无机材料学报 ›› 2025, Vol. 40 ›› Issue (5): 552-562.DOI: 10.15541/jim20240345 CSTR: 32189.14.10.15541/jim20240345
所属专题: 【能源环境】氢能材料(202506)
• 研究快报 • 上一篇
陈莉波1( ), 盛盈1, 伍明1(
), 盛盈1, 伍明1( ), 宋季岭2, 蹇建1, 宋二红3(
), 宋季岭2, 蹇建1, 宋二红3( )
)
收稿日期:2024-07-20
修回日期:2024-10-15
出版日期:2025-05-20
网络出版日期:2024-10-28
通讯作者:
伍 明, 副教授. E-mail: wuming10@mails.jlu.edu.cn;作者简介:陈莉波(2000-), 男, 硕士研究生. E-mail: 1871066627@qq.com
CHEN Libo1( ), SHENG Ying1, WU Ming1(
), SHENG Ying1, WU Ming1( ), SONG Jiling2, JIAN Jian1, SONG Erhong3(
), SONG Jiling2, JIAN Jian1, SONG Erhong3( )
)
Received:2024-07-20
Revised:2024-10-15
Published:2025-05-20
Online:2024-10-28
Contact:
WU Ming, associate professor. E-mail: wuming10@mails.jlu.edu.cn;About author:CHEN Libo (2000-), male, Master candidate. E-mail: 1871066627@qq.com
Supported by:摘要:
元素掺杂可以调控氮化碳(CN)能带结构以获取更好的光催化性能。本研究通过柠檬酸钠和纯CN粉末的固相反应, 在180 ℃空气气氛下制备了Na和O共掺杂CN(Na/O-CNx, x=1.0、2.0、3.0、4.0)。Na/O-CN3.0的比表面积达到18.8 m2/g, 比纯CN(11.7 m2/g)提升了60.7%。Na/O-CN3.0样品的能带宽度为2.68 eV, 略低于纯CN(2.70 eV), 前者有助于可见光吸收。Na和O元素共掺杂有效抑制了材料的光生电子-空穴对复合, 提升了太阳光的利用率。因此, Na/O-CNx样品在可见光条件下的光催化制氢效率得到了显著提升, 最优催化剂Na/O-CN3.0的光催化制氢效率为103.2 μmol∙g-1∙h-1, 相比于纯CN(11.2 μmol∙g-1∙h-1)提升了8.2倍, 同时表现出良好的催化稳定性。此外, 通过调节反应气体中氧含量制备了一系列Na/O-CN3.0-yO2(y=0、20%、40%、60%、80%、100%)样品, 催化性能结果揭示了在Na/O-CNx样品中掺杂Na和O原子均有助于提升光催化性能。本工作为较低温度下制备金属原子掺杂CN材料提供了新思路, 揭示了Na和O原子在Na/O-CNx光催化制氢过程中的协同效应。
中图分类号:
陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562.
CHEN Libo, SHENG Ying, WU Ming, SONG Jiling, JIAN Jian, SONG Erhong. Na and O Co-doped Carbon Nitride for Efficient Photocatalytic Hydrogen Evolution[J]. Journal of Inorganic Materials, 2025, 40(5): 552-562.
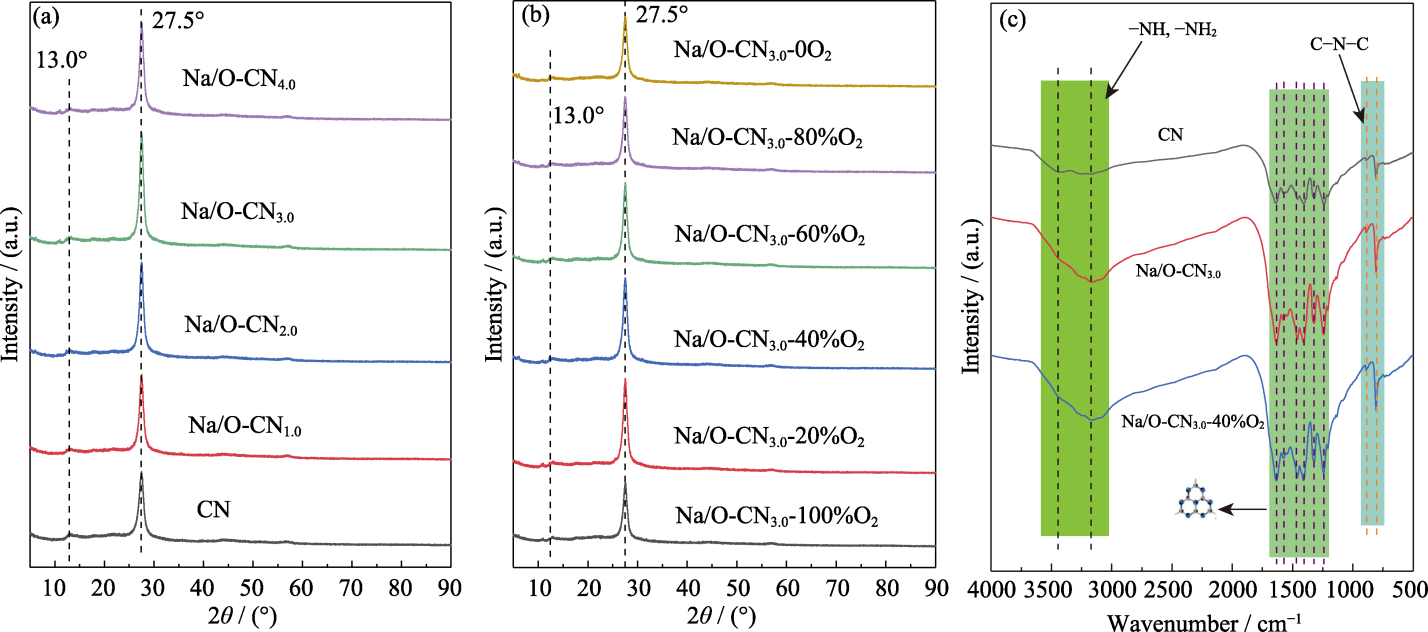
Fig. 2 Structure analyses of samples (a, b) XRD patterns of (a) pure CN and Na/O-CNx, and (b) Na/O-CN3.0-yO2; (c) FT-IR spectra of pure CN, Na/O-CN3.0 and Na/O-CN3.0-40%O2
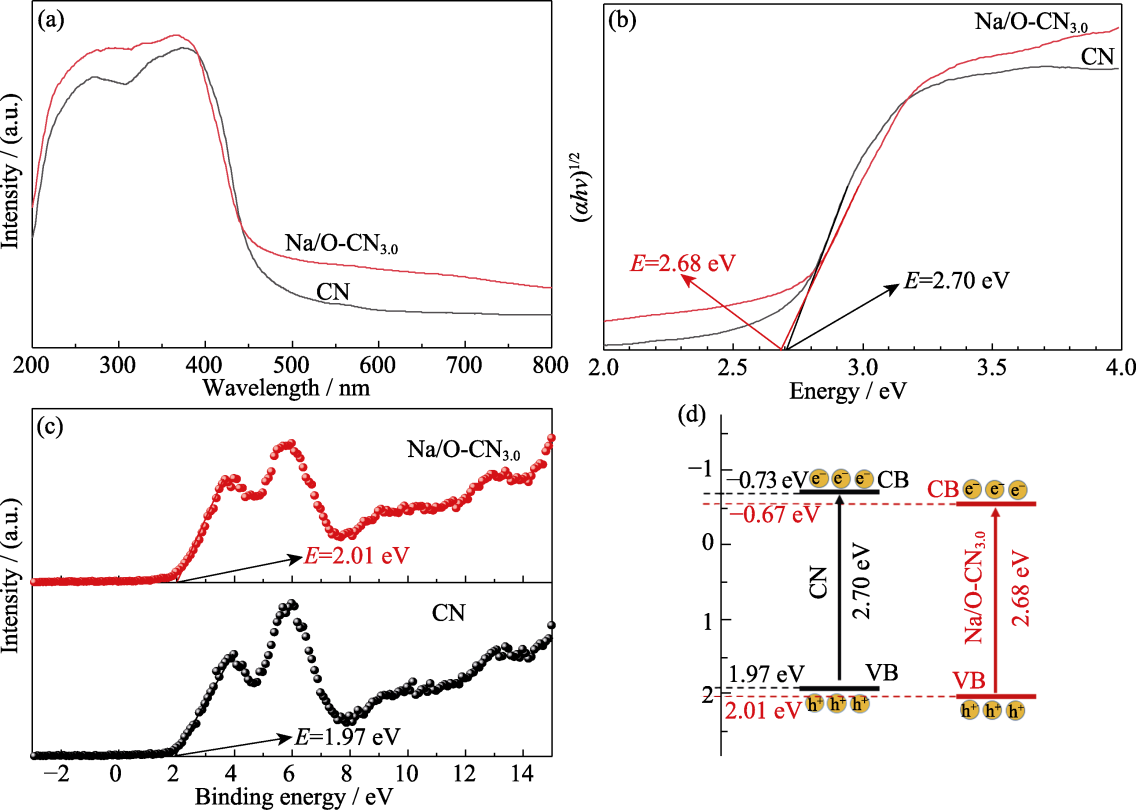
Fig. 5 Band structure analysis of pure CN and Na/O-CN3.0 (a) UV-Vis DRS spectra; (b) Related Tauc plots; (c) VB XPS spectra; (d) Band structure diagram
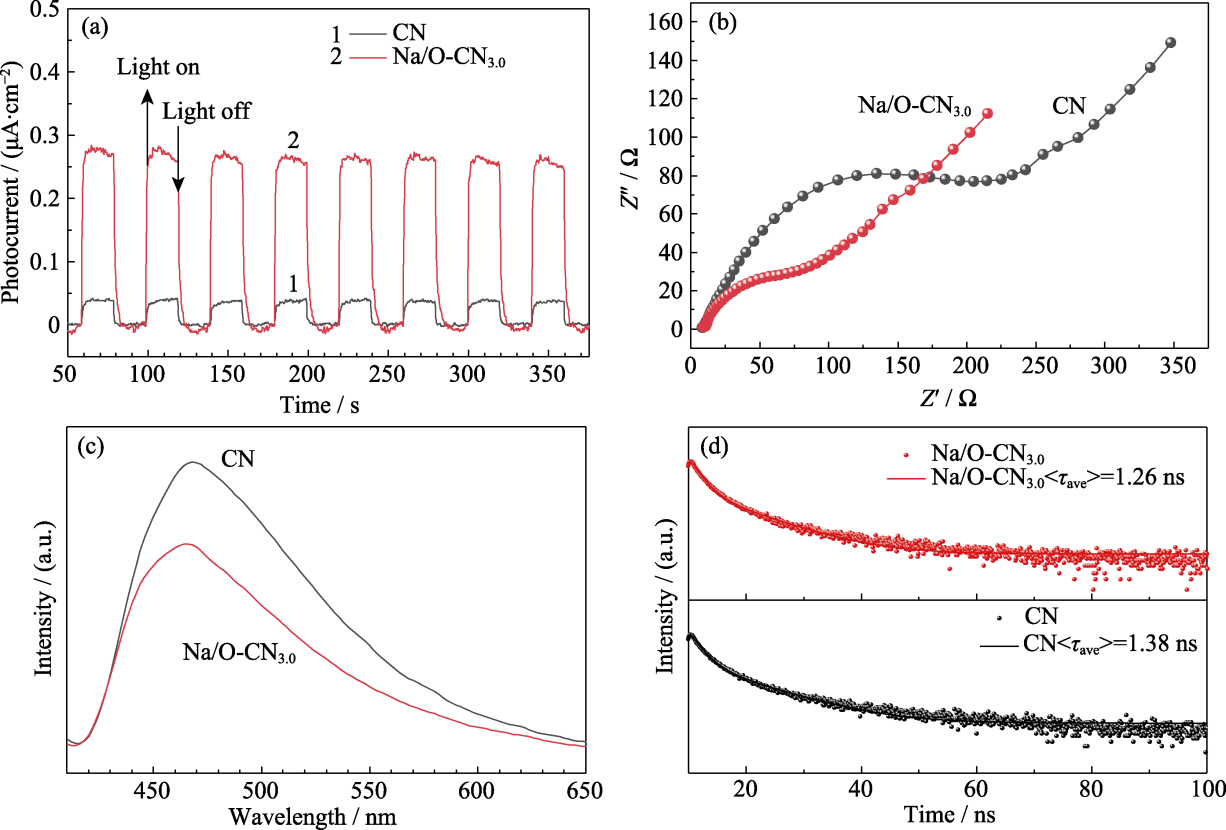
Fig. 6 Photocurrent and PL spectra analysis of pure CN and Na/O-CN3.0 samples (a) Photocurrent test; (b) EIS plots; (c) PL spectra; (d) Time-resolved PL spectra
