无机材料学报 ›› 2021, Vol. 36 ›› Issue (4): 431-435.DOI: 10.15541/jim20200380 CSTR: 32189.14.10.15541/jim20200380
所属专题: 能源材料论文精选(2021); 【虚拟专辑】钙钛矿材料(2020~2021); 【虚拟专辑】超级电容器(2020~2021)
收稿日期:2020-07-07
修回日期:2020-10-23
出版日期:2021-04-20
网络出版日期:2020-11-05
通讯作者:
苗 洋, 副教授. E-mail: miaoyang@tyut.edu.cn
作者简介:郭 猛(1997-), 男, 硕士研究生. E-mail: 18235120868@163.com
基金资助:
GUO Meng( ), ZHANG Fengnian, MIAO Yang(
), ZHANG Fengnian, MIAO Yang( ), LIU Yufeng, YU Jun, GAO Feng
), LIU Yufeng, YU Jun, GAO Feng
Received:2020-07-07
Revised:2020-10-23
Published:2021-04-20
Online:2020-11-05
Contact:
MIAO Yang, associate professor. E-mail: miaoyang@tyut.edu.cn
About author:GUO Meng(1997-), male, Master candidate. E-mail: 18235120868@163.com
Supported by:摘要:
采用共沉淀法结合煅烧工艺制备La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3钙钛矿结构高熵陶瓷粉体, 显著降低了材料的合成温度。采用不同手段对其进行物相及形貌表征, 研究结果表明, 当煅烧温度为800 ℃时, 样品已经形成钙钛矿结构, 但有少量第二相; 当煅烧温度为1000 ℃时, La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3陶瓷粉体形成了纯钙钛矿结构。以La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3为电极材料制成工作电极, 采用三电极体系对工作电极进行电学性能测试, 包括循环伏安(CV)及恒流充放电(GCD)测试, 结果显示该电极材料在1 A/g电流密度下具有154.8 F/g的比容量;当电流密度增大到10 A/g时, 该电极材料仍然能保持初始比容量的47%(73 F/g), 说明La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3高熵陶瓷具有较好的倍率性能。
中图分类号:
郭猛, 张丰年, 苗洋, 刘宇峰, 郁军, 高峰. La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3钙钛矿高熵陶瓷粉体的制备及其电学性能[J]. 无机材料学报, 2021, 36(4): 431-435.
GUO Meng, ZHANG Fengnian, MIAO Yang, LIU Yufeng, YU Jun, GAO Feng. Preparation and Electrical Properties of High Entropy La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 Perovskite Ceramics Powder[J]. Journal of Inorganic Materials, 2021, 36(4): 431-435.
| Sample | La | Co | Cr | Fe | Mn | Ni |
|---|---|---|---|---|---|---|
| La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 | 5 | 1 | 1 | 1 | 1 | 1 |
| La(Cr0.25Fe0.25Mn0.25Ni0.25)O3 La(Co0.25Fe0.25Mn0.25Ni0.25)O3 La(Co0.25Cr0.25Mn0.25Ni0.25)O3 La(Co0.25Cr0.25Fe0.25Ni0.25)O3 La(Co0.25Cr0.25Fe0.25Mn0.25)O3 | 4 4 4 4 4 | No 1 1 1 1 | 1 No 1 1 1 | 1 1 No 1 1 | 1 1 1 No 1 | 1 1 1 1 No |
表1 六组样品各元素组分摩尔之比
Table 1 Molar ratios of each element component of the six samples
| Sample | La | Co | Cr | Fe | Mn | Ni |
|---|---|---|---|---|---|---|
| La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 | 5 | 1 | 1 | 1 | 1 | 1 |
| La(Cr0.25Fe0.25Mn0.25Ni0.25)O3 La(Co0.25Fe0.25Mn0.25Ni0.25)O3 La(Co0.25Cr0.25Mn0.25Ni0.25)O3 La(Co0.25Cr0.25Fe0.25Ni0.25)O3 La(Co0.25Cr0.25Fe0.25Mn0.25)O3 | 4 4 4 4 4 | No 1 1 1 1 | 1 No 1 1 1 | 1 1 No 1 1 | 1 1 1 No 1 | 1 1 1 1 No |
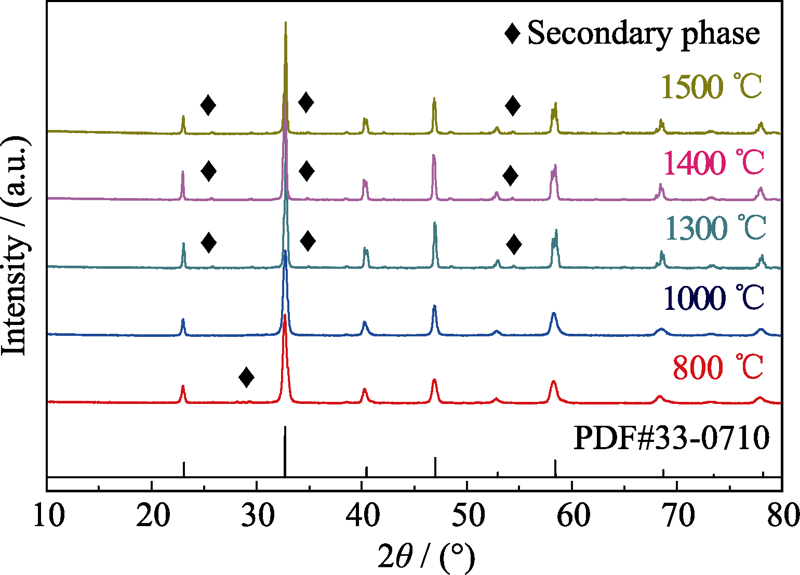
图1 不同温度煅烧所得La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3陶瓷粉体的XRD图谱
Fig. 1 XRD patterns of the La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 ceramic powders calcined at different temperatures

图4 800 ℃煅烧La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3样品的SEM照片(a), 1000 ℃煅烧La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3样品的SEM照片(b)及其元素的EDS分布(c~g)
