无机材料学报 ›› 2025, Vol. 40 ›› Issue (4): 397-404.DOI: 10.15541/jim20240370 CSTR: 32189.14.10.15541/jim20240370
所属专题: 【能源环境】污染物催化去除(202506)
收稿日期:2024-08-12
修回日期:2024-11-05
出版日期:2025-04-20
网络出版日期:2024-12-11
作者简介:贾相华(1981-), 女, 副教授. E-mail: jiaxianghua2000@163.com
基金资助:
JIA Xianghua( ), ZHANG Huixia, LIU Yanfeng, ZUO Guihong
), ZHANG Huixia, LIU Yanfeng, ZUO Guihong
Received:2024-08-12
Revised:2024-11-05
Published:2025-04-20
Online:2024-12-11
About author:JIA Xianghua (1981-), female, associate professor. E-mail: jiaxianghua2000@163.com
Supported by:摘要:
窄带隙半导体作为可见光光催化剂, 可以有效地将太阳能转化为化学能, 在缓解能源短缺和环境污染方面具有潜在的应用前景。以氯化铜(CuCl2·H2O)为前驱体, 盐酸羟胺(H3NO·HCl)和硼氢化钠(NaBH4)为还原剂, 采用一锅无模板湿化学还原法制备Cu2O/Cu空心球异质结光催化剂。采用不同表征手段对样品形貌、晶体结构、组成、比表面积和光学性质进行分析。加入NaBH4使Cu2O/Cu形貌从中空截角八面体逐渐演化为中空纳米球。通过改变NaBH4加入量可以控制Cu2O表面Cu层厚度。NaBH4直接还原Cu2O使Cu2O和Cu界面紧密结合, 这有利于载流子分离和传输, Cu的表面等离子体共振(SPR)效应使Cu2O/Cu对可见光的吸收能力增强, 因此Cu2O/Cu对甲基橙(MO)和无色恩诺沙星(ENR)均表现出良好的光催化活性。连续循环使用5次, Cu2O/Cu对MO的降解率影响不大。捕获实验表明∙O2-和空穴是降解MO过程中主要的活性物质。Cu2O/Cu的良好光催化活性归因于Cu2O和Cu的协同作用。本研究为异质结光催化剂的制备提供了一种策略。
中图分类号:
贾相华, 张辉霞, 刘艳凤, 左桂鸿. 湿化学法制备Cu2O/Cu空心球异质结光催化剂[J]. 无机材料学报, 2025, 40(4): 397-404.
JIA Xianghua, ZHANG Huixia, LIU Yanfeng, ZUO Guihong. Cu2O/Cu Hollow Spherical Heterojunction Photocatalysts Prepared by Wet Chemical Approach[J]. Journal of Inorganic Materials, 2025, 40(4): 397-404.
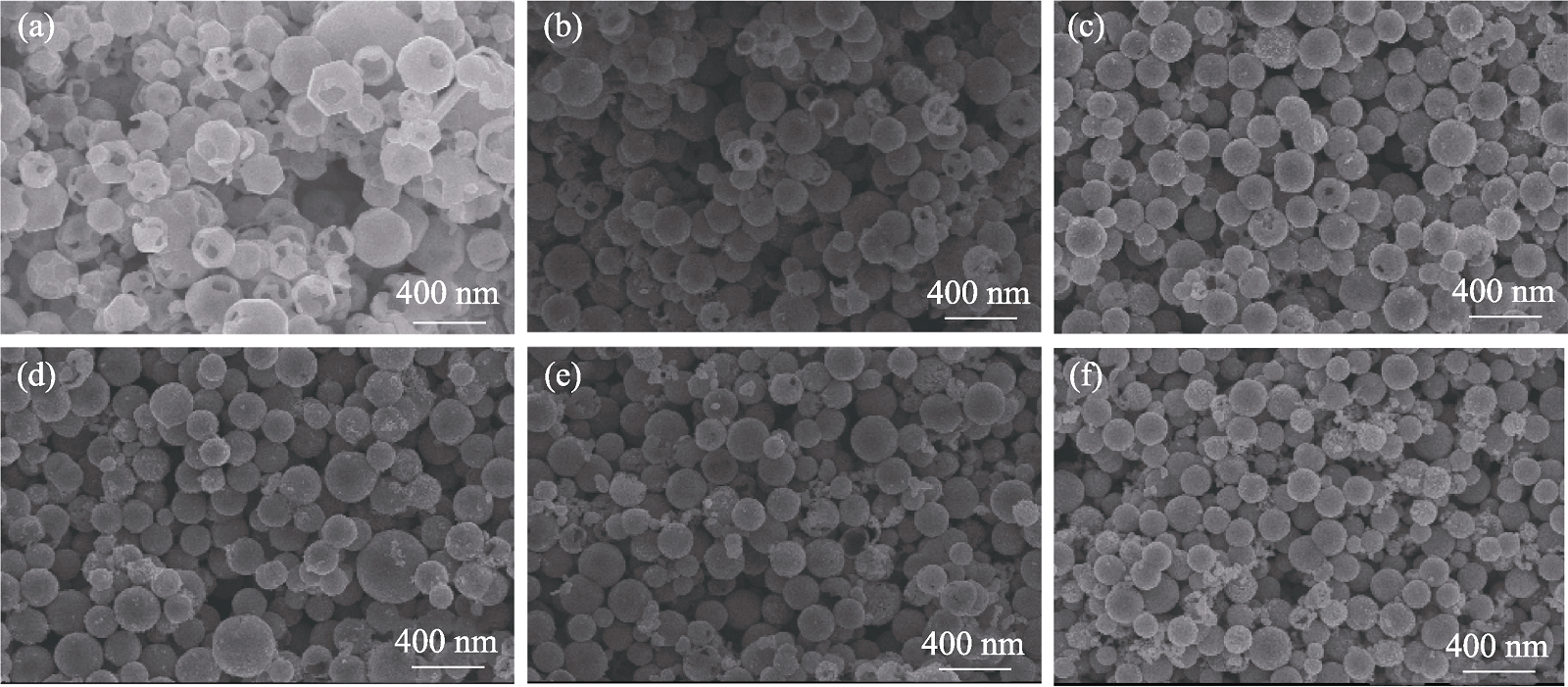
图2 纯Cu2O和Cu2O/Cu样品的SEM照片
Fig. 2 SEM images of the pure Cu2O and Cu2O/Cu samples (a) Pure Cu2O; (b-f) Cu2O/Cu with 50 mL addition of NaBH4 at concentration of (b) 0.0067, (c) 0.0080, (d) 0.0100, (e) 0.0130, and (f) 0.0200 mol/L
