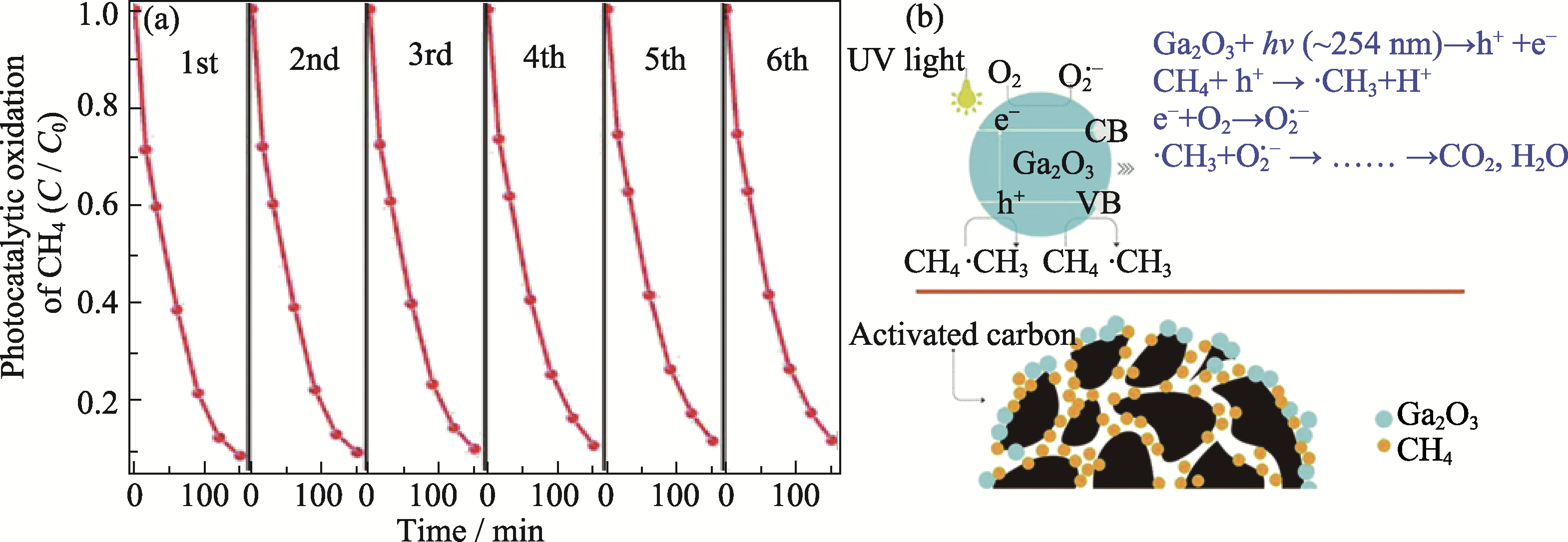
图6 Ga2O3/AC甲烷完全光催化氧化性能测试及氧化机理示意图[60]
Fig. 6 Ga2O3/AC photocatalytic total oxidation of methane and schematic diagram of oxidation mechanism[60] (a) Recycled test of photocatalytic oxidation of CH4 over 15% Ga2O3/AC; (b) Proposed mechanism for photocatalytic oxidation of CH4 over Ga2O3/AC composites. Reprinted from Ref. [60] with permission, Copyright 2017 Royal Society of Chemistry
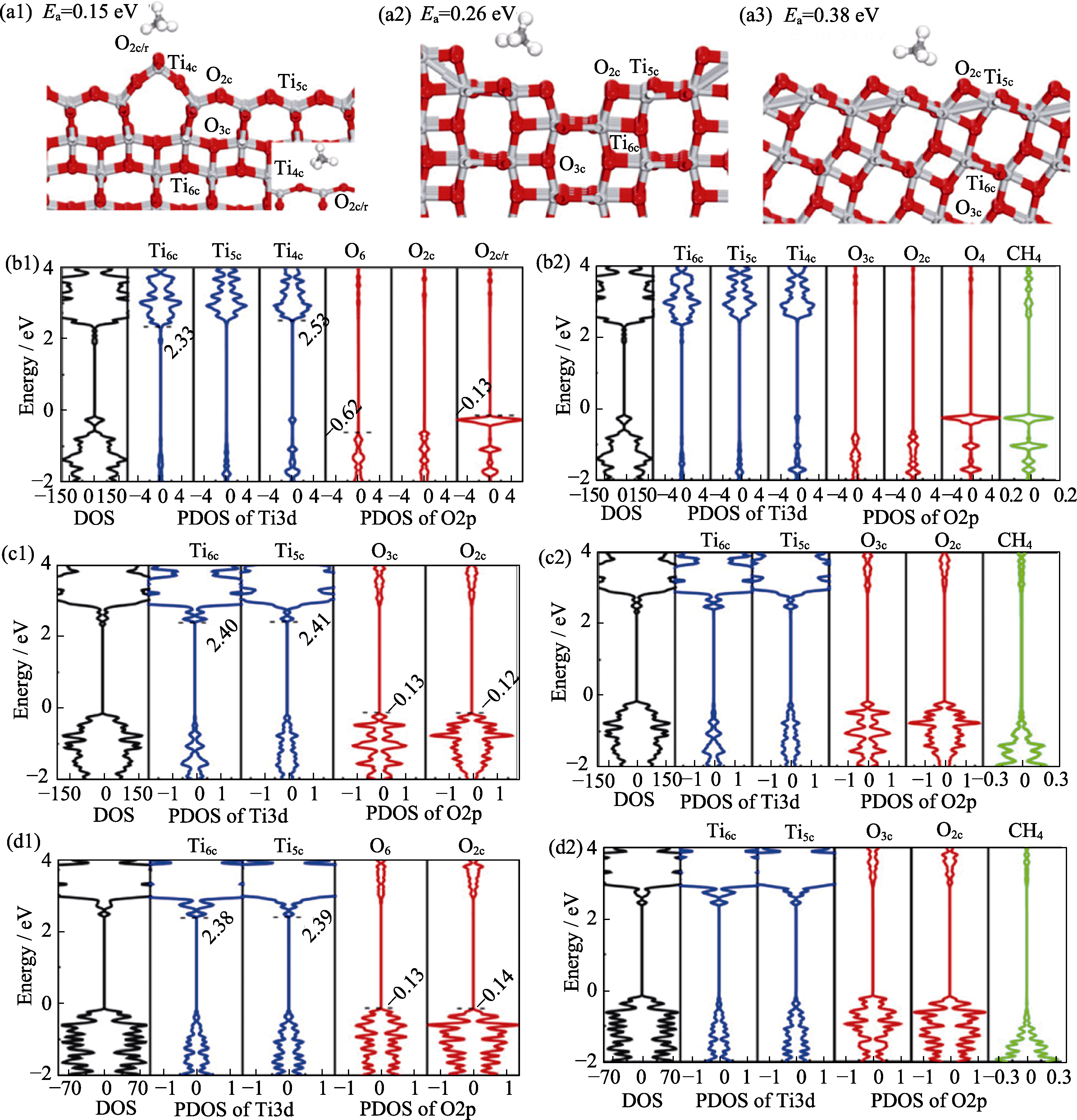
图7 不同TiO2表面甲烷吸附能以及DFT计算[61]
Fig. 7 Adsorption energy calculations of surface methane and DFT calculation of different TiO2 [61] (a1-a3) Most stable adsorption configurations of CH4 on (a1) anatase TiO2(001)-(1×4), (a2) anatase TiO2(100)-(1×2), and (a3) anatase TiO2(101) surfaces. Gray and red balls represent Ti and O atoms, respectively; (b1, b2, c1,c2, d1, d2) Calculated PDOS of (b1) bare and (b2) CH4-adsorbed anatase TiO2(001)-(1×4) surfaces, (c1) bare and (c2) CH4-adsorbed anatase TiO2(100)-(12) surfaces, and (d1) bare and (d2) CH4-adsorbed anatase TiO2-(101) surfaces. Reprinted from Ref. 61 with permission, Copyright 2022 American Chemical Society