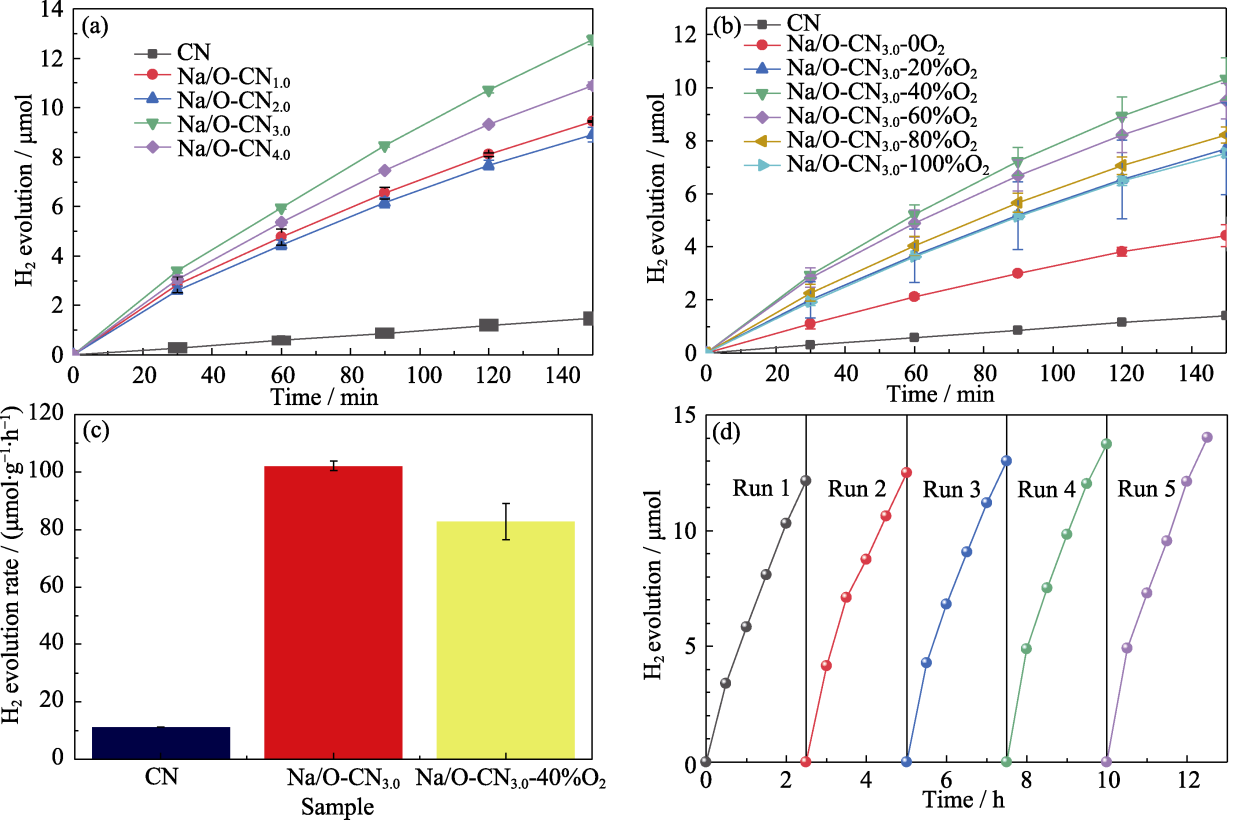
Fig. 7 Photocatalytic hydrogen activity (a, b) Photocatalytic hydrogen evolution of (a) pure CN and Na/O-CNx, and (b) Na/O-CN3.0-yO2; (c) PHER of pure CN, Na/O-CN3.0 and Na/O-CN3.0-40%O2; (d) Cycling performance of Na/O-CN3.0
| Catalyst | Synthesis temperature/℃ | Catalyst weight/mg | Sacrificial reagent/% (in volume) | Surface area/ (m2·g-1) | Activity compared with CN (times) | Ref. |
|---|---|---|---|---|---|---|
| Na/O-CN3.0 | 180 | 50 | TEOA 10% | 18.8 | 9.2 | This work |
| 3% NaCl-CN | 550 | 10 | TEOA 17% | 76.8 | 4.3 | [S1] |
| KCN-10 | 550 | 50 | TEOA 10% | 11 | 5.6 | [S2] |
| K(0.05)-CN | 550 | 50 | TEOA 17% | 11.1 | 5 | [S3] |
| LiNa-K-CN2 | 520 | 50 | TEOA 10% | 116.2 | 15 | [S4] |
| Na(30)-MCN | 550 | 100 | TEOA 10% | 56.1 | 12.9 | [S5] |
| Na0.1-CNNTs | 650 | 20 | TEOA 10% | 94 | 11 | [S6] |
| CN-Na-7 | 550 | 50 | TEOA 10% | 11.7 | 9.9 | [S7] |
| CN-100 | 520 | 50 | TEOA 10% | 14.9 | 9.2 | [S8] |
| (Na,O)g-C3N4 | 160 | 50 | ethyl alcohol 40% | - | 7 | [S9] |
| CN0.05 | 550 | 20 | TEOA | 46.7 | 1.9 | [S10] |
| GCN-Na-5 | 550 | 50 | TEOA | 10.3 | 1.5 | [S11] |
| K@C3N4 | 600 | 100 | TEOA | 27.5 | 8 | [S12] |
Table S1 Photocatalytic hydrogen evolution performance under visible light irradiation (> 420 nm) and surface area of alkali metal doped carbon nitride[S1-S12]
| Catalyst | Synthesis temperature/℃ | Catalyst weight/mg | Sacrificial reagent/% (in volume) | Surface area/ (m2·g-1) | Activity compared with CN (times) | Ref. |
|---|---|---|---|---|---|---|
| Na/O-CN3.0 | 180 | 50 | TEOA 10% | 18.8 | 9.2 | This work |
| 3% NaCl-CN | 550 | 10 | TEOA 17% | 76.8 | 4.3 | [S1] |
| KCN-10 | 550 | 50 | TEOA 10% | 11 | 5.6 | [S2] |
| K(0.05)-CN | 550 | 50 | TEOA 17% | 11.1 | 5 | [S3] |
| LiNa-K-CN2 | 520 | 50 | TEOA 10% | 116.2 | 15 | [S4] |
| Na(30)-MCN | 550 | 100 | TEOA 10% | 56.1 | 12.9 | [S5] |
| Na0.1-CNNTs | 650 | 20 | TEOA 10% | 94 | 11 | [S6] |
| CN-Na-7 | 550 | 50 | TEOA 10% | 11.7 | 9.9 | [S7] |
| CN-100 | 520 | 50 | TEOA 10% | 14.9 | 9.2 | [S8] |
| (Na,O)g-C3N4 | 160 | 50 | ethyl alcohol 40% | - | 7 | [S9] |