| Sample | Specific surface area/(cm2·g-1) | Average pore diameter/nm | Pore volume/ (cm3·g-1) |
|---|---|---|---|
| Pure Cu2O | 34.23 | 85.23 | 0.286 |
| Cu2O/Cu-0.0067 | 37.11 | 55.34 | 0.279 |
| Cu2O/Cu-0.0080 | 39.01 | 34.24 | 0.274 |
| Cu2O/Cu-0.0100 | 41.19 | 32.56 | 0.263 |
| Cu2O/Cu-0.0130 | 64.45 | 30.26 | 0.246 |
| Cu2O/Cu-0.0200 | 88.79 | 26.35 | 0.221 |
表1 纯Cu2O和Cu2O/Cu样品的比表面积、平均孔径和孔容
Table 1 Specific surface area, average pore diameter and pore volume of the pure Cu2O and Cu2O/Cu samples
| Sample | Specific surface area/(cm2·g-1) | Average pore diameter/nm | Pore volume/ (cm3·g-1) |
|---|---|---|---|
| Pure Cu2O | 34.23 | 85.23 | 0.286 |
| Cu2O/Cu-0.0067 | 37.11 | 55.34 | 0.279 |
| Cu2O/Cu-0.0080 | 39.01 | 34.24 | 0.274 |
| Cu2O/Cu-0.0100 | 41.19 | 32.56 | 0.263 |
| Cu2O/Cu-0.0130 | 64.45 | 30.26 | 0.246 |
| Cu2O/Cu-0.0200 | 88.79 | 26.35 | 0.221 |
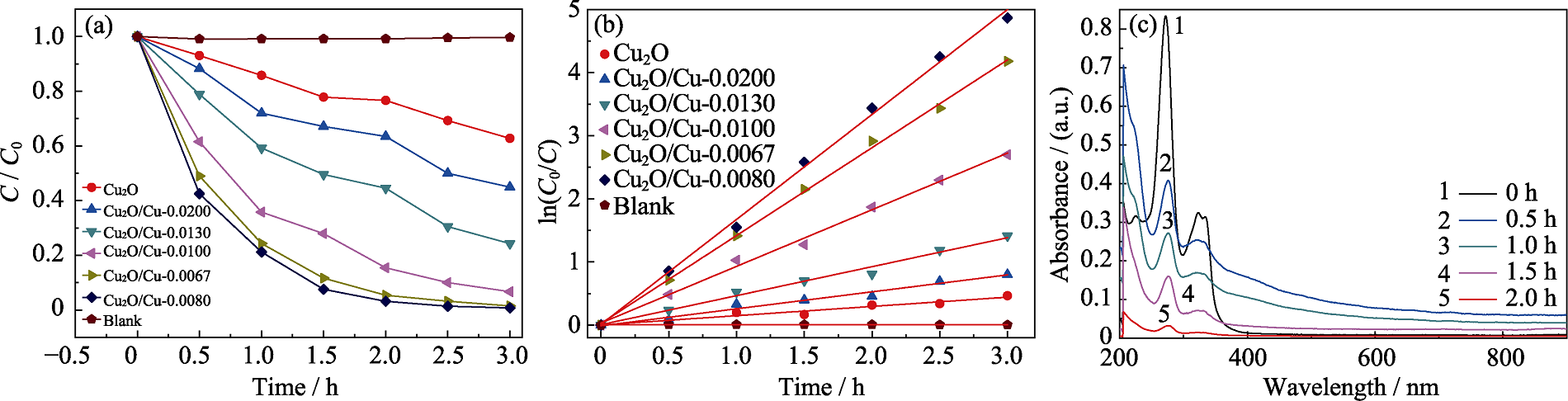
图8 纯Cu2O和Cu2O/Cu样品对MO和ENR的催化性能
Fig. 8 Catalytic performance of the pure Cu2O and Cu2O/Cu catalysts for MO and ENR (a) Catalytic degradation curves of the pure Cu2O and Cu2O/Cu catalysts for MO; (b) Fitting plots of pseudo-first-order kinetics; (c) Absorption spectra of Cu2O/Cu-0.0080 catalyst for ENR
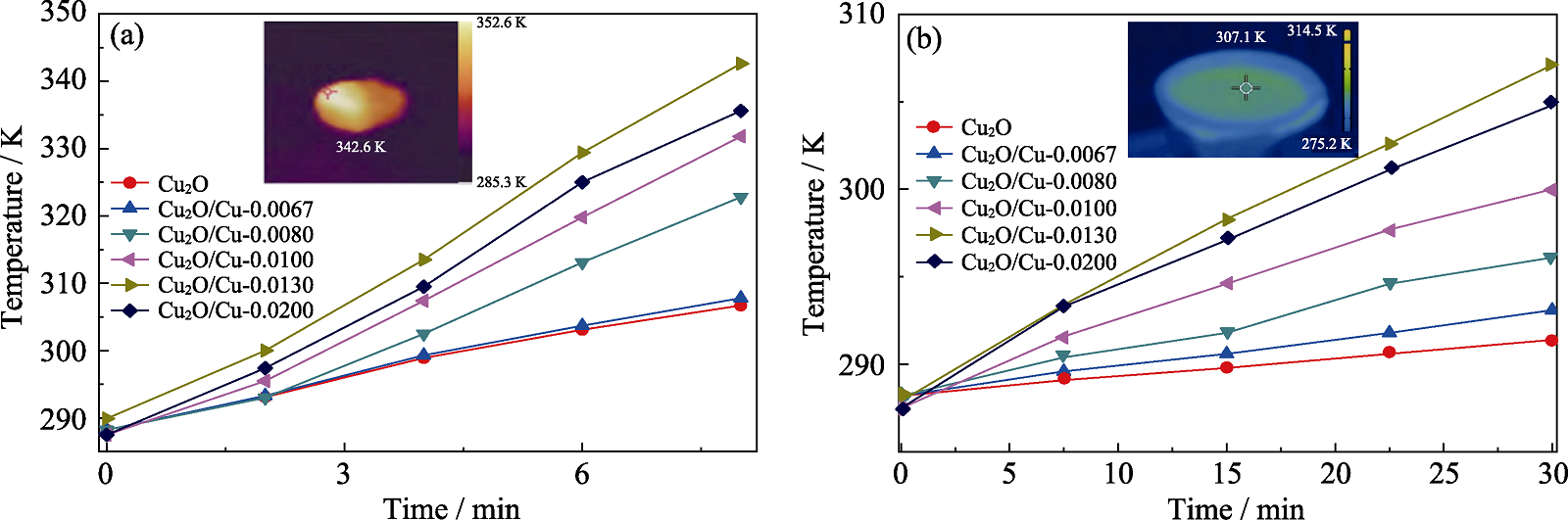
图10 纯Cu2O和Cu2O/Cu样品表面温度随时间的变化
Fig. 10 Time-dependent surface temperature changes of the pure Cu2O and Cu2O/Cu samples (a) Time-dependent surface temperature under irradiation with inset showing infrared thermal image of Cu2O/Cu-0.0130 sample irradiated for 8 min; (b) Time-dependent surface temperature during photocatalysis with inset showing infrared thermal image of Cu2O/Cu-0.0130 sample photocatalytic degradation for 30 min