| Catalyst | Reaction conditions | Yield/(μmol·h-1) | Ref. |
|---|---|---|---|
| TiO2 | Batch reactor, 3×105 Pa CH4, Xe lamp, RT | 1.1 | [ |
| TiO2 | Batch reactor, 2×106 Pa CH4, 5 bar O2, Xe lamp, RT | 23 | [ |
| ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4 in air, Xe lamp, RT | 2 | [ |
| Ag/ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4, Xe lamp, RT | 22 | [ |
| CuO/ZnO | Batch reactor, 1×105 Pa, 100 μg/mL CH4, Xe lamp, RT | 4 | [ |
| Au-CeO2/ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4, Xe lamp, RT | 0.6 | [ |
| Ag/AgCl | Batch reactor, 1×105 Pa, 500 μg/mL CH4, Xe lamp, RT | 5.4 | [ |
| SrCO3/SrTiO3 | Batch reactor, 1×105 Pa, 200 μg/mL CH4, Xe lamp, RT | 0.8 | [ |
| BiVO4 | Batch reactor, 1×105 Pa, 20 μg/mL CH4, visible light, RT | 0.05 | [ |
表2 甲烷完全催化氧化光催化剂的性能比较
Table 2 Comparison of performances of photocatalysts for total oxidation of methane by photocatalysis
