无机材料学报 ›› 2021, Vol. 36 ›› Issue (4): 355-364.DOI: 10.15541/jim20200366 CSTR: 32189.14.10.15541/jim20200366
收稿日期:2020-07-02
修回日期:2020-09-27
出版日期:2021-04-20
网络出版日期:2020-09-20
通讯作者:
王一光, 教授. E-mail: wangyiguang@bit.edu.cn
作者简介:王皓轩(1994-), 男, 博士研究生. E-mail: wanghaoxuan@mail.nwpu.edu.cn
基金资助:
WANG Haoxuan1( ), LIU Qiaomu2, WANG Yiguang3(
), LIU Qiaomu2, WANG Yiguang3( )
)
Received:2020-07-02
Revised:2020-09-27
Published:2021-04-20
Online:2020-09-20
Contact:
WANG Yiguang, professor. E-mail: wangyiguang@bit.edu.cn
About author:WANG Haoxuan(1994-), male, PhD candidate. E-mail: wanghaoxuan@mail.nwpu.edu.cn
Supported by:摘要:
高熵陶瓷作为新型材料, 较大的构型熵赋予其独特的性能, 其中高熵过渡金属碳化物有望成为高超声速飞行器热防护系统的备选材料。相比于单组元碳化物陶瓷, 高熵化的单相陶瓷在综合性能上有较大地提高。目前, 高熵过渡金属碳化物陶瓷的研究还处于初始阶段, 关于高熵过渡金属碳化物的成分设计和理论分析还缺少足够的研究支撑。另外, 如何制备高纯度高熵过渡金属碳化物还需要进一步探索。在高熵过渡金属碳化物陶瓷的性能方面, 还缺少深入的研究。本文针对高熵陶瓷的理论设计和制备方法展开综述, 详细介绍了高熵过渡金属碳化物的力学、热导及抗氧化性能的研究进展, 并指出了高熵过渡金属碳化物陶瓷在超高温陶瓷领域存在的科学问题, 展望了高熵过渡金属碳化物陶瓷未来的发展方向。
中图分类号:
王皓轩, 刘巧沐, 王一光. 高熵过渡金属碳化物陶瓷的研究进展[J]. 无机材料学报, 2021, 36(4): 355-364.
WANG Haoxuan, LIU Qiaomu, WANG Yiguang. Research Progress of High Entropy Transition Metal Carbide Ceramics[J]. Journal of Inorganic Materials, 2021, 36(4): 355-364.
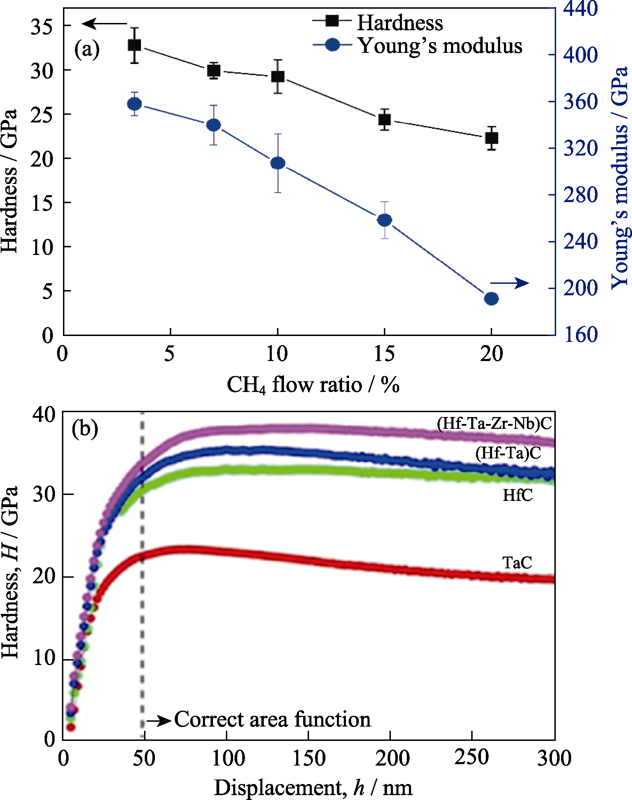
图2 (a)不同碳含量高熵CrNbSiTiZrCx薄膜硬度[84], (b)不同碳化物硬度-深度曲线[87]
Fig. 2 (a) Hardness of the CrNbSiTiZrCx with different carbon contents[84], and (b) hardness depth-profles of the individual, binary and high-entropy carbide[87]
| HEC | Hardness/GPa | Average/GPa |
|---|---|---|
| HfC[ | 25 | — |
| TaC[ | 14 | — |
| ZrC[ | 24 | — |
| TiC[ | 31 | — |
| NbC[ | 17 | — |
| WC[ | 14 | — |
| VC[ | 29 | — |
| Mo2C[ | 27 | — |
| (ZrNbTiV)C[ | 30 | 25 |
| (HfTaZrNb)C[ | 36 | 20 |
| (TiVNbTaW)C[ | 28 | 21 |
| (TiHfTaWZr)C[ | 33 | 22 |
| (TiHfNbTaMo)C[ | 27 | 23 |