| [1] | SINGHA A, KAISHYOP J, KHAN T S, et al. Visible-light-driven toluene oxidation to benzaldehyde over WO3 nanostructures. ACS Applied Nano Materials, 2023, 6(23):21818. |
| [2] | JIAN L, ZHAO H, DONG Y M, et al. Graphite carbon ring modified carbon nitride with a strong built-in electric field for high photocatalysis-self-Fenton performance. Catalysis Science & Technology, 2022, 12(24):7379. |
| [3] | TOE C Y, LAMERS M, DITTRICH T, et al. Facet-dependent carrier dynamics of cuprous oxide regulating the photocatalytic hydrogen generation. Materials Advances, 2022, 3(4): 2200. |
| [4] | ONG W J, TAN L L, CHAI S P, et al. Highly reactive {001} facets of TiO2-based composites: synthesis, formation mechanism and characterization. Nanoscale, 2014, 6(4): 1946. |
| [5] | YANG C, LIU Z L, SU Z L, et al. Combined effect of oxygen vacancies and mesopore sizes in ZnO/SiO2 adsorbents on boosting the H2S removal efficiency in moist conditions. Advanced Functional Materials, 2024, 34(49):2409214. |
| [6] | LI W J, DA P M, ZHANG Y Y, et al. WO3 nanoflakes for enhanced photoelectrochemical conversion. ACS Nano, 2014, 8(11):11770. |
| [7] | BANIAMERIAN H, SHOKROLLAHZADEH S, SAFAVI M, et al. Visible-light-activated Fe2O3-TiO2 nanoparticles enhance biofouling resistance of polyethersulfone ultrafiltration membranes against marine algae Chlorella vulgaris. Scientific Reports, 2024, 14: 24831. |
| [8] | XIONG L K, ZHANG X, CHEN L, et al. Geometric modulation of local CO flux in Ag@Cu2O nanoreactors for steering the CO2RR pathway toward high-efficacy methane production. Advanced Materials, 2021, 33(32):2101741. |
