无机材料学报 ›› 2020, Vol. 35 ›› Issue (10): 1088-1098.DOI: 10.15541/jim20190572 CSTR: 32189.14.10.15541/jim20190572
所属专题: 【虚拟专辑】钙钛矿材料(2020~2021)
收稿日期:2019-11-09
修回日期:2020-02-04
出版日期:2020-10-20
网络出版日期:2020-09-23
作者简介:杨丹丹(1989-), 女, 博士研究生. E-mail:dandan.yang@njust.edu.cn.
基金资助:
YANG Dandan( ),LI Xiaoming(
),LI Xiaoming( ),MENG Cuifang,CHEN Jiaxin,ZENG Haibo
),MENG Cuifang,CHEN Jiaxin,ZENG Haibo
Received:2019-11-09
Revised:2020-02-04
Published:2020-10-20
Online:2020-09-23
About author:YANG Dandan (1989-), female, PhD candidate. E-mail: dandan.yang@njust.edu.cn
Supported by:摘要:
全无机钙钛矿纳米晶具有窄发射、高量子效率及较高的载流子迁移率等优点, 在高清柔性显示器和太阳能电池等领域具有广泛的应用前景。然而, 钙钛矿纳米晶的表面配体处于高度动态结合的状态, 容易在分离和提纯等过程中造成大量配体缺失, 从而导致量子效率和稳定性下降。此外, 钙钛矿材料本身的离子晶体特性使其对极性溶剂非常敏感, 这些问题严重制约了钙钛矿纳米晶在光电器件中的实际应用。本文从钙钛矿纳米晶表面态出发, 结合国内外的研究工作, 分析了路易斯酸、路易斯碱及表面包覆策略对钙钛矿纳米晶光学性质和稳定性的影响, 并对进一步优化提升钙钛矿纳米晶的稳定性进行了展望。
中图分类号:
杨丹丹, 李晓明, 孟翠芳, 陈佳欣, 曾海波. CsPbX3纳米晶稳定性的研究进展[J]. 无机材料学报, 2020, 35(10): 1088-1098.
YANG Dandan, LI Xiaoming, MENG Cuifang, CHEN Jiaxin, ZENG Haibo. Research Progress on the Stability of CsPbX3 Nanocrystals[J]. Journal of Inorganic Materials, 2020, 35(10): 1088-1098.
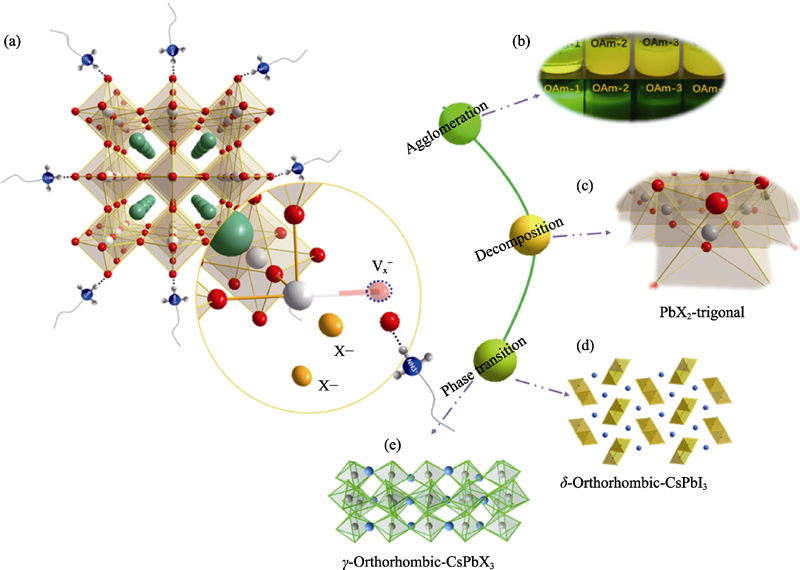
图2 (a) CsPbX3晶体结构不稳定性机理的示意图; (b) CsPbX3纳米晶的团聚现象; (c) CsPbX3纳米晶的分解产物; CsPbX3纳米晶的(d)相转变(非钙钛矿相)和(e)相转变(钙钛矿相)示意图
Fig. 2 (a) Schematic diagram of instability mechanism of CsPbX3 nanocrystals, (b) agglomeration and (c) decomposing product of CsPbX3 nanocrystals, and schematic diagram of (d) phase transition (non-perovskite phase) and (e) phase transition (perovskite phase)
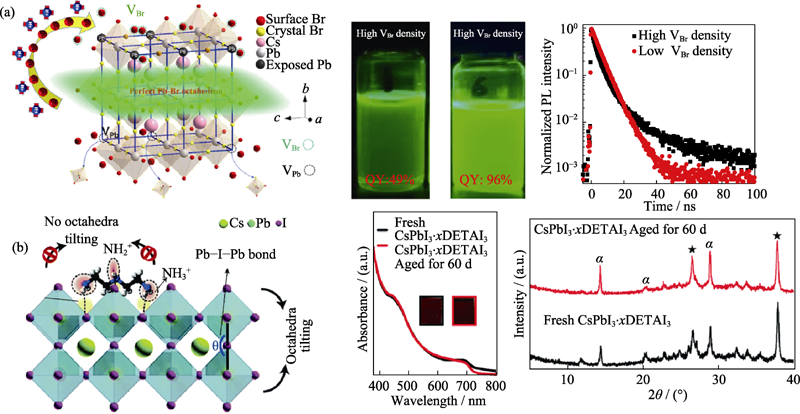
图3 (a)硬路易斯酸配体表面钝化策略(左)、高和低缺陷密度纳米晶的发光照片(激发波长365 nm, 中)和对应的荧光寿命曲线(右)[25]; (b)DETAI3表面钝化策略的原理图(左)、CsPbI3?xDETAI3薄膜和放置60 d后的紫外-可见吸收光谱图(中)、以及相应的XRD图谱(右)[29]