| CN0.05 | 550 | 20 | TEOA | 46.7 | 1.9 | [S10] |
| GCN-Na-5 | 550 | 50 | TEOA | 10.3 | 1.5 | [S11] |
| K@C3N4 | 600 | 100 | TEOA | 27.5 | 8 | [S12] |
| [1] | SU C W, PANG L D, QIN M, et al. The spillover effects among fossil fuel, renewables and carbon markets: evidence under the dual dilemma of climate change and energy crises. Energy, 2023, 274: 127304. |
| [2] | LI Y M, ALHARTHI M, AHMAD I, et al. Nexus between renewable energy, natural resources and carbon emissions under the shadow of transboundary trade relationship from South East Asian economies. Energy Strategy Rev., 2022, 41: 100855. |
| [3] | PANG L D, ZHU M N, YU H Y, et al. Is green finance really a blessing for green technology and carbon efficiency? Energy Econ., 2022, 114: 106272. |
| [4] | INDRAWIRAWAN S, SUN H Q, DUAN X G, et al. Nanocarbons in different structural dimensions (0-3D) for phenol adsorption and metal-free catalytic oxidation. Appl. Catal. B Environ., 2015, 179: 352. |
| [5] | LIN Y Y, HUNG K Y, LIU F Y, et al. Photocatalysts of quaternary composite, bismuth oxyfluoride/bismuth oxyiodide/graphitic carbon nitride: synthesis, characterization, and photocatalytic activity. Mol. Catal., 2022, 528: 112463. |
| [6] | ZHANG Z, YANG L, LIU J R, et al. Improved oxygen electrocatalysis at FeN4 and CoN4 sites via construction of axial coordination. Chin. Chem. Lett., 2025, 36(2): 110013. |
| [7] | XU T, DING X T, CHENG H H, et al. Moisture-enabled electricity from hygroscopic materials: a new type of clean energy. Adv. Mater., 2023, 36(12): 2209661. |
| [8] | MANDAL D, ANDRADA D M. Oil droplets cut to the chase. Nat. Chem., 2020, 12(12): 1089. |
| [9] | NISHIYAMA H, YAMADA T, NAKABAYASHI M, et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature, 2021, 598(7880): 304. |
| [10] | TANG D, TAN G L, LI G W, et al. State-of-the-art hydrogen generation techniques and storage methods: a critical review. J. Energy Storage, 2023, 64: 107196. |
| [11] | LI C Q, DU X, JIANG S, et al. Constructing direct Z-scheme heterostructure by enwrapping ZnIn2S4 on CdS hollow cube for efficient photocatalytic H2 generation. Adv. Sci., 2022, 9(24): 2201773. |