| [9] | LIU W Q, BAI P Y, WEI S L, et al. Electron-rich Cu0-Cu2O heterogeneous interface constructed via controllable electrochemical reconstruction for a single CO2 deep-reduction product ethylene. Applied Catalysis B: Environment and Energy, 2024, 348: 123831. |
| [10] | KIM H E, WI D H, LEE J S, et al. Photoelectrochemical nitrate and nitrite reduction using Cu2O photocathodes. ACS Energy Letters, 2024, 9(5): 1993. |
| [11] | WU E T, HUANG M H. Photocatalytic oxidative amine coupling with 4-nitrophenylacetylene-modified Cu2O polyhedra. ACS Catalysis, 2023, 13(22):14746. |
| [12] | CHEN B H, KUMAR G, WEI Y J, et al. Experimental revelation of surface and bulk lattices in faceted Cu2O crystals. Small, 2023, 19(44):2303491. |
| [13] | ZHANG Y Y, CHEN Y X, WANG X W, et al. Low-coordinated copper facilitates the *CH2CO affinity at enhanced rectifying interface of Cu/Cu2O for efficient CO2-to-multicarbon alcohols conversion. Nature Communications, 2024, 15: 5172. |
| [14] | ZHANG Z H, SONG R, YU Z Y, et al. Crystal-plane effect of Cu2O templates on compositions, structures and catalytic performance of Ag/Cu2O nanocomposites. CrystEngComm, 2019, 21(12): 2002. |
| [15] | ZHU M Y, CHENG Y K, LUO Q, et al. A review of synthetic approaches to hollow nanostructures. Materials Chemistry Frontiers, 2021, 5(6):2552. |
| [16] |
ZHANG F, LIU T Y, LI M Y, et al. Multiscale pore network boosts capacitance of carbon electrodes for ultrafast charging. Nano Letters, 2017, 17(5):3097.