Fig. 3 (a) Passivation strategy based on hard Lewis acid ligands (left), images under 365 nm UV light (middle), and photoluminescence decay curves (right) of nanocrystals with high and low defect densities[25]; (b) Schematic diagram of DETAI3 surface passivation strategy (left), absorption curves (middle) and long-term phase stability (right) of CsPbI3?xDETAI3 thin films[29]
| Ligands | Treating agent | QY/% | Stability | Ref. |
|---|---|---|---|---|
| OA/OAm | DDDMAB | ~100 | 21 d | [ |
| OA/OAm | TOAB | 95 | - | [ |
| OA/OAm | DDAB | 96 | - | [ |
| OA/OAm | NH4BF4 | (95±2) | - | [ |
| OA/OAm | NH4SCN | (99±2) | - | [ |
| OA/OAm | Trimethylsilyl iodine | 85 | 105 d | [ |
| OA/OAm | Oxalic acid | 89 | - | [ |
表1 路易斯酸型配体表面钝化策略
Table 1 Surface passivation strategies of Lewis acid ligands
| Ligands | Treating agent | QY/% | Stability | Ref. |
|---|---|---|---|---|
| OA/OAm | DDDMAB | ~100 | 21 d | [ |
| OA/OAm | TOAB | 95 | - | [ |
| OA/OAm | DDAB | 96 | - | [ |
| OA/OAm | NH4BF4 | (95±2) | - | [ |
| OA/OAm | NH4SCN | (99±2) | - | [ |
| OA/OAm | Trimethylsilyl iodine | 85 | 105 d | [ |
| OA/OAm | Oxalic acid | 89 | - | [ |
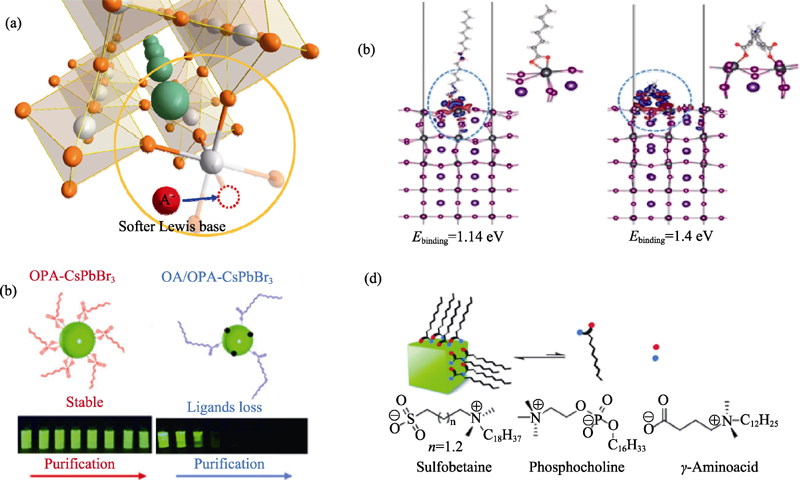
图4 (a)路易斯碱表面钝化CsPbX3纳米晶的策略示意图; (b)理论计算单羧酸和双羧酸吸附在CsPbI3纳米晶表面的束缚能[34]; (c)OPA和OAm-CsPbX3纳米晶表面钝化策略的示意图和多次纯化后的发光照片[35]; (d)两性离子(磺酸甜菜碱, 磷酸胆碱和γ-氨基酸)表面钝化策略[36]
Fig. 4 (a) Schematic diagram of Lewis base surface passivation strategy for CsPbX3 nanocrystals; (b) Theoretical calculation of the binding energy of mono- and dicarboxylic acids on the surface of CsPbI3 nanocrystals[34]; (c) Schematic diagram of OPA and OAm-CsPbX3 surface passivation strategies and photos after multiple purifications[35]; (d) Surface passivation strategy with zwitterionic ligands (sulfobetaines, phosphocholines and γ-amino acids)[36]
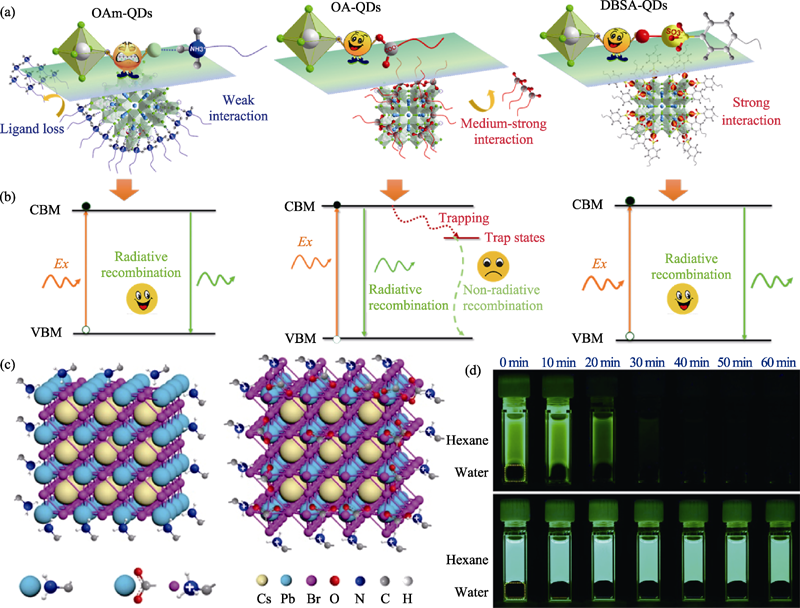