| (TiZrNbTaMo)C[ | 32 | 23 |
| (VNbTaMoW)C[ | 27 | 20 |
| (HfTaZrTiNb)C[ | 32 | 28 |
| (TiZrHfTaW)C[ | 24 | 22 |
| (TiHfNbTaW)C[ | 31 | 20 |
| (TiHfVNbTa)C[ | 29 | 23 |
表1 部分碳化物陶瓷硬度[31,74,88-90]
Table 1 Hardness of some carbide ceramics[31,74,88-90]
| HEC | Hardness/GPa | Average/GPa |
|---|---|---|
| HfC[ | 25 | — |
| TaC[ | 14 | — |
| ZrC[ | 24 | — |
| TiC[ | 31 | — |
| NbC[ | 17 | — |
| WC[ | 14 | — |
| VC[ | 29 | — |
| Mo2C[ | 27 | — |
| (ZrNbTiV)C[ | 30 | 25 |
| (HfTaZrNb)C[ | 36 | 20 |
| (TiVNbTaW)C[ | 28 | 21 |
| (TiHfTaWZr)C[ | 33 | 22 |
| (TiHfNbTaMo)C[ | 27 | 23 |
| (TiZrNbTaMo)C[ | 32 | 23 |
| (VNbTaMoW)C[ | 27 | 20 |
| (HfTaZrTiNb)C[ | 32 | 28 |
| (TiZrHfTaW)C[ | 24 | 22 |
| (TiHfNbTaW)C[ | 31 | 20 |
| (TiHfVNbTa)C[ | 29 | 23 |
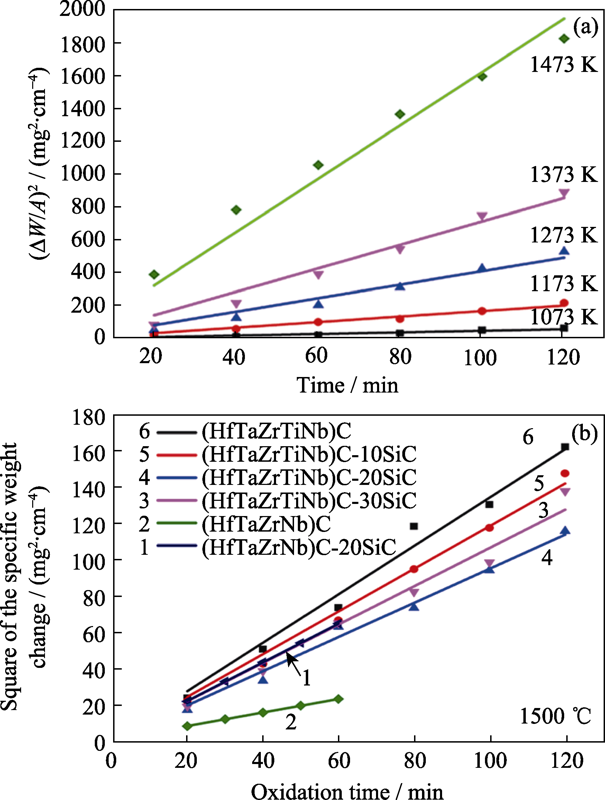
图4 (a) (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C在800~1200 ℃氧化增重曲线[95], 和(b)不同体系碳化物在1500 ℃氧化增重曲线[33,97,99]
Fig. 4 (a) Square of the specific weight change as a function of oxidation time at 800-1200 ℃ for (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C[95], (b) square of the specific weight change as a function of oxidation time at 1500 ℃ for high-entropy carbide in different systems[33,97,99]
| [1] | MIRACLE D B, SENKOV O N. A critical review of high entropy alloys and related concepts. Acta Mater., 2017,122:448-511. |
| [2] | YEH J W, CHEN S K, LIN S J, et al. Nanostructured high- entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater., 2004,6(5):299-303. |
| [3] |
KUCZA W, DABROWA J, CIESLAK G, et al. Studies of “sluggish diffusion” effect in Co-Cr-Fe-Mn-Ni, Co-Cr-Fe-Ni and Co-Fe-Mn-Ni high entropy alloys; determination of tracer diffusivities by combinatorial approach. J. Alloys Compd., 2018,731:920-928.
DOI URL |
