无机材料学报 ›› 2021, Vol. 36 ›› Issue (9): 943-949.DOI: 10.15541/jim20200624 CSTR: 32189.14.10.15541/jim20200624
收稿日期:2020-11-04
修回日期:2020-12-07
出版日期:2021-09-20
网络出版日期:2020-12-30
通讯作者:
史彦涛, 教授. E-mail: shiyantao@dlut.edu.cn
作者简介:刘自若(1996-), 女, 硕士研究生. E-mail: lzr@mail.dlut.edu.cn
基金资助:
LIU Ziruo( ), LIU Wei, HAO Ce, HU Jinwen, SHI Yantao(
), LIU Wei, HAO Ce, HU Jinwen, SHI Yantao( )
)
Received:2020-11-04
Revised:2020-12-07
Published:2021-09-20
Online:2020-12-30
Contact:
SHI Yantao, professor. E-mail: shiyantao@dlut.edu.cn
About author:LIU Ziruo(1996-), female, Master candidate. E-mail: lzr@mail.dlut.edu.cn
Supported by:摘要:
单原子催化剂(SACs)以近100%的原子利用率以及优秀的催化活性等, 在促进多相催化方面受到了广泛关注。然而, 由于金属原子在高温下易烧结, SACs的合成仍然具有挑战性。本研究利用熔融盐(MS)提供的强极性环境, 制备了以氮掺杂碳为载体的铁基单原子催化剂(Fe SA-NC)。结果表明, Fe SA-NC显示出蜂窝状的多孔形貌, 比表面积高达2072 m2·g-1, 其中Fe元素的重量百分比含量为0.57%。通过球差电镜直接观察到了孤立存在的Fe单原子, 并通过X射线吸收精细结构(XAFS)分析确定Fe单原子以Fe-N4配位体形式分散在碳基材料上。Fe SA-NC催化剂在0.1 mol/L KOH 溶液中半波电位为0.85 V, 极限电流密度为5.79 mA·cm-2, 优于商业Pt/C催化剂。Fe SA-NC催化剂不仅对ORR四电子途径显示出高选择性(H2O2产率<2%, 转移电子数为3.9), 同时表现出优秀的抗甲醇性能。
中图分类号:
刘自若, 刘炜, 郝策, 胡金文, 史彦涛. 蜂窝状碳负载铁基单原子催化剂的制备及ORR催化性能研究[J]. 无机材料学报, 2021, 36(9): 943-949.
LIU Ziruo, LIU Wei, HAO Ce, HU Jinwen, SHI Yantao. Honeycomb-like Carbon-supported Fe Single Atom Catalyst: Preparation and Electrocatalytic Performance in Oxygen Reduction Reaction[J]. Journal of Inorganic Materials, 2021, 36(9): 943-949.
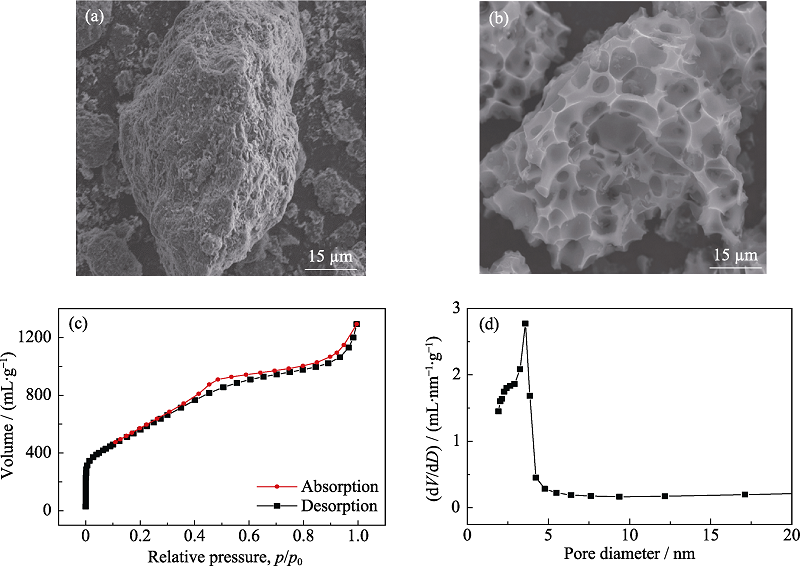
图1 (a)Fe-NC和(b)Fe SA-NC的扫描电镜照片; (c)Fe SA-NC在77 K下的N2吸脱附曲线; (d) Fe SA-NC的孔径分布图