Fig. 4 SEM image of sample La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 calcined at 800 ℃ (a), SEM image (b) and corresponding EDS element mapping (c-g) of La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 calcined at 1000 ℃
| [1] | TSAI M H, YEH J W. High-entropy alloys: a critical review. Materials Research Letters, 2014,2(3):107-123. |
| [2] | MIRACLE D B, SENKOV O N. A critical review of high entropy alloys and related concepts. Acta Materialia, 2017,122:448-511. |
| [3] | HUO W Y, ZHOU H, FANG F, et al. Microstructure and mechanical properties of CoCrFeNiZrx eutectic high-entropy alloys. Materials & Design, 2017,134:226-233. |
| [4] | ABHISHEK S, QINGSONG W, ALEXANDER S, et al. High entropy oxides: fundamental aspects and electrochemical properties. Advanced Materials, 2019,31(26):1806236-1-9. |
| [5] | ROST C M, SACHET E, BORMAN T, et al. Entropy-stabilized oxides. Nature Communications, 2015 , 6:8485. |
| [6] |
ELINOR C, CSANADI TAMAS, SALVATORE G, et al. Processing and properties of high-entropy ultra-high temperature carbides. Scientific Reports, 2018,8(1):8609.
DOI URL PMID |
| [7] | GILD J, ZHANG Y, HARRINGTON T, et al. High-entropy metal diborides: a new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Scientific Reports, 2016,6(1):37946. |
| [8] | POGREBNJAK A D, BAGDASARYAN A A, YAKUSHCHENKO I V, et al. The structure and properties of high-entropy alloys and nitride coatings based on them. Russian Chemical Reviews, 2014,83(11):1027-1061. |
| [9] |
ZHANG R Z, GUCCI F, ZHU H, et al. Data-driven design of ecofriendly thermoelectric high-entropy sulfides. Inorganic Chemistry, 2018,57(20):13027-3033.
DOI URL PMID |
| [10] | BÉRARDAN D, FRANGER S, MEENA A K, et al. Room temperature lithium superionic conductivity in high entropy oxides. Journal of Materials Chemistry A, 2016,4(24) : 9536-541. |
| [11] | CHEN H, QIU N, WU B Z, et al. Tunable pseudocapacitive contribution by dimension control in nanocrystalline-constructed (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O solid solutions to achieve superior lithium-storage properties. RSC Advances, 2019,9(50):28908-28915. |
| [12] | BÉRARDAN D, FRANGER S, DRAGOE D, et al. Colossal dielectric constant in high entropy oxides. Physica Status Solidi, 2016,10(4):328-333. |
| [13] | ZHANG J J, YAN J Q, CALDER S, et al. Long-range antiferromagnetic order in a rocksalt high entropy oxide. Chemistry of Materials, 2019,31(10):3705-3711. |
| [14] | CHEN H, FU J, ZHANG P F, et al. Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability. Journal of Materials Chemistry A, 2018,6(24):11129-11133. |
| [15] | CHEN H, LIN W W, ZHANG Z H, et al. Mechanochemical synthesis of high entropy oxide materials under ambient conditions: dispersion of catalysts via entropy maximization. ACS Materials Letters, 2019,1(1):83-88. |
| [16] |
SARKAR A, LOHO C, VELASCO L, et al. Multicomponent equiatomic rare earth oxides with narrow band gap and associated praseodymium multivalency. Dalton Transactions, 2017,46(36):12167-12176.