| [4] | UTT D, STUKOWSKI A, ALBE K. Grain boundary structure and mobility in high-entropy alloys: a comparative molecular dynamics study on a 11 symmetrical tilt grain boundary in face-centered cubic CuNiCoFe. Acta Mater., 2020,186:11-19. |
| [5] |
GLUDOVATZ B, HOHENWARTER A, CATOOR D, et al. A fracture-resistant high-entropy alloy for cryogenic applications. Science, 2014,345(6201):1153-1158.
URL PMID |
| [6] |
LI Z M, PRADEEP K G, DENG Y, et al. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature, 2016,534(7606):227-230.
URL PMID |
| [7] | ZHANG R Z, REECE M J. Review of high entropy ceramics: design, synthesis, structure and properties. J. Mater. Chem. A, 2019,7(39):22148-22162. |
| [8] | MURTY B S, YEH J W, RANGANATHAN S, et al. High-entropy Alloys. United Kingdom: Butterworth-Heinemann, 2019: 165-176. |
| [9] | OSES C, TOHER C, CURTAROLO S. High-entropy ceramics. Nat. Rev. Mater., 2020,5(4):295-309. |
| [10] | LAL M S, SUNDARA R. High entropy oxides-a cost-effective catalyst for the growth of high yield carbon nanotubes and their energy applications. ACS Appl. Mater. Inter., 2019,11(34):30846-30857. |
| [11] |
SARKAR A, VELASCO L, WANG D, et al. High entropy oxides for reversible energy storage. Nat. Commun., 2018,9(1):3400.
URL PMID |
| [12] | BERARDAN D, FRANGER S, DRAGOE D, et al. Colossal dielectric constant in high entropy oxides. Phys. Status Solidi-R, 2016,10(4):328-333. |
| [13] | BIESUZ M, SPIRIDIGLIOZZI L, DELLAGLI G, et al. Synthesis and sintering of (Mg,Co,Ni,Cu,Zn)O entropy-stabilized oxides obtained by wet chemical methods. J. Mater. Sci., 2018,53(11):8074-8085. |
| [14] |
OKEJIRI F, ZHANG Z H, LIU J X, et al. Room-temperature synthesis of high-entropy perovskite oxide nanoparticle catalysts via ultrasonication-based method. ChemSusChem, 2020,13(1):111-115.