DOI PMID |
| [17] | POOLAKKANDY R R, MENAMPARAMBATH M M. Soft- template-assisted synthesis: a promising approach for the fabrication of transition metal oxides. Nanoscale Advances, 2020, 2(11):5015. |
| [18] |
CUI L L, WANG C C, ZHANG H W, et al. Facile one-step dialysis strategy for fabrication of hollow complex nanoparticles. Chemical Communications, 2019, 55(62):9120.
DOI PMID |
| [19] | FANG Y J, YU X Y, LOU X W. Formation of hierarchical Cu-doped CoSe2 microboxes via sequential ion exchange for high-performance sodium-ion batteries. Advanced Materials, 2018, 30(21):1706668. |
| [20] | DONG Y L, TAO F F, WANG L X, et al. One-pot preparation of hierarchical Cu2O hollow spheres for improved visible-light photocatalytic properties. RSC Advances, 2020, 10(38):22387. |
| [21] | LV T T, XING H Z, YANG H M, et al. Rapid synthesis of Cu2O hollow spheres at low temperature and their catalytic performance for the decomposition of ammonium perchlorate. CrystEngComm, 2021, 23(45):7985. |
| [22] | LIU B Q, YAO X, ZHANG Z J, et al. Synthesis of Cu2O nanostructures with tunable crystal facets for electrochemical CO2 reduction to alcohols. ACS Applied Materials & Interfaces, 2021, 13(33):39165. |
| [23] | ZHOU B, LIU Z G, ZHANG H J, et al. One-pot synthesis of Cu2O/Cu self-assembled hollow nanospheres with enhanced photocatalytic performance. Journal of Nanomaterials, 2014, 2014: 291964. |
| [24] | CHANG J Y, BAO Q W, ZHANG C, et al. Rapid preparation and photocatalytic properties of octahedral Cu2O@Cu powders. Advanced Powder Technology, 2021, 32(1):144. |
| [25] |
CHANG Y, TEO J J, ZENG H C. Formation of colloidal CuO nanocrystallites and their spherical aggregation and reductive transformation to hollow Cu2O nanospheres. Langmuir, 2005, 21(3):1074.
PMID |
| [26] | ZHU S C, CHEN Z Y, LIU Z P, et al. Thermodynamics and catalytic activity of the reduced Cu on a Cu2O surface from machine learning atomic simulation. ACS Materials Letters, 2024, 6(8):3690. |
| [27] | YUE Y M, ZHANG P X, WANG W, et al. Enhanced dark adsorption and visible-light-driven photocatalytic properties of narrower-band-gap Cu2S decorated Cu2O nanocomposites for efficient removal of organic pollutants. Journal of Hazardous Materials, 2020, 384: 121302. |
| [28] | BAO Y C, CHEN K Z. A novel Z-scheme visible light driven Cu2O/Cu/g-C3N4 photocatalyst using metallic copper as a charge transfer mediator. Molecular Catalysis, 2017, 432: 187. |
| [29] | SARAEV A A, KURENKOVA A Y, MISHCHENKO D D, et al. Cu/TiO2 photocatalysts for CO2 reduction: structure and evolution of the cocatalyst active form. Transactions of Tianjin University, 2024, 30(2):140. |
| [30] | XU B G, WANG B, ZHANG H Y, et al. Z-scheme Cu2O nanoparticle/graphite carbon nitride nanosheet heterojunctions for photocatalytic hydrogen evolution. ACS Applied Nano Materials, 2022, 5(6):8475. |