DOI URL |
| [17] |
GILD J, SAMIEE M, BRAUN J L, et al. High-entropy fluorite oxides. Journal of the European Ceramic Society, 2018,38(10):3578-3584.
DOI URL |
| [18] |
WANG D, JIANG S D, DUAN C Q, et al. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. Journal of Alloys and Compounds, 2020,844:156158.
DOI URL |
| [19] |
MAO A Q, QUAN F, XIANG H Z, et al. Facile synthesis and ferrimagnetic property of spinel (CoCrFeMnNi)3O4 high-entropy oxide nanocrystalline powder. Journal of Molecular Structure, 2019,1194:11-18.
DOI URL |
| [20] |
WANG J B, STENZEL D, AZMI R, et al. Spinel to rock-salt transformation in high entropy oxides with Li incorporation. Electrochem, 2020,1(1):60-74.
DOI URL |
| [21] |
LI F, ZHOU L, LIU J X, et al. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. Journal of Advanced Ceramics, 2019,8(4):576-582.
DOI URL |
| [22] | CHEN H, ZHAO Z F, XIANG H M, et al. High entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12: a novel high temperature stable thermal barrier material. Journal of Materials Science & Technology, 2020,48:57-62. |
| [23] |
JIANG S C, HU T, GILD J, et al. A new class of high-entropy perovskite oxides. Scripta Materialia, 2018,142:116-120.
DOI URL |
| [24] |
SARKAR A, DJENADIC R, WANG D, et al. Rare earth and transition metal based entropy stabilised perovskite type oxides. Journal of the European Ceramic Society, 2018,38(5):2318-2327.
DOI URL |
| [25] |
IRFAN S, AJAZUNNABI M, JAMIL Y, et al. Synthesis of Mn1-xZnxFe2O4 ferrite powder by co-precipitation method. IOP Conference Series: Materials Science and Engineering, 2014,60:12048.
DOI URL |
| [26] |
MASASHI, KOTOBUKI, MASAKI, et al. Preparation of Li1.5Al0.5Ti1.5(PO4)3 solid electrolyte via a co-precipitation method. Ionics, 2013,19(12):1945-1948.
DOI URL |
| [27] |
ZHOU S Y, PU Y P, ZHANG Q W, et al. Microstructure and dielectric properties of high entropy Ba(Zr0.2Ti0.2Sn0.2Hf0.2Me0.2)O3 perovskite oxides. Ceramics International, 2020,46(6):7430-7437.
DOI URL |
| [28] |
ZHAO S H, YANG Z B, ZHAO X M. Green preparation and supercapacitive performance of NiCo2S4@ACF heterogeneous electrode materials. Journal of Inorganic Materials, 2019,34(2):130-136.
DOI URL |
| [29] |
TAO K Y, LI P Y, KANG L T, et al. Facile and low-cost combustion-synthesized amorphous mesoporous NiO/carbon as high mass-loading pseudocapacitor materials. Journal of Power Sources, 2015,293:23-32.
DOI URL |
| [30] |
MA X J, KONG L B, ZHANG W B, et al. Design and synthesis of 3D Co3O4@MMoO4 (M=Ni, Co) nanocomposites as high-performance supercapacitor electrodes. Electrochimica Acta, 2014,130:660-669.
DOI URL |
| [31] |
ZHOU R, HAN C J, WANG X M. Hierarchical MoS2-coated three-dimensional graphene network for enhanced supercapacitor performances. Journal of Power Sources, 2017,352:99-110.
DOI URL |
| [32] |
HUO H H, ZHAO Y Q, XU C L. 3D Ni3S2 nanosheet arrays supported on Ni foam for high-performance supercapacitor and non-enzymatic glucose detection. Journal of Materials Chemistry A, 2014,2(36):15111-15117.