| [12] | HISATOMI T, DOMEN K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal., 2019, 2(5): 387. |
| [13] | YI S S, ZHANG B X, WULAN B R, et al. Non-noble metals applied to solar water splitting. Energy Environ. Sci., 2016, 9(11): 145. |
| [14] | JIAO Y Y, LI Y K, WANG J S, et al. Exfoliation-induced exposure of active sites for g-C3N4/N-doped carbon dots heterojunction to improve hydrogen evolution activity. Mol. Catal., 2020, 497: 111223. |
| [15] | CHU X Y, LUAN B B, HUANG A X, et al. Controlled synthesis of 2D-2D conductive metal-organic framework/g-C3N4 heterojunctions for efficient photocatalytic hydrogen evolution. Dalton Trans., 2024, 53(6): 2534. |
| [16] | YANG Y, ZHOU C Y, WANG W J, et al. Recent advances in application of transition metal phosphides for photocatalytic hydrogen production. Chem. Eng. J., 2021, 405: 126547. |
| [17] | SONG B, CHEN M, ZENG G M, et al. Using graphdiyne (GDY) as a catalyst support for enhanced performance in organic pollutant degradation and hydrogen production: a review. J. Hazard. Mater., 2020, 398: 122957. |
| [18] | XIANG Q J, YU J G, JARONIEC M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc., 2012, 134(15): 6575. |
| [19] | XIAO S N, DAI W R, LIU X Y, et al. Microwave-induced metal dissolution synthesis of core-shell copper nanowires/ZnS for visible light photocatalytic H2 evolution. Adv. Energy Mater., 2019, 9(22): 1900775. |
| [20] | LI H H, WU Y, LI L, et al. Adjustable photocatalytic ability of monolayer g-C3N4 utilizing single-metal atom: density functional theory. Appl. Surf. Sci., 2018, 457: 735. |
| [21] | SHANG Y Y, MA Y J, CHEN X, et al. Effect of sodium doping on the structure and enhanced photocatalytic hydrogen evolution performance of graphitic carbon nitride. Mol. Catal., 2017, 433: 128. |
| [22] | YE Z W, YUE W H, TAYYAB M, et al. Simple one-pot, high-yield synthesis of 2D graphitic carbon nitride nanosheets for photocatalytic hydrogen production. Dalton Trans., 2022, 51(48): 18542. |
| [23] | SHI J Y, ZHANG J, CUI Z W, et al. In situ growth of MOF- derived sulfur vacancy-rich CdS nanoparticles on 2D polymers for highly efficient photocatalytic hydrogen generation. Dalton Trans., 2022, 51(15): 5841. |