图5 (a)不同配体(OAm, OA和DBSA)在CsPbBr3纳米晶表面的钝化策略和(b)对应的激子复合过程[37]; (c) OAm-CsPbBr3纳米晶表面富含PbBrx(左)和OAm/OA-CsPbBr3纳米晶表面富溴(右)的示意图[11]; (d) OAm/OA-CsPbBr3纳米晶(上)和OAm-CsPbBr3 纳米晶(下)在水溶液中保持稳定的发光照片[11]
Fig. 5 (a) Passivation strategies with different ligands (OAm, OA and DBSA) on the surface of CsPbBr3 nanocrystals and (b) the corresponding exciton recombination processes[37]; (c) Schematic diagram of PbBrx-rich surface of OAm-CsPbBr3 nanocrystals (left) and Br-rich surface of OAm/OA-CsPbBr3 nanocrystals (right)[11]; (d) Photographs showing the resistance of different samples against water treatment of OAm/OA-CsPbBr3 nanocrystals (above) and OAm-CsPbBr3 nanocrystals (below)[11]
| Ligands | QY/% | Stability | Ref. |
|---|---|---|---|
| OAm | ~100 % | - | [ |
| OA | 70 | - | [ |
| IDA/OAm | 95 | 40 d | [ |
| OPA/TOPO | >90 | - | [ |
| Zwitterion | >90 | 28 d | [ |
| DBSA | 95 | 5 m | [ |
| TMPPA/OAm | 90 | 28 d | [ |
| OA/TOPO | 90 | - | [ |
| TDPA/OAm | 68 | - | [ |
| DA | 94.3 | 70 d | [ |
表2 路易斯碱表面钝化策略
Table 2 Surface passivation strategies of Lewis base ligands
| Ligands | QY/% | Stability | Ref. |
|---|---|---|---|
| OAm | ~100 % | - | [ |
| OA | 70 | - | [ |
| IDA/OAm | 95 | 40 d | [ |
| OPA/TOPO | >90 | - | [ |
| Zwitterion | >90 | 28 d | [ |
| DBSA | 95 | 5 m | [ |
| TMPPA/OAm | 90 | 28 d | [ |
| OA/TOPO | 90 | - | [ |
| TDPA/OAm | 68 | - | [ |
| DA | 94.3 | 70 d | [ |
| Strategies | Characteristics | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|
| Lewis acid ligands | Quaternary ammonium salt | Large steric hindrance | High QY* | Instable | [ |
| Lewis base ligands | Carboxylic acids | Hard base, weak acid | Simple synthesis | Instable, low QY | [ |
| Phosphoric acid | Soft alkali, moderately strong acid | High QY, stable | TOPO assisted dissolution | [ | |