DOI URL |
| [33] | ZHANG L X, ZHENG W H, JIU H F, et al. The synthesis of NiO and NiCo2O4 nanosheets by a new method and their excellent capacitive performance for asymmetric supercapacitor. Electrochimica Acta, 2016,215:212-222. |
| [34] | ZHANG G X, CHEN Y M, HE Z N, et al. Surfactant dependence of nanostructured NiCo2S4 films on Ni foam for superior electrochemical performance. Journal of Inorganic Materials, 2018,33(3):289-294. |
| [1] | 余乐洋阳, 赵芳霞, 张舒心, 徐以祥, 牛亚然, 张振忠, 郑学斌. 感应等离子球化技术制备喷涂用高熵硼化物粉体[J]. 无机材料学报, 2025, 40(7): 808-816. |
| [2] | 樊文楷, 杨潇, 李宏华, 李永, 李江涛. 无压烧结制备(Y0.2Gd0.2Er0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷及其高温抗CMAS腐蚀性能[J]. 无机材料学报, 2025, 40(2): 159-167. |
| [3] | 张宇晨, 陆知遥, 赫晓东, 宋广平, 朱春城, 郑永挺, 柏跃磊. 硫族MAX相硼化物的物相稳定性和性能预测[J]. 无机材料学报, 2024, 39(2): 225-232. |
| [4] | 郭凌翔, 唐颖, 黄世伟, 肖博澜, 夏东浩, 孙佳. C/C复合材料高熵氧化物涂层抗烧蚀性能[J]. 无机材料学报, 2024, 39(1): 61-70. |
| [5] | 王马超, 唐扬敏, 邓明雪, 周真真, 刘小峰, 王家成, 刘茜. 共沉淀法制备Cs2Ag0.1Na0.9BiCl6:Tm3+双钙钛矿及其近红外发光性能[J]. 无机材料学报, 2023, 38(9): 1083-1088. |
| [6] | 付宇坤, 曾敏, 饶先发, 钟盛文, 张慧娟, 姚文俐. 锂离子电池高镍LiNi0.8Mn0.2O2正极材料的微波合成及其Co、Al共改性[J]. 无机材料学报, 2021, 36(7): 718-724. |
| [7] | 朱嘉桐, 楼志豪, 张萍, 赵佳, 孟轩宇, 许杰, 高峰. 稀土钽酸盐(RETaO4)高熵陶瓷的制备与热学性能研究[J]. 无机材料学报, 2021, 36(4): 411-417. |
| [8] | 桑玮玮, 张红松, 陈华辉, 温斌, 李新春. (Sm0.2Gd0.2Dy0.2Y0.2Yb0.2)3TaO7高熵陶瓷的制备及热物理性能[J]. 无机材料学报, 2021, 36(4): 405-410. |
| [9] | 张晓燕, 刘馨玥, 闫金华, 谷耀行, 齐西伟. 钙钛矿型(La0.2Li0.2Ba0.2Sr0.2Ca0.2)TiO3高熵氧化物陶瓷的制备及性能研究[J]. 无机材料学报, 2021, 36(4): 379-385. |
| [10] | 孙娅楠, 叶丽, 赵文英, 陈凤华, 邱文丰, 韩伟健, 刘伟, 赵彤. 液相聚合物前驱体法制备高熵碳化物纳米粉体[J]. 无机材料学报, 2021, 36(4): 393-398. |
| [11] | 孙鲁超, 任孝旻, 杜铁锋, 罗颐秀, 张洁, 王京阳. 高熵化设计: 稀土硅酸盐材料关键性能优化新策略[J]. 无机材料学报, 2021, 36(4): 339-346. |
| [12] | 郭晓杰, 鲍伟超, 刘吉轩, 王新刚, 张国军, 许钫钫. 高熵陶瓷固溶结构的透射电镜研究[J]. 无机材料学报, 2021, 36(4): 365-371. |
| [13] | 刘子玉, TOCI Guido, PIRRI Angela, PATRIZI Barbara, 冯亚刚, 陈肖朴, 胡殿君, 田丰, 吴乐翔, VANNINIMatteo, 李江. 固体激光用Nd:Lu2O3透明陶瓷的制备和光学性能研究[J]. 无机材料学报, 2021, 36(2): 210-216. |
| [14] | 黄新友, 刘玉敏, 刘洋, 李晓英, 冯亚刚, 陈肖朴, 陈鹏辉, 刘欣, 谢腾飞, 李江. 醇水共沉淀法制备Yb:YAG透明陶瓷及其性能研究[J]. 无机材料学报, 2021, 36(2): 217-224. |
| [15] | 陈磊,王恺,苏文韬,张文,徐晨光,王玉金,周玉. 过渡金属非氧化物高熵陶瓷的研究进展[J]. 无机材料学报, 2020, 35(7): 748-758. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||