Fig. 1 SEM images of (a) Fe-NC and (b)Fe SA-NC, (c)nitrogen adsorption/desorption plots of Fe SA-NC at 77 K, and (d) pore size distribution of Fe SA-NC
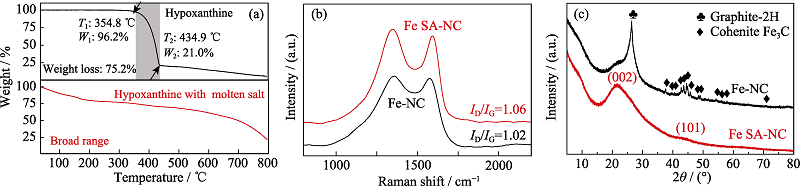
图2 (a)次黄嘌呤以及次黄嘌呤和熔融盐共存时的TGA曲线; Fe SA-NC和Fe-NC的催化剂的(b)拉曼光谱以及(c)XRD图谱
Fig. 2 (a) TGA curves of hypoxanthine and hypoxanthine with molten salt; (b) Raman spectra and (c) XRD patterns of Fe SA-NC and Fe-NC
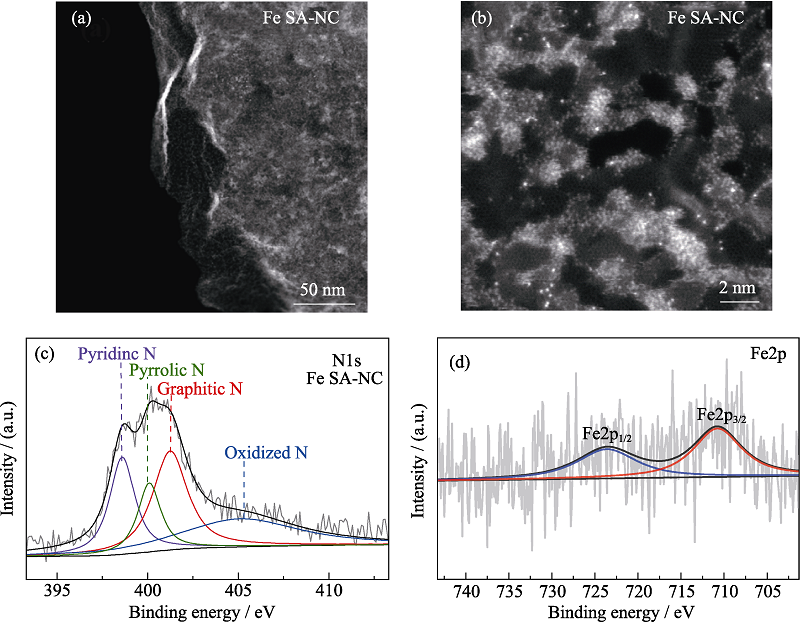
图3 Fe SA-NC的(a, b)球差矫正高角环形暗场扫描透射电镜照片, (c)N1s和(d)Fe2p的高分辨XPS图谱
Fig. 3 (a, b) AC HAADF-STEM images, high-resolution X-ray photoelectron spectra (XPS) of (c) N1s and (c) Fe2p of the Fe SA-NC catalyst
| Element | C | N | O | Fe |
|---|---|---|---|---|
| Atomatic percent/% | 74.54 | 14.18 | 10.15 | 0.60 |
表1 Fe SA-NC催化剂中各元素含量的XPS测试结果
Table 1 XPS results of element composition in Fe SA-NC catalyst
| Element | C | N | O | Fe |
|---|---|---|---|---|
| Atomatic percent/% | 74.54 | 14.18 | 10.15 | 0.60 |
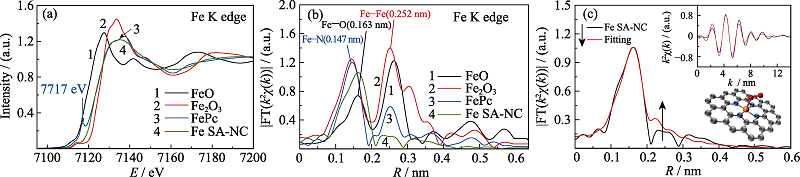
图4 Fe SA-NC、FePc、FeO和Fe2O3的Fe K边(a)X射线吸收近边结构(XANES)光谱和(b)径向结构函数; (c)Fe SA-NC的R空间EXAFS拟合曲线
Fig. 4 Fe K-edge (a) XANES spectra (b) k2-weighted FT spectra for the Fe SA-NC, FePc, FeO and Fe2O3 and (c) EXAFS fitting curves in R space for Fe SA-NC Insets in (c): EXAFS fitting curves in k space (up) and schematic models of Fe SA-NC (down); C (gray), N (blue), Fe (orange), O (red)
| Sample | Path | N | R/nm | σ2/(×10-5, nm2) | ΔE0/eV | R factor |
|---|---|---|---|---|---|---|