| Zwitterionic ligands | Surfactant | High QY, stable | Complex process | [ | |
| Sulfonic acid | Soft alkali, strong acid | High QY, stable | High temperature | [ | |
| Neutral ligands | Lone pair electrons | High QY, stable | Room temperature | [ | |
表3 不同钝化策略的特点及其优缺点
Table 3 Characteristics, advantages and disadvantages of different passivation strategies
| Strategies | Characteristics | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|
| Lewis acid ligands | Quaternary ammonium salt | Large steric hindrance | High QY* | Instable | [ |
| Lewis base ligands | Carboxylic acids | Hard base, weak acid | Simple synthesis | Instable, low QY | [ |
| Phosphoric acid | Soft alkali, moderately strong acid | High QY, stable | TOPO assisted dissolution | [ | |
| Zwitterionic ligands | Surfactant | High QY, stable | Complex process | [ | |
| Sulfonic acid | Soft alkali, strong acid | High QY, stable | High temperature | [ | |
| Neutral ligands | Lone pair electrons | High QY, stable | Room temperature | [ | |
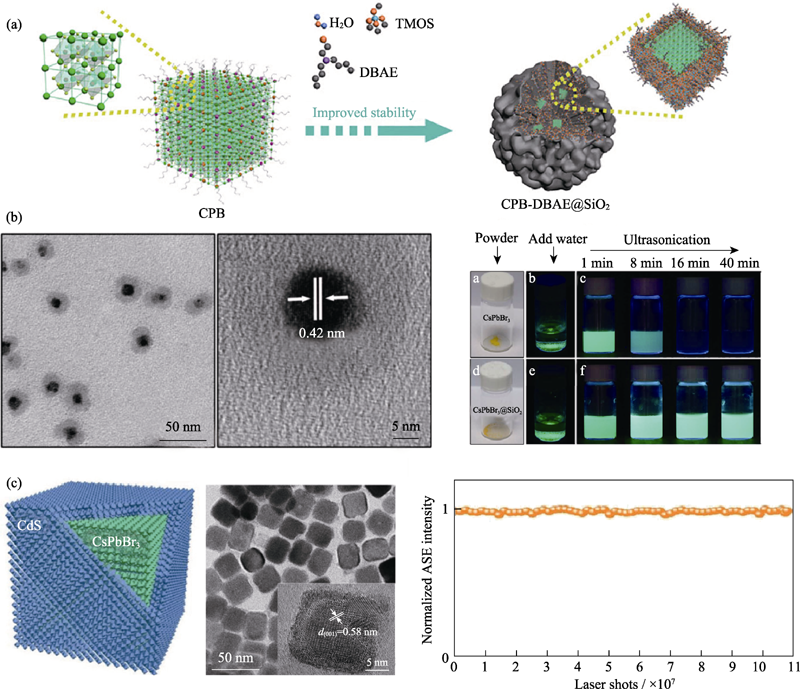
图6 (a) CPB-DBAE@SiO2纳米复合材料的制备过程示意图[51]; (b) CsPbBr3@SiO2纳米晶的普通透射电镜照片(TEM)和高分辨透射电镜照片(HRTEM)以及水稳定性的实物照片[60]; (c) CsPbBr3/CdS纳米晶的示意图(左)、HRTEM照片(中)以及连续脉冲激光照射CsPbBr3/CdS纳米晶的稳定性曲线(右)[67]
Fig. 6 (a) Schematic diagram of CPB-DBAE@SiO2 preparation process[51]; (b) Transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) images of CsPbBr3@SiO2 nanocrystals and photographs of water stability[60]; (c) Schematic representation (left), HRTEM image (middle), and plot of emission intensity under continuous pulsed laser irradiation (right) of CsPbBr3/CdS nanocrystals[67]
| [1] |
KOJIMA A, TESHIMA K, SHIRAI Y , et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. Journal of the American Chemical Society, 2009,131(17):6050-6051.