| [31] | LI Z J, WANG M Z, JIA Y Y, et al. CeO2/Cu2O/Cu tandem interfaces for efficient water-gas shift reaction catalysis. ACS Applied Materials & Interfaces, 2023, 15(26):31584. |
| [32] | LI D Y, ZAN J, WU L P, et al. Heterojunction tuning and catalytic efficiency of g-C3N4-Cu2O with glutamate. Industrial & Engineering Chemistry Research, 2019, 58(10):4000. |
| [33] | ZHANG Z J, WANG W Z, GAO E P, et al. Photocatalysis coupled with thermal effect induced by SPR on Ag-loaded Bi2WO6 with enhanced photocatalytic activity. The Journal of Physical Chemistry C, 2012, 116(49):25898. |
| [34] | WANG Y B, ZHAO X, CAO D, et al. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Applied Catalysis B: Environmental, 2017, 211: 79. |
| [35] | MEI W D, LI D Y, XU H M, et al. Effect of electronic migration of MIL-53(Fe) on the activation of peroxymonosulfate under visible light. Chemical Physics Letters, 2018, 706: 694. |
| [36] | ZHANG H, DIAO J F, LIU Y H, et al. In situ-grown Cu dendrites plasmonically enhance electrocatalytic hydrogen evolution on facet-engineered Cu2O. Advanced Materials, 2023, 35(42):2305742. |
| [1] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [2] | 范小暄, 郑永炅, 徐丽荣, 姚子敏, 曹硕, 王可心, 王绩伟. 基于富氧空位LiYScGeO4: Bi3+长余辉光催化剂的自激活余辉驱动有机污染物芬顿降解[J]. 无机材料学报, 2025, 40(5): 481-488. |
| [3] | 马彬彬, 钟婉菱, 韩涧, 陈椋煜, 孙婧婧, 雷彩霞. ZIF-8/TiO2复合介观晶体的制备及光催化活性[J]. 无机材料学报, 2024, 39(8): 937-944. |
| [4] | 曹青青, 陈翔宇, 吴健豪, 王筱卓, 王乙炫, 王禹涵, 李春颜, 茹菲, 李兰, 陈智. SiO2增强自敏性氮化碳微球可见光降解盐酸四环素的研究[J]. 无机材料学报, 2024, 39(7): 787-792. |
| [5] | 王兆阳, 秦鹏, 蒋胤, 冯小波, 杨培志, 黄富强. 三明治结构钌插层二氧化钛光催化四环素降解性能研究[J]. 无机材料学报, 2024, 39(4): 383-389. |
| [6] | 叶茂森, 王耀, 许冰, 王康康, 张胜楠, 冯建情. II/Z型Bi2MoO6/Ag2O/Bi2O3异质结可见光催化降解四环素[J]. 无机材料学报, 2024, 39(3): 321-329. |
| [7] | 李秋实, 殷广明, 吕伟超, 王怀尧, 李婧琳, 杨红光, 关芳芳. Na+/g-C3N4材料的制备及光催化降解亚甲基蓝机理[J]. 无机材料学报, 2024, 39(10): 1143-1150. |
| [8] | 李跃军, 曹铁平, 孙大伟. S型异质结Bi4O5Br2/CeO2的制备及其光催化CO2还原性能[J]. 无机材料学报, 2023, 38(8): 963-970. |
| [9] | 伍林, 胡明蕾, 王丽萍, 黄少萌, 周湘远. TiHAP@g-C3N4异质结的制备及光催化降解甲基橙[J]. 无机材料学报, 2023, 38(5): 503-510. |
| [10] | 凌洁, 周安宁, 王文珍, 贾忻宇, 马梦丹. Cu/Mg比对Cu/Mg-MOF-74的CO2吸附性能的影响[J]. 无机材料学报, 2023, 38(12): 1379-1386. |
| [11] | 孙晨, 赵昆峰, 易志国. 甲烷完全催化氧化研究进展[J]. 无机材料学报, 2023, 38(11): 1245-1256. |
| [12] | 贾鑫, 李晋宇, 丁世豪, 申倩倩, 贾虎生, 薛晋波. Pd纳米颗粒协同氧空位增强TiO2光催化CO2还原性能[J]. 无机材料学报, 2023, 38(11): 1301-1308. |
| [13] | 马润东, 郭雄, 施凯旋, 安胜利, 王瑞芬, 郭瑞华. MoS2/g-C3N4 S型异质结的构建及光催化性能研究[J]. 无机材料学报, 2023, 38(10): 1176-1182. |
| [14] | 马心全, 李喜宝, 陈智, 冯志军, 黄军同. S型异质结BiOBr/ZnMoO4的构建及光催化降解性能研究[J]. 无机材料学报, 2023, 38(1): 62-70. |
| [15] | 陈瀚翔, 周敏, 莫曌, 宜坚坚, 李华明, 许晖. CoN/g-C3N4 0D/2D复合结构及其光催化制氢性能研究[J]. 无机材料学报, 2022, 37(9): 1001-1008. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||