| Catalyst | Reaction conditions | Yield/(μmol·h-1) | Ref. |
|---|---|---|---|
| TiO2 | Batch reactor, 3×105 Pa CH4, Xe lamp, RT | 1.1 | [ |
| TiO2 | Batch reactor, 2×106 Pa CH4, 5 bar O2, Xe lamp, RT | 23 | [ |
| ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4 in air, Xe lamp, RT | 2 | [ |
| Ag/ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4, Xe lamp, RT | 22 | [ |
| CuO/ZnO | Batch reactor, 1×105 Pa, 100 μg/mL CH4, Xe lamp, RT | 4 | [ |
| Au-CeO2/ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4, Xe lamp, RT | 0.6 | [ |
| Ag/AgCl | Batch reactor, 1×105 Pa, 500 μg/mL CH4, Xe lamp, RT | 5.4 | [ |
| SrCO3/SrTiO3 | Batch reactor, 1×105 Pa, 200 μg/mL CH4, Xe lamp, RT | 0.8 | [ |
| BiVO4 | Batch reactor, 1×105 Pa, 20 μg/mL CH4, visible light, RT | 0.05 | [ |
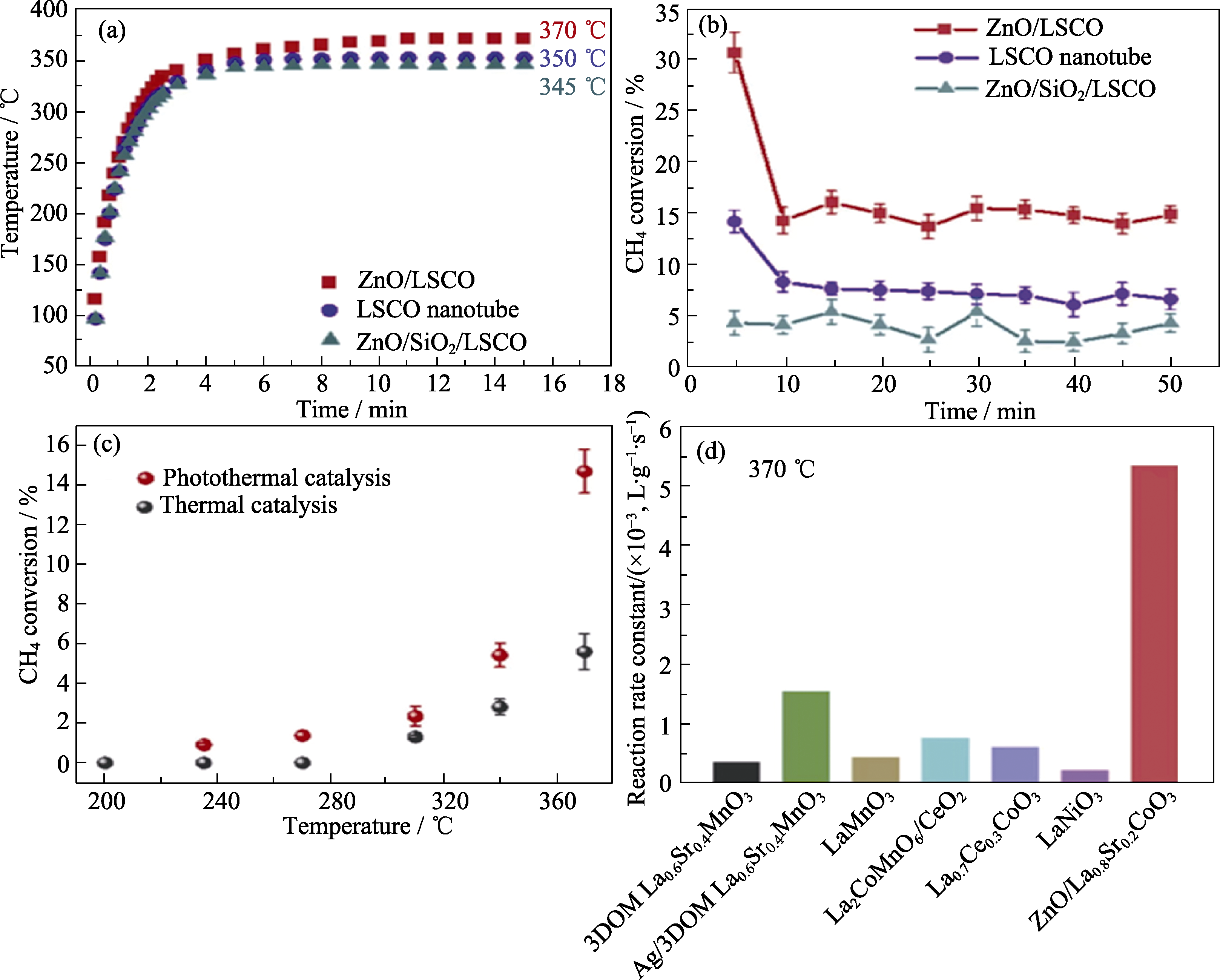
图8 ZnO/LSCO光催化及光热协同催化甲烷完全氧化性能测试[70]