DOI URL PMID |
| [2] |
SCHMIDT L C, PERTEGAS A, GONZALEZ-CARRERO S , et al. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. Journal of the American Chemical Society, 2014,136(3):850-853.
DOI URL PMID |
| [3] |
PROTESESCU L, YAKUNIN S, BODNARCHUK M I , et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Letters, 2015,15(6):3692-3696.
DOI URL PMID |
| [4] | DOU L, LAI M, KLEY C S , et al. Spatially resolved multicolor CsPbX3 nanowire heterojunctions via anion exchange. Proceedings of the National Academy of Sciences of the United States of America, 2017,114(28):7216-7221. |
| [5] |
ALMEIDA G, INFANTE I, MANNA L . Resurfacing halide perovskite nanocrystals. Science, 2019,364(6443):833-834.
DOI URL PMID |
| [6] | LI X, WU Y, ZHANG S , et al. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Advanced Functional Materials, 2016,26(15):2435-2445. |
| [7] |
DE ROO J, IBANEZ M, GEIREGAT P , et al. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano, 2016,10(2):2071-2081.
DOI URL PMID |
| [8] |
RAVI V K, SANTRA P K, JOSHI N , et al. Origin of the substitution mechanism for the binding of organic ligands on the surface of CsPbBr3 perovskite nanocubes. The Journal of Physical Chemistry Letters, 2017,8(20):4988-4994.
DOI URL PMID |
| [9] | IMRAN M, IJAZ P, GOLDONI L , et al. Simultaneous cationic and anionic ligand exchange for colloidally stable CsPbBr3 nanocrystals. ACS Energy Letters, 2019,4(4):819-824. |
| [10] |
NENON D P, PRESSLER K, KANG J , et al. Design principles for trap-free CsPbX3 nanocrystals: enumerating and eliminating surface halide vacancies with softer Lewis bases. Journal of the American Chemical Society, 2018,140(50):17760-17772.
DOI URL PMID |
| [11] |
ZHONG Q, CAO M, XU Y , et al. L-type ligand-assisted acid-free synthesis of CsPbBr3 nanocrystals with near-unity photoluminescence quantum yield and high stability. Nano Letters, 2019,19(6):4151-4157.
DOI URL PMID |
| [12] |
WEI Y, CHENG Z, LIN J . An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chemical Society Reviews, 2019,48(1):310-350.
DOI URL PMID |
| [13] |
ZHAO Y, ZHU K . Organic-inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chemical Society Reviews, 2016,45(3):655-689.
DOI URL PMID |
| [14] | ZHOU Y, ZHAO Y . Chemical stability and instability of inorganic halide perovskites. Energy & Environmental Science, 2019,12(5):1495-1511. |
| [15] | DUTTA A, PRADHAN N . Phase-stable red-emitting CsPbI3 nanocrystals: successes and challenges. ACS Energy Letters, 2019,4(3):709-719. |
| [16] | LI F, LIU Y, WANG H , et al. Postsynthetic surface trap removal of CsPbX3(X=Cl, Br, or I) quantum dots via a ZnX2/hexane solution toward an enhanced luminescence quantum yield. Chemistry of Materials, 2018,30(23):8546-8554. |
| [17] | SUN J K, HUANG S, LIU X Z , et al. Polar solvent induced lattice distortion of cubic CsPbI3 nanocubes and hierarchical self-assembly into orthorhombic single-crystalline nanowires. Journal of the American Chemical Society, 2018,140(37):11705-11715. |
| [18] |
HOU Y, ZHOU Z R, WEN T Y , et al. Enhanced moisture stability of metal halide perovskite solar cells based on sulfur-oleylamine surface modification. Nanoscale Horizons, 2019,4(1):208-213.