DOI URL PMID |
| [15] | DEMIRSKYI D, BORODIANSKA H, SUZUKI T S, et al. High- temperature flexural strength performance of ternary high-entropy carbide consolidated via spark plasma sintering of TaC, ZrC and NbC. Scripta Mater., 2019,164:12-16. |
| [16] | LI F, BAO W C, SUN S K, et al. Synthesis of single-phase metal oxycarbonitride ceramics. Scripta Mater., 2020,176:17-22. |
| [17] | KUMAR A, GUPTA M. An insight into evolution of light weight high entropy alloys: a review. Metals Basel, 2016,6(9):199. |
| [18] | ROST C M, SACHET E, BORMAN T, et al. Entropy-stabilized oxides. Nat. Commun., 2015,6(1):8485. |
| [19] | WEI X F, QIN Y, LIU J X, et al. Gradient microstructure development and grain growth inhibition in high-entropy carbide ceramics prepared by reactive spark plasma sintering . J. Eur. Ceram. Soc., 2020,40(4):935-941. |
| [20] | LI F, LU Y, WANG X G, et al. Liquid precursor-derived high- entropy carbide nanopowders. Ceram. Int., 2019,45(7):22437-22441. |
| [21] | WEI X F, LIU J X, LI F, et al. High entropy carbide ceramics from different starting materials. J. Eur. Ceram. Soc., 2019,39(10):2989-2994. |
| [22] | DUSZA J, SVEC P, GIRMAN V, et al. Microstructure of (Hf-Ta-Zr-Nb)C high-entropy carbide at micro and nano/atomic level. J. Eur. Ceram. Soc., 2018,38(12):4303-4307. |
| [23] | ZHOU J Y, ZHANG J Y, ZHANG F, et al. High-entropy carbide: a novel class of multicomponent ceramics. Ceram. Int., 2018,44(17):22014-22018. |
| [24] | JIANG S C, HU T, GILD J, et al. A new class of high-entropy perovskite oxides. Scripta Mater., 2018,142:116-120. |
| [25] | ZHAO Z F, CHEN H, XIANG H M, et al. (Y0.25Yb0.25Er0.25Lu0.25)2(Zr0.5Hf0.5)2O7: a defective fluorite structured high entropy ceramic with low thermal conductivity and close thermal expansion coefficient to Al2O3. J. Mater. Sci. Technol., 2020,39:167-172. |
| [26] | DABROWA J, STYGAR M, MIKULA A, et al. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett., 2018,216:32-36. |
| [27] | ZHAO Z F, CHEN H, XIANG H M, et al. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)PO4: a high-entropy rare-earth phosphate monazite ceramic with low thermal conductivity and good compatibility with Al2O3. J. Mater. Sci. Technol., 2020,38:80-85. |
| [28] | GILD J, KAUFMANN K, Vecchio K, et al. Reactive flash spark plasma sintering of high-entropy ultrahigh temperature ceramics. Scripta. Mater., 2019,170:106-110. |
| [29] | YAN J L, LIU F S, MA G H, et al. Suppression of the lattice thermal conductivity in NbFeSb-based half-Heusler thermoelectric materials through high entropy effects. Scripta Mater., 2018,157:129-134. |
| [30] | GILD J, BRAUN J L, KAUFMANN K, et al. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater., 2019,5(3):337-343. |
| [31] |
SARKER P, HARRINGTON T, TOHER C, et al. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun., 2018,9(1):4980.
URL PMID |
| [32] |
SARKAR A, VELASCO L, WANG D, et al. High entropy oxides for reversible energy storage. Nat. Commun., 2018,9(1):3400.
URL PMID |
| [33] |
WANG H X, CAO Y J, LIU W, et al. Oxidation behavior of (Hf0.2Ta0.2Zr0.2Ti0.2Nb0.2)C-xSiC ceramics at high temperature. Ceram. Int., 2020,46(8):11160-11168.