Fig. 8 Tests of ZnO/LSCO photocatalysis and photothermal cocatalysis for methane total oxidation[70] (a) Temperature profiles on these monolithic catalysts under irradiation of Xe lamp; (b) CH4 photothermal conversions over these monolithic catalysts under Xe lamp irradiation; (c) Comparison of CH4 conversion for ZnO/LSCO under Xe lamp irradiation and direct thermal heating (furnace) at the same temperature; (d) Activity comparison of methane oxidation with previous studies by normalized reaction rate constant. Reprinted from Ref. [70] with permission, Copyright 2008 American Chemical Society
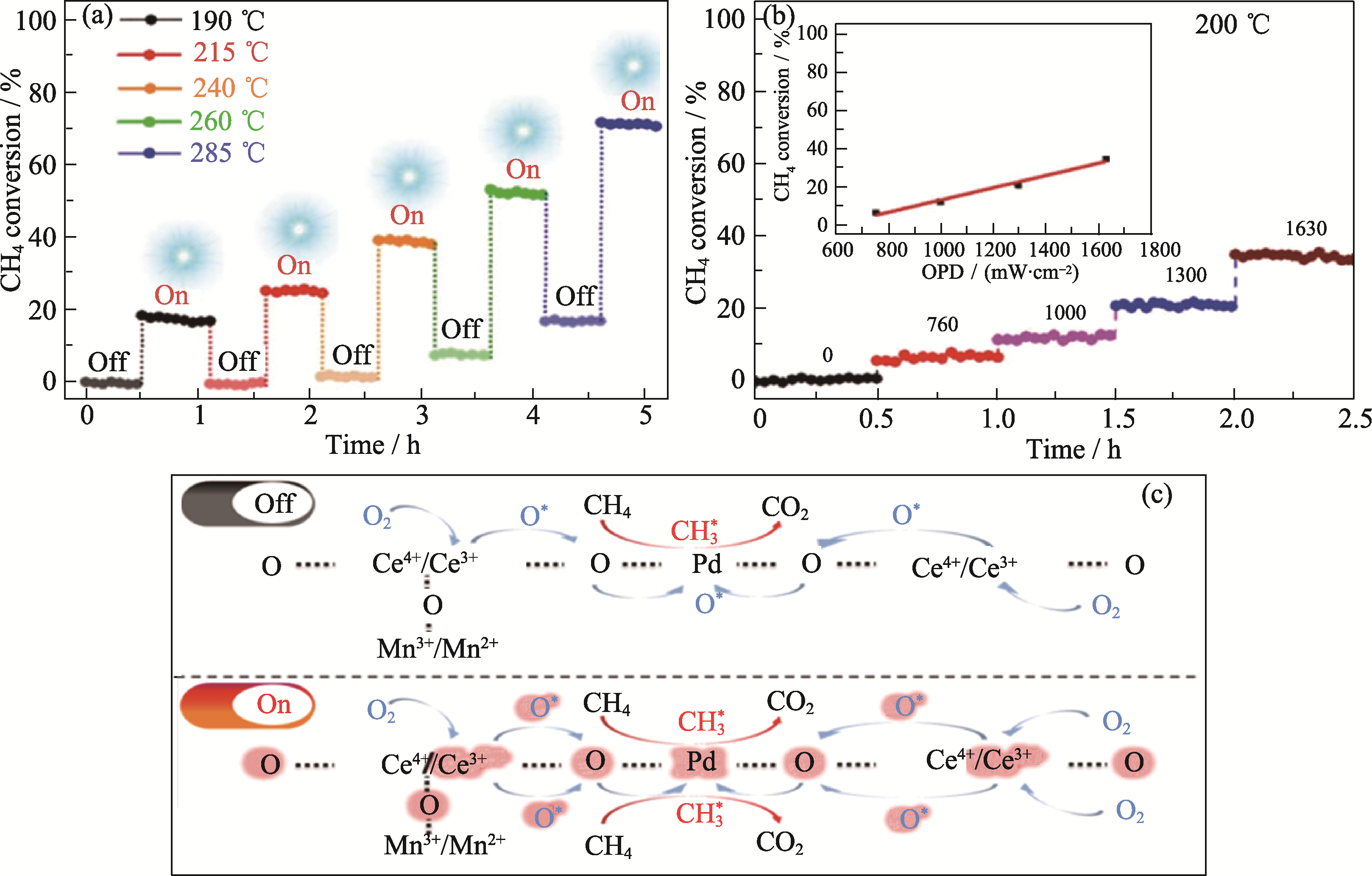
图9 HPMC光热协同催化甲烷完全氧化性能(a,b)及其机理示意图(c)[71]
Fig. 9 HPMC photothermal co-catalyzed methane total oxidation performance (a, b) and its mechanism (c)[71] (a) Cycling stability test of HPMC; (b) Methane conversion measured at 200 ℃ with different optical power (OPD); (c) Reaction mechanism of HPMC catalyzed methane combustion. Reprinted from Ref. [71] with permission, Copyright 2021 Wiley
| [1] |
SONG H, MENG X, WANG Z J, et al. Solar-energy-mediated methane conversion. Joule, 2019, 3(7): 1606.