| [24] | HUANG X, WU K Y, SU C, et al. Metal-organic framework Cu-BTC for overall water splitting: a density functional theory study. Chin. Chem. Lett., 2025, 36(4): 109720. |
| [25] | WANG X C, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater., 2009, 8(1): 76. |
| [26] | YI S S, YAN J M, WULAN B R, et al. Noble-metal-free cobalt phosphide modified carbon nitride: an efficient photocatalyst for hydrogen generation. Appl. Catal. B Environ., 2017, 200: 477. |
| [27] | HE J J, SUN H Q, INDRAWIRAWAN S, et al. Novel polyoxometalate@g-C3N4 hybrid photocatalysts for degradation of dyes and phenolics. J. Colloid Interface Sci., 2015, 456: 15. |
| [28] | LI H H, WU Y, LI C, et al. Design of Pt/t-ZrO2/g-C3N4 efficient photocatalyst for the hydrogen evolution reaction. Appl. Catal. B Environ., 2019, 251: 305. |
| [29] | ZHOU A Q, YANG J M, ZHU X W, et al. Self-assembly construction of NiCo LDH/ultrathin g-C3N4 nanosheets photocatalyst for enhanced CO2 reduction and charge separation mechanism study. Rare Met., 2022, 41(6):2118. |
| [30] | ZHU X W, XU H M, BI C Z, et al. Piezo-photocatalysis for efficient charge separation to promote CO2 photoreduction in nanoclusters. Ultrason. Sonochem., 2023, 101: 106653. |
| [31] | WU M, YAN J M, TANG X N, et al. Synthesis of potassium- modified graphitic carbon nitride with high photocatalytic activity for hydrogen evolution. ChemSusChem, 2014, 7(9): 2654. |
| [32] | ZHU Y L, SUN Y Y, KHAN J, et al. NaClO-induced sodium-doped cyano-rich graphitic carbon nitride nanosheets with nitrogen vacancies to boost photocatalytic hydrogen peroxide production. Chem. Eng. J., 2022, 443: 136501. |
| [33] | ZHAO X L, ZHANG Y G, LI F, et al. Salt-air template synthesis of Na and O doped porous graphitic carbon nitride nanorods with exceptional photocatalytic H2 evolution activity. Carbon, 2021, 179: 42. |
| [34] | HAN X, KANG Y, SONG S, et al. Sodium ion doped graphitic carbon nitride with high crystallinity for superior photocatalytic hydrogen evolution efficiency. J. Mater. Chem. A, 2023, 11(34): 18213. |