DOI URL |
| [34] | TAN Y Q, CHEN C, Li S G, et al. Oxidation behaviours of high-entropy transition metal carbides in 1200 ℃ water vapor. J. Alloys Compd., 2020,816:152523. |
| [35] | REN K, WANG Q K, SHAO G, et al. Multicomponent high- entropy zirconates with comprehensive properties for advanced thermal barrier coating. Scripta Mater., 2020,178:382-386. |
| [36] | DONG Y, REN K, LU Y H, et al. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc., 2018,39(7):2574-2579. |
| [37] | TENG Z, ZHU L N, TAN Y Q, et al. Synjournal and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc., 2020,40(4):1639-1643. |
| [38] | ZHAO Z F, XIANG H M, DAI F Z, et al. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol., 2019,35(11):2647-2651. |
| [39] | SAVINO R, FUMO M D S, PATERNA D, et al. Aerothermodynamic study of UHTC-based thermal protection systems. Aerosp. Sci. Technol., 2005,9(2):151-160. |
| [40] | OPRKA M M, TALMY I G, ZAYKOSKI J A. Oxidation-based materials selection for 2000 ℃ + hypersonic aerosurfaces: theoretical considerations and historical experience. J. Mater. Sci., 2004,39(19):5887-5904. |
| [41] | KUBOTA Y, YANO M, INOUE R, et al. Oxidation behavior of ZrB2-SiC-ZrC in oxygen-hydrogen torch environment. J. Eur. Ceram. Soc., 2017,38(4):1095-1102. |
| [42] | RAMA RAO G A, VENUGOPAL V. Kinetics and mechanism of the oxidation of ZrC. J. Alloys Compd., 1994,206(2):237-242. |
| [43] | VOITOVICH R F, PUGACH E A. High-temperature oxidation of ZrC and HfC. Powder Metall. Met. C, 1973,12(11):916-921. |
| [44] | CHEN L Y, GU Y L, SHI L, et al. Synjournal and oxidation of nanocrystalline HfB2. J. Alloys Compd., 2004,368(1):353-356. |
| [45] | SHIMADA S. Interfacial reaction on oxidation of carbides with formation of carbon. Solid State Ionics, 2001,141:99-104. |
| [46] | PARTHASARATHY T A, RAPP R A, OPEKA M M, et al. A model for the oxidation of ZrB2, HfB2 and TiB2. Acta Mater., 2007,55(17):5999-6010. |
| [47] | PARTHASARATHY T A, RAPP R A, OPEKA M M, et al. Effect of phase change and oxygen permeability in oxide scales on oxidation kinetics of ZrB2 and HfB2. J. Am. Ceram. Soc., 2009,92(5):1079-1086. |
| [48] | JING Y, YUAN H B, LIAN Z S. Microstructure and mechanical properties of ZrB2-HfC ceramics influenced by HfC addition. Materials, 2018,11(10):2046. |
| [49] | MALLIK M, RAY K K, MITRA R. Oxidation behavior of hot pressed ZrB2-SiC and HfB2-SiC composites. J. Eur. Ceram. Soc., 2011,31(1):199-215. |
| [50] | TRIPP W C, GRAHAM H C. Thermogravimetric study of oxidation of ZrB2 in temperature range of 800 ℃ to 1500 ℃. J. Electrochem. Soc., 1971,118(7):1195-1199. |
| [51] | FAHRENHOLTZ W G. The ZrB2 volatility diagram. J. Am. Ceram. Soc., 2005,88(12):3509-3512. |
| [52] | FAHRENHOLTZ W G. Thermodynamic analysis of ZrB2-SiC oxidation: formation of a SiC-depleted region . J. Am. Ceram. Soc., 2007,90(1):143-148. |
| [53] | HU P, GUOLIN W, WANG Z. Oxidation mechanism and resistance of ZrB2-SiC composites. Corros. Sci., 2009,51(11):2724-2732. |