| Fe SA-NC | Fe-N | 3.9 | 0.209 | 5.7 | 4.7 | 0.093 |
| Fe-O | 1.0 | 0.190 | 5.7 | 4.7 | 0.093 |
表2 Fe SA-NC样品的EXAFS拟合参数
Table 2 EXAFS fitting parameters of Fe SA-NC sample
| Sample | Path | N | R/nm | σ2/(×10-5, nm2) | ΔE0/eV | R factor |
|---|---|---|---|---|---|---|
| Fe SA-NC | Fe-N | 3.9 | 0.209 | 5.7 | 4.7 | 0.093 |
| Fe-O | 1.0 | 0.190 | 5.7 | 4.7 | 0.093 |
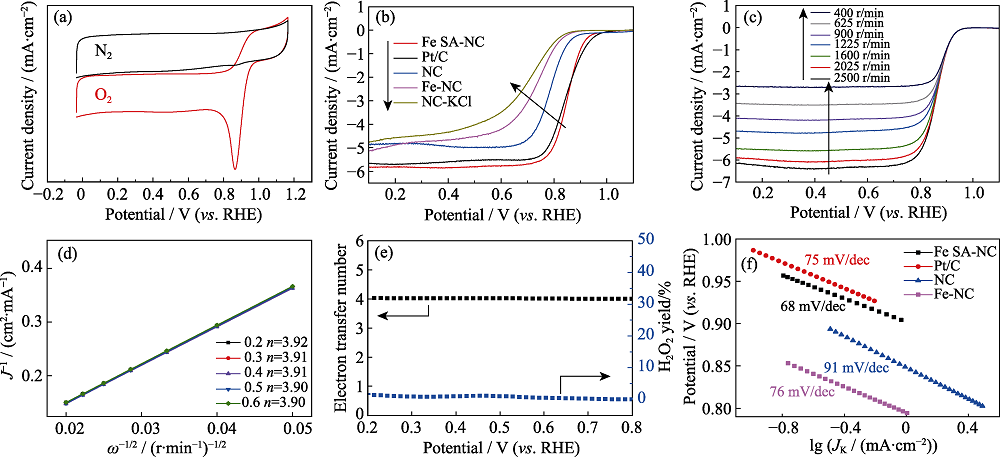
图5 (a)Fe SA-NC在氮气和氧气饱和的0.1 mol/L KOH电解质溶液中的 CV 曲线; (b)氧饱和的0.1 mol/L KOH电解质溶液(1600 r/min, 10 mV s-1)中不同催化剂的LSV曲线; (c)Fe SA-NC在氧气饱和0.1 mol/L KOH 溶液条件中, 不同转速条件的LSV曲线; (d)图(c)对应的K-L方程图; (e)Fe SA-NC的ORR转移电子数和H2O2产率; (f)氧气饱和溶液中不同催化剂材料的塔菲尔斜率
Fig. 5 (a) CV curves of the Fe SA-NC in a N2-saturated and O2-saturated 0.1 mol/L KOH solution, respectively; (b) LSV curves of Fe SA-NC, NC and Pt/C in O2-saturated 0.1 mol/L KOH solution (1600 r/min, 10 mV·s-1); (c) ORR polarization curves of Fe SA-NC recorded at different rotating speeds; (d) K-L plots derived from Fig. (c); (e) ORR electron transfer number and H2O2 yield of Fe SA-NC; (f) Tafel slopes of Fe SA-NC, NC and Pt/C in O2-saturated 0.1 mol/L KOH solution
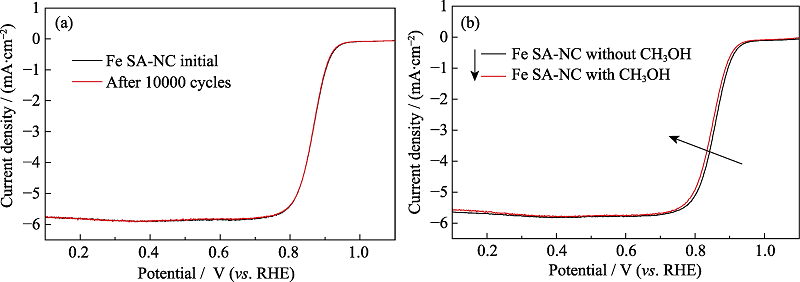
图6 Fe SA-NC在1600 r/min转速下氧气饱和0.1 mol/L KOH溶液中的(a)耐久性试验和(b)抗甲醇能力测试
Fig. 6 (a) Durability test and (b) methanol resistance test for Fe SA-NC in O2-saturated 0.1 mol/L KOH solution (1600 r/min)
| [1] |
YANG X, WANG A, QIAO B, et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Accounts of Chemical Research, 2013, 46(8):1740-1748.