| [35] | DOU Q, HOU J H, HUSSAIN A, et al. One-pot synthesis of sodium-doped willow-shaped graphitic carbon nitride for improved photocatalytic activity under visible-light irradiation. J. Colloid Interface Sci., 2022, 624: 79. |
| [36] | SUN Z Z, TAN Y Y, SHI X K, et al. General method to introduce π-electrons into oxygen-doped porous carbon nitride for photocatalytic hydrogen evolution and toluene oxidation. ACS Sustainable Chem. Eng., 2024, 12(2): 1051. |
| [37] | WANG Y X, WANG H, CHEN F Y, et al. Facile synthesis of oxygen doped carbon nitride hollow microsphere for photocatalysis. Appl. Catal. B Environ., 2018, 231: 43. |
| [38] | REN J X, ZHENG Y M, LIN H W, et al. Near-infrared light- activated g-C3N4 with effective n → π* electron transition for H2O2 production. Appl. Surf. Sci., 2023, 638: 158053. |
| [39] | WU M, HE X, JING B H, et al. Novel carbon and defects co-modified g-C3N4 for highly efficient photocatalytic degradation of bisphenol A under visible light. J. Hazard. Mater., 2020, 384: 121323. |
| [40] | LI Y H, HO W K, LV K L, et al. Carbon vacancy-induced enhancement of the visible light-driven photocatalytic oxidation of NO over g-C3N4 nanosheets. Appl. Surf. Sci., 2018, 430: 380. |
| [41] | LU X J, WANG Y, ZHANG X Y, et al. NiS and MoS2 nanosheet co-modified graphitic C3N4 ternary heterostructure for high efficient visible light photodegradation of antibiotic. J. Hazard. Mater., 2018, 341: 10. |
| [42] | HU S Y, YU A C, LU R. A comparison study of sodium ion- and potassium ion-modified graphitic carbon nitride for photocatalytic hydrogen evolution. RSC Adv., 2021, 11(26): 15701. |
| [43] | FANG W J, LIU J Y, YU L, et al. Novel (Na, O) co-doped g-C3N4 with simultaneously enhanced absorption and narrowed bandgap for highly efficient hydrogen evolution. Appl. Catal. B Environ., 2017, 209: 631. |
| [44] | MA R D, GUO X, SHI K X, et al. S-type heterojunction of MOS2/g-C3N4: construction and photocatalysis. J. Inorg. Mater., 2023, 38(10): 1176. |
| [45] | CHEN X, LI H K, WU Y X, et al. Facile fabrication of novel porous graphitic carbon nitride/copper sulfide nanocomposites with enhanced visible light driven photocatalytic performance. J. Colloid Interface Sci., 2016, 476: 132. |