| [54] | JACOBSON N S, MYERS D L. Active oxidation of SiC. Oxid. Met., 2011,75(1):1-25. |
| [55] | JACOBSON N S, HARDER B, MYERS D L, et al. Oxidation transitions for SiC. Part I. Active-to-passive transitions. J. Am. Ceram. Soc., 2013,96(3):838-844. |
| [56] | WANG Y G, LUO L, SUN J, et al. ZrB2-SiC(Al) ceramics with high resistance to oxidation at 1500 ℃. Corros. Sci., 2013,74:154-158. |
| [57] | HE J B, WANG Y G, LUO L, et al. Oxidation behaviour of ZrB2-SiC (Al/Y) ceramics at 1700 ℃. J. Eur. Ceram. Soc., 2016,36(15):3769-3774. |
| [58] | WANG Y G, MA B S, LI L L, et al. Oxidation behavior of ZrB2-SiC-TaC ceramics . J. Am. Ceram. Soc., 2012,95(1):374-378. |
| [59] |
TONG Z W, HE R J, CHENG T B, et al. High temperature oxidation behavior of ZrB2-SiC added MoSi2 ceramics. Ceram. Int., 2018,44(17):21076-21082.
DOI URL |
| [60] | ZAPATASOLVAS E, JAYASEELAN D D, BROWN P, et al. Effect of La2O3 addition on long-term oxidation kinetics of ZrB2-SiC and HfB2-SiC ultra-high temperature ceramics . J. Eur. Ceram. Soc., 2014,34(15):3535-3548. |
| [61] | GILD J, ZHANG Y Y, HARRINGTON T, et al. High-entropy metal diborides: a new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Sci. Rep-UK, 2016,6(1):37946-37946. |
| [62] | YE B L, WEN T Q, HUANG K H, et al. First-principles study, fabrication, and characterization of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high- entropy ceramic. J. Am. Ceram. Soc., 2019,102(7):4344-4352. |
| [63] | HOSKING F M. Sodium compatibility of refractory-metal alloy- type 304l stainless-steel joints. Int. J. Refract. Met. H., 1985,64(7):S181-S190. |
| [64] | WERNER E A. Introduction to the thermodynamics of materials. Mat. Sci. Eng., 2008,494(1/2):464. |
| [65] |
CAR R, PARRINELLO M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett., 1985,55(22):2471-2474.
URL PMID |
| [66] | LIU X J, WANG C P, GAO F, et al. Thermodynamic calculation of phase equilibria in the Sn-Ag-Cu-Ni-Au System . J. Electron. Mater., 2007,36(11):1429-1441. |
| [67] | WANG C P, WANG J, GUO S H, et al. Experimental investigation and thermodynamic calculation of the phase equilibria in the Co-Mo-W system. Intermetallics, 2009,17(8):642-650. |
| [68] | FENG R, GAO M C, LEE C, et al. Design of light-weight high- entropy alloys. Entropy Switz., 2016,18(9):333-353. |
| [69] | KIM J. Applicability of special quasi-random structure models in thermodynamic calculations using semi-empirical Debye-Grüneisen theory . J. Alloys Compd., 2015,650:564-571. |
| [70] | VOAS B K, USHER T M, LIU X M, et al. Special quasirandom structures to study the (K0.5Na0.5)NbO3 random alloy. Phys. Rev. B, 2014,90(2):024105-1-6. |
| [71] | SAHARA R, EMURA S, LI S, et al. First-principles study of electronic structures and stability of body-centered cubic Ti-Mo alloys by special quasirandom structures. Sci. Technol. Adv. Mat., 2014,15(3):035014-1-10. |
| [72] |
VITOS L, ABRIKOSOV I A, JOHANSSON B. Anisotropic lattice distortions in random alloys from first-principles theory. Phys. Rev. Lett., 2001,87(15):156401-1-4.