DOI |
| [2] |
NEWTON M A, KNORPP A J, SUSHKEVICH V L, et al. Active sites and mechanisms in the direct conversion of methane to methanol using Cu in zeolitic hosts: a critical examination. Chemical Society Reviews, 2020, 49(5): 1449.
DOI PMID |
| [3] |
SCHWACH P, PAN X, BAO X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chemical Reviews, 2017, 117(13): 8497.
DOI PMID |
| [4] |
VOOSE P. Ominous feedback loop may be accelerating methane emissions. Science, 2022, 377(6603): 250.
DOI PMID |
| [5] |
PENG S, LIN X, THOMPSON R L, et al. Wetland emission and atmospheric sink changes explain methane growth in 2020. Nature, 2022, 612(7940): 477.
DOI |
| [6] |
LASHOF D A, AHUJA D. R. Relative contributions of greenhouse gas emissions to global warming. Nature, 1990, 344(5): 529.
DOI |
| [7] |
ALLEN G H, Cause of the 2020 rise in atmospheric methane. Nature, 2022, 612(15): 13.
DOI |
| [8] |
HE L, FAN Y, BELLETTRE J, et al. A review on catalytic methane combustion at low temperatures: catalysts, mechanisms, reaction conditions and reactor designs. Renewable and Sustainable Energy Reviews, 2020, 119: 109589.
DOI URL |
| [9] |
RAVI M, RANOCCHIARI M, VAN BOKHOVEN J A. The direct catalytic oxidation of methane to methanol-a critical assessment. Angewandte Chemie International Edition, 2017, 56(52): 16464.
DOI URL |
| [10] |
IGLESIAS-JUEZ, FRESNO A F, CORONADO J M, et al. Emerging high-prospect applications in photothermal catalysis. Current Opinion in Green and Sustainable Chemistry, 2022, 37: 100652.
DOI URL |
| [11] | LUO Y R. Comprehensive handbook of chemical bond energies. Boca Raton: CRC Press, 2007, 19. |
| [12] | DIETL N, ENGESER M, SCHWARZ H. Competitive hydrogen- atom abstraction versus oxygen-atom and electron transfers in gas-phase reactions of [X4O10] (X = P, V) with C2H4. Chemistry Europe, 2010, 16(15): 4452. |
| [13] |
DIETL N, SCHLANGEN M, SCHWARZ H. Thermal hydrogen- atom transfer from methane: the role of radicals and spin states in oxo-cluster chemistry. Angewandte Chemie International Edition, 2012, 51(23): 5544.
DOI URL |
| [14] |
YULIATI L, YOSHIDA H. Photocatalytic conversion of methane. Chemical Society Reviews, 2008, 37(8): 1592.
DOI PMID |
| [15] |
CHEN J, ARANDIYAN H, GAO X, et al. Recent advances in catalysts for methane combustion. Catalysis Surveys from Asia, 2015, 19(3): 140.
DOI URL |
| [16] |
WANG C, XU Y, TANG J. Catalytic methane removal to mitigate its environmental effect. Science China Chemistry, 2023, 66: 1032.
DOI |
| [17] |
FENG X, JIANG L, LI D, et al. Progress and key challenges in catalytic combustion of lean methane. Journal of Energy Chemistry, 2022, 75: 173.
DOI |
| [18] |
SCHWARTZ W R, CIUPARU D, PFEFFERLE L D. Combustion of methane over palladium-based catalysts: catalytic deactivation and role of the support. The Journal of Physical Chemistry C, 2012, 116(15): 8587.