| [46] | KRÖGER J, JIMÉNEZ‐SOLANO A, SAVASCI G, et al. Interfacial engineering for improved photocatalysis in a charge storing 2D carbon nitride: melamine functionalized poly(heptazine imide). Adv. Energy Mater., 2021, 11(6): 2003016. |
| [47] | LIU M Q, JIAO Y Y, QIN J C, et al. Boron doped C3N4 nanodots/ nonmetal element (S, P, F, Br) doped C3N4 nanosheets heterojunction with synergistic effect to boost the photocatalytic hydrogen production performance. Appl. Surf. Sci., 2021, 541: 148558. |
| [48] | YANG C W, XUE Z, QIN J Q, et al. Heterogeneous structural defects to prompt charge shuttle in g-C3N4 plane for boosting visible-light photocatalytic activity. Appl. Catal. B Environ., 2019, 259: 118094. |
| [49] | SHI L, LIU G, ZHANG Y H, et al. Na, O co-doping and cyano groups synergistically adjust the band structure of g-C3N4 for improving photocatalytic oxygen evolution. Mater. Res. Bull., 2023, 167: 112423. |
| [50] | GU J, CHEN H, JIANG F, et al. Visible light photocatalytic mineralization of bisphenol A by carbon and oxygen dual-doped graphitic carbon nitride. J. Colloid Interface Sci., 2019, 540: 97. |
| [51] | IQBAL O, ALI H, LI N, et al. A review on the synthesis, properties, and characterizations of graphitic carbon nitride (g-C3N4) for energy conversion and storage applications. Mater. Today Phys., 2023, 34: 101080. |
| [52] | OU H H, LIN L H, ZHENG Y, et al. Tri-s-triazine-based crystalline carbon nitride nanosheets for an improved hydrogen evolution. Adv. Mater., 2017, 29(22): 1700008. |
| [53] | ZHOU J, YANG Y, ZHANG C Y. A low-temperature solid-phase method to synthesize highly fluorescent carbon nitride dots with tunable emission. Chem. Commun., 2013, 49(77): 8605. |
| [54] | ZHANG Y Z, ZONG S C, CHENG C, et al. One-pot annealing preparation of Na-doped graphitic carbon nitride from melamine and organometallic sodium salt for enhanced photocatalytic H2 evolution. Int. J. Hydrog. Energy, 2018, 43(30): 13953. |
| [55] | GUO F, CHEN J L, ZHANG M W, et al. Deprotonation of g-C3N4 with Na ions for efficient nonsacrificial water splitting under visible light. J. Mater. Chem. A, 2016, 4(28): 10806. |