URL PMID |
| [73] | ABRIKOSOV I A, JOHANSSON B. Applicability of the coherent- potential approximation in the theory of random alloys. Phys. Rev. B, 1998,57(22):14164-14173. |
| [74] | YE B L, WEN T Q, NGUYEN M C, et al. First-principles study, fabrication and characterization of (Zr0.25Nb0.25Ti0.25V0.25)C high- entropy ceramics. Acta Mater., 2019,170:15-23. |
| [75] |
BRAIC V, VLADESCU A, BALACEANU M, et al. Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surf. Coat. Tech., 2012,211:117-121.
DOI URL |
| [76] |
LIN S Y, CHANG S Y, HUANG Y C, et al. Mechanical performance and nanoindenting deformation of (AlCrTaTiZr)NCy multi-component coatings co-sputtered with bias. Surf. Coat. Tech., 2012,206(24):5096-5102.
DOI URL |
| [77] |
ZHOU J Y, ZHANG J Y, ZHANG F, et al. high-entropy carbide: a novel class of multicomponent ceramics. Ceram. Int., 2018,44(17):22014-22018.
DOI URL |
| [78] |
FENG L, FAHRENHOLTZ W G, HILMAS G E, et al. Synthesis of single-phase high-entropy carbide powders. Scripta Mater., 2019,162(12):90-93.
DOI URL |
| [79] | YE B L, NING S S, LIU D, et al. One-step synthesis of coral-like high-entropy metal carbide powders. J.Am. Ceram. Soc., 102(10):6372-6378. |
| [80] |
DU B, LIU H H, CHU Y H. Fabrication and characterization of polymer-derived high-entropy carbide ceramic powders . J. Am. Ceram. Soc., 2020,103:4063-4068.
DOI URL |
| [81] | NING S S, WEN T Q, YE B L, et al. Low-temperature molten salt synjournal of high-entropy carbide nanopowders . J. Am. Ceram. Soc., 2020,103(3):2244-2251. |
| [82] | JAGADEESH S, VISHNU D S M, KIM H K, et al. Facile electrochemical synthesis of nanoscale (TiNbTaZrHf)C high-entropy carbide powder. Angew. Chem. Int. Ed., 2020 59(29):11830-11835. |
| [83] | BRAIC M, BRAIC V, BALACEANU M, et al. Characteristics of (TiAlCrNbY)C films deposited by reactive magnetron sputtering. Surf. Coat. Tech., 2010,204(12):2010-2014. |
| [84] | JHONG Y S, HUANG C W, LIN S J, et al. Effects of CH4 flow ratio on the structure and properties of reactively sputtered (CrNbSiTiZr)Cx coatings. Mater. Chem. Phys., 2017,210:348-352. |
| [85] | BRAIC M, BALACEANU M, VLADESCU A, et al. Deposition and characterization of multi-principal-element (CuSiTiYZr)C coatings. Appl. Surf. Sci., 2013 284:671-678. |
| [86] | BRAIC V, PARAU A C, PANA I, et al. Effects of substrate temperature and carbon content on the structure and properties of (CrCuNbTiY)C multicomponent coatings. Surf. Coat. Tech., 2014 258:996-1005. |
| [87] | CSANADI T, CASTLE E G, REECE M J, et al. Strength enhancement and slip behaviour of high-entropy carbide grains during micro-compression. Sci. Rep-UK, 2019,9(1):10200. |
| [88] | WANG C, YE Y, GUAN X, et al. An analysis of tribological performance on Cr/GLC film coupling with Si3N4, SiC, WC, Al2O3 and ZrO2 in seawater. Tribol. Int., 2016 96:77-86. |
| [89] | HARRINGTON T J, GILD J, SARKER P, et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater., 2019 166:271-280. |
| [90] | WANG K, CHEN L, XU C G, et al. Microstructure and mechanical properties of (TiZrNbTaMo)C high-entropy ceramic. J. Mater. Sci. Technol., 2020,39:99-105. |
| [91] | HAN X X, VLADIMIR G, RICHARD S, et al. Improved creep resistance of high entropy transition metal carbides . J. Eur. Ceram. Soc., 2020,40(7):2709-2715. |
| [92] | YAN X L, CONSTANTIN L, LU Y F, et al. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics with low thermal conductivity. J. Am. Ceram. Soc., 2018,101(10):4486-4491. |
| [93] | CHEN H, XIANG H M, DAI F Z, et al. Porous high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)B2: a novel strategy towards making ultrahigh temperature ceramics thermal insulating. J. Mater. Sci. Technol., 2019,35(10):2404-2408. |
| [94] | CHEN H, XIANG H M, DAI F Z, et al. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol., 2019,35(8):1700-1705. |
| [95] |
YE B L, WEN T Q, LIU D, et al. Oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics at 1073-1473 K in air. Corros. Sci., 2019,153:327-332.