DOI URL |
| [19] |
GARETTO T F, APESTEGUIA C R. Oxidative catalytic removal of hydrocarbons over Pt/Al2O3 catalysts. Catalysis Today, 2000, 62: 189.
DOI URL |
| [20] |
NEUBERG S, PENNEMANN H, SHANMUGAM V, et al. Promoting effect of Rh on the activity and stability of Pt-based methane combustion catalyst in microreactors. Catalysis Communications, 2021, 149: 106202.
DOI URL |
| [21] |
CHOUDHARY R V, PATIL V P, JANA P, et al. Nano-gold supported on Fe2O3: a highly active catalyst for low temperature oxidative destruction of methane green house gas from exhaust/waste gases. Applied Catalysis A: General, 2008, 350(2): 186.
DOI URL |
| [22] |
DING Y, WU Q, LIN B, et al. Superior catalytic activity of a Pd catalyst in methane combustion by fine-tuning the phase of ceria-zirconia support. Applied Catalysis B: Environmental, 2020, 266: 118631.
DOI URL |
| [23] |
XIAO L H, SUN K P, XU X L, et al. Low-temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition- precipitation method. Catalysis Communications, 2005, 6(12): 796.
DOI URL |
| [24] |
KINNUNEN N M, SUVANTO M, MORENO M A, et al. Methane oxidation on alumina supported palladium catalysts: effect of Pd precursor and solvent. Applied Catalysis A: General, 2009, 370(1/2): 78.
DOI URL |
| [25] |
HONG E, KIM C, LIM D H, et al. Catalytic methane combustion over Pd/ZrO2 catalysts: effects of crystalline structure and textural properties. Applied Catalysis B: Environmental, 2018, 232: 544.
DOI URL |
| [26] |
VENEZIA A M, DI CARLO G, PANTALEO G, et al. Oxidation of CH4 over Pd supported on TiO2-doped SiO2: effect of Ti(IV) loading and influence of SO2. Applied Catalysis B: Environmental, 2009, 88(3/4): 430.
DOI URL |
| [27] |
SEKIZAWA KOSHI, WIDJIAJA H, SHINGO MAEDA, et al. Low temperature oxidation of methane over Pd catalyst supported on metal oxides. Catalysis Today, 2000, 59: 69.
DOI URL |
| [28] |
HUANG W X, JOHNSTON-PECK A C, WOLTER T, et al. Steam-created grain boundaries for methane C-H activation in palladium catalysts. Science, 2021, 373(6562): 1518.
DOI PMID |
| [29] |
LUO L, WANG S, FAN C, et al. Promoting effect of alkali metal cations on the catalytic performance of Pd/H-ZSM-5 in the combustion of lean methane. Applied Catalysis A: General, 2020, 602: 117678.
DOI URL |
| [30] | CORRO G, TORRALBA R, PAL U, et al. Total oxidation of methane over Pt/Cr2O3 catalyst at low temperature: effect of Pt0-Ptx+ dipoles at the metal-support interface. The Journal of Physical Chemistry C, 2019, 123(5): 28823. |
| [31] | PECCHIA G, REYES P, GOÂMEZB R, et al. Methane combustion on Rh/ZrO2 catalysts. Applied Catalysis B: Environmental, 1998, 17: L13. |
| [32] |
GRISEL R J H, KOOYMAN P J, NIEUWENHUYS B E. Influence of the preparation of Au/Al2O3 on CH4oxidation activity. Journal of Catalysis, 2000, 191(2): 430.
DOI URL |
| [33] |
ZHANG Y, WANG X, ZHU Y, et al. Thermal evolution crystal structure and Fe crystallographic sites in LaFexAl12-xO19 hexaaluminates. The Journal of Physical Chemistry C, 2014, 118(20): 10792.
DOI URL |
| [34] |
WANG Z, HAO Z, SHI F, et al. Boosting the oxygen evolution reaction through migrating active sites from the bulk to surface of perovskite oxides. Journal of Energy Chemistry, 2022, 69: 434.
DOI |
| [35] |
YU Q, LIU C, LI X, et al. N-doping activated defective Co3O4 as an efficient catalyst for low-temperature methane oxidation. Applied Catalysis B: Environmental, 2020, 269: 118757.
DOI URL |
| [36] | HUANG F, WANG X, WANG A, et al. A two-step synthesis of Fe-substituted hexaaluminates with enhanced surface area and activity in methane catalytic combustion. Catalysis Science & Technology, 2016, 6(13): 4962. |
| [37] |
MIAO F, WANG F, MAO D, et al. Effect of different reaction conditions on catalytic activity of La(Mn, Fe)O3+λ catalyst for methane combustion. Materials Research Express, 2019, 6(5): 055001.