| [56] | WU M, CHEN L B, LUO X, et al. Defective carbon nitride with dual-surface engineering for highly efficient photocatalytic hydrogen evolution under visible light irradiation. Langmuir, 2024, 40(34): 18153. |
| [57] | MAKUŁA P, PACIA M, MACYK W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra. J. Phys. Chem. Lett., 2018, 9(23): 6814. |
| [58] | YU H J, SHI R, ZHAO Y X, et al. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater., 2017, 29(26): 1605148. |
| [59] | WULANA B R, YI S S, LI S J, et al. Amorphous nickel pyrophosphate modified graphitic carbon nitride: an efficient photocatalyst for hydrogen generation from water splitting. Appl. Catal. B Environ., 2018, 231: 43. |
| [1] | 张文宇, 郭瑞华, 岳全鑫, 黄雅荣, 张国芳, 关丽丽. 高熵磷化物双功能催化剂的制备及高效电解水性能[J]. 无机材料学报, 2024, 39(11): 1265-1274. |
| [2] | 孙强强, 陈子璇, 杨子玥, 王毅梦, 曹宝月. 金属镍铜负载钒氧化物的高效电解产氢性能[J]. 无机材料学报, 2023, 38(6): 647-655. |
| [3] | 安琳, 吴淏, 韩鑫, 李耀刚, 王宏志, 张青红. 非贵金属Co5.47N/N-rGO助催化剂增强TiO2光催化制氢性能[J]. 无机材料学报, 2022, 37(5): 534-540. |
| [4] | 蔡苗, 陈子航, 曾实, 杜江慧, 熊娟. CuS纳米片修饰Bi5O7I复合材料用于光催化还原Cr(VI)水溶液[J]. 无机材料学报, 2021, 36(6): 665-672. |
| [5] | 董正明, 李修, 陈晨, 曹明贺, 易志国. NBT-BNT陶瓷的光致形变性能[J]. 无机材料学报, 2021, 36(3): 277-282. |
| [6] | 李翠霞, 孙会珍, 金海泽, 史晓, 李文生, 孔文慧. 3D多级孔rGO/TiO2复合材料的构筑及其光催化性能研究[J]. 无机材料学报, 2021, 36(10): 1039-1046. |
| [7] | 王苹,李心宇,时占领,李海涛. Ag与Ag2O协同增强TiO2光催化制氢性能的研究[J]. 无机材料学报, 2020, 35(7): 781-788. |
| [8] | 张塞, 邹英桐, 陈中山, 李冰峰, 顾鹏程, 文涛. 可见光驱动RGO/g-C3N4活化过硫酸盐降解水中双酚A[J]. 无机材料学报, 2020, 35(3): 329-336. |
| [9] | 魏鑫, 卢占会, 王路平, 方明. 可见光下Bi2WO6纳米片高效光降解四环素的机理研究[J]. 无机材料学报, 2020, 35(3): 324-328. |
| [10] | 李志锋, 谭杰, 杨晓飞, 蔺祖弘, 郇正来, 张婷婷. 高暴露(001)面BiOBr/Ti3C2复合光催化剂的制备及其可见光催化性能[J]. 无机材料学报, 2020, 35(11): 1247-1254. |
| [11] | 魏居孟, 吕强, 王奔驰, 潘家乐, 叶祥桔, 宋常春. 高可见光催化活性立方体浮雕状Ag3PO4的合成[J]. 无机材料学报, 2019, 34(7): 786-790. |
| [12] | 李纳, 刘斌, 施佼佼, 薛艳艳, 赵衡煜, 施张丽, 侯文涛, 徐晓东, 徐军. 可见光波段稀土激光晶体的研究进展[J]. 无机材料学报, 2019, 34(6): 573-589. |
| [13] | 李晓萍, 李跃军, 曹铁平, 孙大伟, 王霞, 席啸天. 简易合成Bi/Bi2MoO6/TiO2复合纳米纤维及其增强的可见光催化性能[J]. 无机材料学报, 2019, 34(11): 1193-1199. |
| [14] | 柯银环, 曾敏, 姜宏, 熊春荣. N掺杂TiO2纳米纤维高可见光催化CO2合成甲醇[J]. 无机材料学报, 2018, 33(8): 839-844. |
| [15] | 李健, 张刚华, 范立坤, 黄国全, 高志鹏, 曾涛. 不同pH下KBiFe2O5可见光催化性能研究[J]. 无机材料学报, 2018, 33(7): 805-810. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||