DOI URL |
| [2] |
QIAO B, WANG A, YANG X, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nature Chemistry, 2011, 3(8):634-641.
DOI URL |
| [3] |
LIU P, ZHAO Y, QIN R, et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science, 2016, 352(6287):797-801.
DOI URL |
| [4] |
WEI H, LIU X, WANG A, et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun., 2014, 5:5634.
DOI URL |
| [5] | JIANG Z, WANG T, PEI J, et al. Discovery of main group single Sb-N4 active sites for CO2 electroreduction to formate with high efficiency. Energy & Environmental Science, 2020, 13(9):2856-2863. |
| [6] |
FEI H, DONG J, ARELLANO-JIMENEZ M J, et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun., 2015, 6:8668.
DOI URL |
| [7] |
BAI L, HSU C S, ALEXANDER D T L, et al. A cobalt-iron double-atom catalyst for the oxygen evolution reaction. Journal of the American Chemical Society, 2019, 141(36):14190-14199.
DOI URL |
| [8] |
ZHANG J, ZHAO Y, CHEN C, et al. Tuning the coordination environment in single-atom catalysts to achieve highly efficient oxygen reduction reactions. J. Am. Chem. Soc., 2019, 141(51):20118-20126.
DOI URL |
| [9] |
PENG Y, LU B, CHEN S. Carbon-supported single atom catalysts for electrochemical energy conversion and storage. Advanced Materials, 2018, 30(48):1801995.
DOI URL |
| [10] |
GUPTA S, ZHAO S, OGOKE O, et al. Engineering favorable morphology and structure of Fe-N-C oxygen-reduction catalysts through tuning of nitrogen/carbon precursors. ChemSusChem, 2017, 10(4):774-785.
DOI URL |
| [11] |
ZHAO K, NIE X, WANG H, et al. Selective electroreduction of CO2 to acetone by single copper atoms anchored on N-doped porous carbon. Nat. Commun., 2020, 11(1):2455.
DOI URL |
| [12] |
LI J, CHEN S, YANG N, et al. Ultrahigh-loading zinc single-atom catalyst for highly efficient oxygen reduction in both acidic and alkaline media. Angewandte Chemie International Edition, 2019, 58(21):7035-7039.
DOI URL |
| [13] |
LIU X, FECHLER N, ANTONIETTI M. Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chemical Society Reviews, 2013, 42(21):8237-8265.
DOI URL |
| [14] |
HU J, WU D, ZHU C, et al. Melt-salt-assisted direct transformation of solid oxide into atomically dispersed FeN4 sites on nitrogen-doped porous carbon. Nano Energy, 2020, 72:104670.
DOI URL |
| [15] | HE W, MA R, ZHU Y, et al. Renewable porous carbons prepared by KOH activation as oxygen reduction electrocatalysts. Journal of Inorganic Materials, 2019, 34(10):1115-1122. |
| [16] |
MORAIS R G, REY-RAAP N, FIGUEIREDO J L, et al. Highly electroactive N-Fe hydrothermal carbons and carbon nanotubes for the oxygen reduction reaction. Journal of Energy Chemistry, 2020, 50:260-270.
DOI URL |
| [17] | CHEN Y, LI Z, ZHU Y, et al. Atomic Fe dispersed on N-doped carbon hollow nanospheres for high-efficiency electrocatalytic oxygen reduction. Adv. Mater., 2019, 31(8):e1806312. |
| [18] |
TANG F, LEI H, WANG S, et al. A novel Fe-N-C catalyst for efficient oxygen reduction reaction based on polydopamine nanotubes. Nanoscale, 2017, 9(44):17364-17370.