DOI URL |
| [38] |
JIANG L, LI D, DENG G, et al. Design of hybrid La1-xCexCoO3- catalysts for lean methane combustion via creating active Co and Ce species. Chemical Engineering Journal, 2023, 456: 141054.
DOI URL |
| [39] |
PENG H, RAO C, ZHANG N, et al. Confined ultrathin Pd-Ce nanowires with outstanding moisture and SO2 tolerance in methane combustion. Angewandte Chemie International Edition, 2018, 57(29): 8953.
DOI URL |
| [40] | XIAO Y, LI J, WANG C, et al. Construction and evolution of active palladium species on phase-regulated reducible TiO2 for methane combustion. Catalysis Science & Technology, 2021, 11(3): 836. |
| [41] | PETROV A W, FERRI D, KRUMEICH F, et al. Stable complete methane oxidation over palladium based zeolite catalysts. Nature Communcations, 2018, 9(1): 2545. |
| [42] |
XIONG H, KUNWAR D, JIANG D, et al. Engineering catalyst supports to stabilize PdOx two-dimensional rafts for water-tolerant methane oxidation. Nature Catalysis, 2021, 4(10): 830.
DOI |
| [43] |
CHEN J, WANG X, ZHANG L, et al. Strong metal-support interaction assisted redispersion strategy for obtaining ultrafine and stable IrO2/Ir active sites with exceptional methane oxidation activity. Applied Catalysis B: Environmental, 2021, 297: 120410.
DOI URL |
| [44] |
MACHOCKI A, IOANNIDES T, STASINKSA B, et al. Manganese- lanthanum oxides modified with silver for the catalytic combustion of methane. Journal of Catalysis, 2004, 227(2): 282.
DOI URL |
| [45] |
HAO Y J, TIAN L G, DUAN E, et al. Low-temperature methane oxidation triggered by peroxide radicals over noble-metal-free MgO Catalyst. ACS Applied Materials Interfaces, 2020, 12(19): 21761.
DOI URL |
| [46] |
WANG Y G, REN J R, WANG Y Q, et al. Nanocasted synthesis of mesoporous LaCoO3 perovskite with extremely high surface area and excellent activity in methane combustion. The Journal of Physical Chemistry C, 2008, 112: 15293.
DOI URL |
| [47] | TAO F F, SHAN J J, NGUYEN L, et al. Understanding complete oxidation of methane on spinel oxides at a molecular level. Nature Communcations, 2015, 6: 7798. |
| [48] |
ARANDIYAN H, DAI H, DENG J, et al. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 with high surface areas: active catalysts for the combustion of methane. Journal of Catalysis, 2013, 307: 327.
DOI URL |
| [49] |
FAN X, LI L, JING F, et al. Effects of preparation methods on CoAlOx/CeO2catalysts for methane catalytic combustion. Fuel, 2018, 225: 588.
DOI URL |
| [50] |
COLUSSI S, FORNASIERO P, TROVARELLI A. Structure- activity relationship in Pd/CeO2 methane oxidation catalysts. Chinese Journal of Catalysis, 2020, 41(6): 938.
DOI URL |
| [51] |
CHEN J, WU Y, HU W, et al. New insights into the role of Pd-Ce interface for methane activation on monolithic supported Pd catalysts: a step forward the development of novel PGM three-way catalysts for natural gas fueled engines. Applied Catalysis B: Environmental, 2020, 264: 118475.
DOI URL |
| [52] |
EISWIRTH M, BURGER P, STRASSER P, et al. Oscillating langmuir-hinshelwood mechanisms. Journal of Physical Chemistry, 1996, 100: 19118.
DOI URL |
| [53] |
YANG J, HU S, SHI L, et al. Oxygen vacancies and lewis acid sites synergistically promoted catalytic methane combustion over perovskite oxides. Environmental Science & Technology, 2021, 55(13): 9243.
DOI URL |
| [54] |
KWON Y, KIM T Y, KWON G, et al. Selective activation of methane on single-atom catalyst of rhodium dispersed on zirconia for direct conversion. Journal of the American Chemical Society, 2017, 139(48): 17694.
DOI PMID |
| [55] |
LI Q, OUYANG Y, LI H, et al. Photocatalytic conversion of methane: recent advancements and pospects. Angewandte Chemie International Edition, 2022, 61(2): e202108069.