DOI URL |
| [96] | YE B L, WEN T Q, CHU Y H. High-temperature oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics in air. J. Am. Ceram. Soc., 2019,103(1):500-507. |
| [97] | WANG H X, HAN X, LIU W, et al. Oxidation behavior of high-entropy carbide (Hf0.2Ta0.2Er0.2Ti0.2Nb0.2)C at 1400-1600 ℃. DOI: 10.1016/j.ceramint.2020.12.201. |
| [98] | BACKMAN L, GILD J, LUO J, et al. Theoretical predictions of preferential oxidation in refractory high entropy materials. Acta Mater., 2020,197:20-27. |
| [99] | WANG H X, WANG S Y, CAO Y J, et al. Oxidation behaviors of (Hf0.25Zr0.25Ta0.25Nb0.25)C and (Hf0.25Zr0.25Ta0.25Nb0.25)C-SiC at 1300-1500 ℃. J. Mater. Sci. Technol., 2021,60:147-155. |
| [100] |
BRAIC V, BALACEANU M, BRAIC M, et al. Characterization of multi-principal-element (TiZrNbHfTa)N and (TiZrNbHfTa)C coatings for biomedical applications. J. Mech. Behav. Biomed., 2019,10:197-205.
DOI URL |
| [101] | WANG F, YAN X L, WANG T Y, et al. Irradiation damage in (Zr0.25Ta0.25Nb0.25Ti0.25)C high-entropy carbide ceramics. Acta Mater., 2020,195:739-749. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 陈义, 邱海鹏, 陈明伟, 徐昊, 崔恒. SiC/SiC复合材料基体硼改性方法及其力学性能研究[J]. 无机材料学报, 2025, 40(5): 504-510. |
| [8] | 崔宁, 张玉新, 王鲁杰, 李彤阳, 于源, 汤华国, 乔竹辉. (TiVNbMoW)Cx高熵陶瓷的单相形成过程与碳空位调控[J]. 无机材料学报, 2025, 40(5): 511-520. |
| [9] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [10] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [11] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [12] | 李紫薇, 弓伟露, 崔海峰, 叶丽, 韩伟健, 赵彤. 前驱体法制备(Zr, Hf, Nb, Ta, W)C-SiC复相陶瓷及性能研究[J]. 无机材料学报, 2025, 40(3): 271-280. |
| [13] | 高晨光, 孙晓亮, 陈君, 李达鑫, 陈庆庆, 贾德昌, 周玉. 基于湿法纺丝技术的SiBCN-rGO陶瓷纤维的组织结构、力学和吸波性能[J]. 无机材料学报, 2025, 40(3): 290-296. |
| [14] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [15] | 穆浩洁, 张源江, 喻彬, 付秀梅, 周世斌, 李晓东. ZrO2掺杂Y2O3-MgO纳米复相陶瓷的制备及性能研究[J]. 无机材料学报, 2025, 40(3): 281-289. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||