DOI URL |
| [19] |
DING S, NING K, YUAN B, et al. Durability of Fe-N/C catalysts with different nanostructures for electrochemical oxygen reduction in alkaline solution. Journal of Inorganic Materials, 2020, 35(8):953-961.
DOI URL |
| [20] |
SEYAMA H, SOMA M. Fe2P spectra of silicate minerals. Journal of Electron Spectroscopy and Related Phenomena, 1987, 42(1):97-101.
DOI URL |
| [21] |
MA L, ZHI C. Fe, N doped 2D porous carbon bifunctional catalyst for zinc-air battery. Journal of Inorganic Materials, 2019, 34(1):103-108.
DOI URL |
| [22] |
FEI H, DONG J, FENG Y, et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nature Catalysis, 2018, 1(1):63-72.
DOI URL |
| [23] |
CHEN Y, JI S, WANG Y, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angewandte Chemie International Edition, 2017, 56(24):6937-6941.
DOI URL |
| [24] |
LI Q, WANG T, HAVAS D, et al. High-performance direct methanol fuel cells with precious-metal-free cathode. Advanced Science, 2016, 3(11):1600140.
DOI URL |
| [1] | 杨光, 张楠, 陈舒锦, 王义, 谢安, 严育杰. 基于多孔ITO电极的WO3薄膜的制备及其电致变色性能[J]. 无机材料学报, 2025, 40(7): 781-789. |
| [2] | 郭子玉, 朱云洲, 王力, 陈健, 李红, 黄政仁. Zn2+催化剂对酚醛树脂/乙二醇制备多孔碳微观孔结构的影响[J]. 无机材料学报, 2025, 40(5): 466-472. |
| [3] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [4] | 刘磊, 郭瑞华, 王丽, 王艳, 张国芳, 关丽丽. Pt3Co高指数晶面氧还原过程的密度泛函理论研究[J]. 无机材料学报, 2025, 40(1): 39-46. |
| [5] | 靳宇翔, 宋二红, 朱永福. 3d过渡金属单原子掺杂石墨烯缺陷电催化还原CO2的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 845-852. |
| [6] | 赵日达, 汤素芳. 多孔碳陶瓷化改进反应熔渗法制备陶瓷基复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 623-633. |
| [7] | 徐州, 刘宇轩, 池俊霖, 张婷婷, 王姝越, 李伟, 马春慧, 罗沙, 刘守新. 双模板-水热炭化制备马蹄形中空多孔炭及其电化学性能[J]. 无机材料学报, 2023, 38(8): 954-962. |
| [8] | 王新玲, 周娜, 田亚文, 周明冉, 韩静茹, 申远升, 胡执一, 李昱. SnS2/ZIF-8衍生二维多孔氮掺杂碳纳米片复合材料的锂硫电池性能研究[J]. 无机材料学报, 2023, 38(8): 938-946. |
| [9] | 吴振, 李慧芳, 张中晗, 张振, 李阳, 蓝江河, 苏良碧, 武安华. 面向磁光应用的CeF3晶体生长与性能表征[J]. 无机材料学报, 2023, 38(3): 296-302. |
| [10] | 杨代辉, 孙甜, 田合鑫, 史晓斐, 马东伟. 铁氮共掺杂介孔碳材料的简易制备及其氧还原反应催化性能[J]. 无机材料学报, 2023, 38(11): 1309-1315. |
| [11] | 王鲁凯, 冯军宗, 姜勇刚, 李良军, 冯坚. 直写3D打印陶瓷基多孔结构的研究进展[J]. 无机材料学报, 2023, 38(10): 1133-1148. |
| [12] | 吴松泽, 周洋, 李润丰, 刘晓倩, 李翠伟, 黄振莺. 铁尾矿及其反应烧结多孔陶瓷的制备与性能研究[J]. 无机材料学报, 2023, 38(10): 1193-1199. |
| [13] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [14] | 孙炼, 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵. 二维过渡金属硫属化合物氧还原反应催化剂的研究进展[J]. 无机材料学报, 2022, 37(7): 697-709. |
| [15] | 蒋丽丽, 徐帅帅, 夏宝凯, 陈胜, 朱俊武. 缺陷调控石墨烯复合催化剂在氧还原反应中的作用[J]. 无机材料学报, 2022, 37(2): 215-222. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||