DOI URL |
| [56] | CHEN X, LI Y, PAN X, et al. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nature Communcations, 2016, 7: 12273. |
| [57] |
LIANG X, WANG P, GAO Y, et al. Design and synthesis of porous M-ZnO/CeO2 microspheres as efficient plasmonic photocatalysts for nonpolar gaseous molecules oxidation: insight into the role of oxygen vacancy defects and M=Ag, Au nanoparticles. Applied Catalysis B: Environmental, 2020, 260: 118151.
DOI URL |
| [58] |
LI Z, PAN X, YI Z. Photocatalytic oxidation of methane over CuO-decorated ZnO nanocatalysts. Journal of Materials Chemistry A, 2019, 7(2): 469.
DOI |
| [59] | LI Z, BODA M A, PAN X, et al. Photocatalytic oxidation of small molecular hydrocarbons over ZnO nanostructures: the difference between methane and ethylene and the impact of polar and nonpolar facets. ACS Sustainable Chemistry & Engineering, 2019, 7(23): 19042. |
| [60] |
WEI J, YANG J, WEN Z, et al. Efficient photocatalytic oxidation of methane over β-Ga2O3/activated carbon composites. RSC Advances, 2017, 7(60): 37508.
DOI URL |
| [61] |
FU C, LI F, YANG J, et al. Spontaneous bulk-surface charge separation of TiO2-{001} nanocrystals leads to high activity in photocatalytic methane combustion. ACS Catalysis, 2022, 12(11): 6457.
DOI URL |
| [62] |
YU X, ZHOLOBENKE V L, OLDOVAN S, et al. Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature. Nature Energy, 2020, 5(7): 511.
DOI |
| [63] |
JIANG Y, ZHAO W, LI S, et al. Elevating photooxidation of methane to formaldehyde via TiO2 crystal phase engineering. Journal of the American Chemical, 2022, 144(35): 15977.
DOI URL |
| [64] |
WANG F, LIANG X, WANG P, et al. Ag/AgCl as an efficient plasmonic photocatalyst for greenhouse gaseous methane oxidation. Journal of Environmental Chemical Engineering, 2021, 9(6): 106435.
DOI URL |
| [65] |
PAN X, CHEN X, YI Z. Photocatalytic oxidation of methane over SrCO3 decorated SrTiO3 nanocatalysts via a synergistic effect. Physical Chemistry Chemical Physics, 2016, 18(46): 31400.
DOI URL |
| [66] |
WANG J, XU R, XIA Y, et al. Ti2CTx MXene: a novel p-type sensing material for visible light-enhanced room temperature methane detection. Ceramics International, 2021, 47(24): 34437.
DOI URL |
| [67] |
YANG Z, ZHANG Q, REN L, et al. Efficient photocatalytic conversion of CH4 into ethanol with O2 over nitrogen vacancy-rich carbon nitride at room temperature. Chemical Communication, 2021, 57(7): 871.
DOI URL |
| [68] |
ZHANG W, FU C, LOW J, et al. High-performance photocatalytic nonoxidative conversion of methane to ethane and hydrogen by heteroatoms-engineered TiO2. Nature Communications, 2022, 13(1): 2806.
DOI PMID |
| [69] |
MAYERNICK A D, JANIK M J. Methane oxidation on Pd-ceria: a DFT study of the mechanism over PdxCe1-xO2, Pd, and PdO. Journal of Catalysis, 2011, 278(1): 16.
DOI URL |
| [70] |
YANG J, XIAO W, CHI X, et al. Solar-driven efficient methane catalytic oxidation over epitaxial ZnO/La0.8Sr0.2CoO3 heterojunctions. Applied Catalysis B: Environmental, 2020, 265: 118469.
DOI URL |
| [71] |
FENG X, LIU D, YAN B, et al. Highly active PdO/Mn3O4/CeO2 nanocomposites supported on one dimensional halloysite nanotubes for photoassisted thermal catalytic methane combustion. Angewandte Chemie International Edition, 2021, 60(34): 18552.
DOI URL |
| [72] |
KANG L, LIU X Y, WANG A, et al. Photo-thermo catalytic oxidation over a TiO2-WO3-supported platinum catalyst. Angewandte Chemie International Edition, 2020, 59(31): 12909.
DOI URL |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [8] | 范小暄, 郑永炅, 徐丽荣, 姚子敏, 曹硕, 王可心, 王绩伟. 基于富氧空位LiYScGeO4: Bi3+长余辉光催化剂的自激活余辉驱动有机污染物芬顿降解[J]. 无机材料学报, 2025, 40(5): 481-488. |
| [9] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [10] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [11] | 贾相华, 张辉霞, 刘艳凤, 左桂鸿. 湿化学法制备Cu2O/Cu空心球异质结光催化剂[J]. 无机材料学报, 2025, 40(4): 397-404. |
| [12] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [13] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [14] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [15] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||