DOI URL PMID |
| [19] |
ZHOU W, SUI F, ZHONG G , et al. Lattice dynamics and thermal stability of cubic-phase CsPbI3 quantum dots. The Journal of Physical Chemistry Letters, 2018,9(17):4915-4920.
DOI URL PMID |
| [20] |
RUAN L, SHEN W, WANG A , et al. Stable and conductive lead halide perovskites facilitated by X-type ligands. Nanoscale, 2017,9(21):7252-7259.
DOI URL PMID |
| [21] | YANG D, LI X, ZENG H . Surface chemistry of all inorganic halide perovskite nanocrystals: passivation mechanism and stability . Advanced Materials Interfaces, 2018,5(8):1701662. |
| [22] |
KANG J, WANG L W . High defect tolerance in lead halide perovskite CsPbBr3. The Journal of Physical Chemistry Letters, 2017,8(2):489-493.
DOI URL PMID |
| [23] |
RAN C, XU J, GAO W , et al. Defects in metal triiodide perovskite materials towards high-performance solar cells: origin, impact, characterization, and engineering. Chemical Society Reviews, 2018,47(12):4581-4610.
DOI URL PMID |
| [24] |
LI X, CAO F, YU D , et al. All inorganic halide perovskites nanosystem: synthesis, structural features, optical properties and optoelectronic applications. Small, 2017,13(9):1603996.
DOI URL |
| [25] |
YANG D, LI X, WU Y , et al. Surface halogen compensation for robust performance enhancements of CsPbX3 perovskite quantum dots. Advanced Optical Materials, 2019,7(11):1900276.
DOI URL |
| [26] |
WU H, ZHANG Y, LU M , et al. Surface ligand modification of cesium lead bromide nanocrystals for improved light-emitting performance. Nanoscale, 2018,10(9):4173-4178.
DOI URL PMID |
| [27] | AHMED T, SETH S, SAMANTA A . Boosting the photoluminescence of CsPbX3 (X=Cl, Br, I) perovskite nanocrystals covering a wide wavelength range by postsynthetic treatment with tetrafluoroborate salts. Chemistry of Materials, 2018,30(11):3633-3637. |
| [28] |
KOSCHER B A, SWABECK J K, BRONSTEIN N D , et al. Essentially trap-free CsPbBr3 colloidal nanocrystals by postsynthetic thiocyanate surface treatment. Journal of the American Chemical Society, 2017,139(19):6566-6569.
DOI URL PMID |
| [29] | DING X, CHEN H, WU Y , et al. Triple cation additive NH 3+C 2H 4NH 2+C2H4NH 3+-induced phase-stable inorganic α-CsPbI3 perovskite films for use in solar cells. Journal of Materials Chemistry A , 2018,6(37):18258-18266. |
| [30] |
PAN J, QUAN L N, ZHAO Y , et al. Highly efficient perovskite quantum dot light-emitting diodes by surface engineering. Advanced Materials, 2016,28(39):8718-8725.
DOI URL PMID |
| [31] | ZHU J, ZHU Y, HUANG J , et al. Synthesis of CsPbBr3 perovskite nanocrystals with the sole ligand of protonated (3-aminopropyl) triethoxysilane. Journal of Materials Chemistry C, 2019,7(24):7201-7206. |
| [32] | WANG S, SHEN X, ZHANG Y , et al. Oxalic acid enabled emission enhancement and continuous extraction of chloride from cesium lead chloride/bromide perovskite nanocrystals. Small, 2019,15(34):1901828. |
| [33] | YASSITEPE E, YANG Z, VOZNYY O , et al. Amine-free synthesis of cesium lead halide perovskite quantum dots for efficient light- emitting diodes. Advanced Functional Materials, 2016,26(47):8757-8763. |
| [34] | PAN J, SHANG Y, YIN J , et al. Bidentate ligand-passivated CsPbI3 perovskite nanocrystals for stable near-unity photoluminescence quantum yield and efficient red light-emitting diodes. Journal of the American Chemical Society, 2018,140(2):562-565. |
| [35] |
TAN Y, ZOU Y, WU L , et al. Highly luminescent and stable perovskite nanocrystals with octylphosphonic acid as a ligand for efficient light-emitting diodes. ACS Applied Materials & Interfaces, 2018,10(4):3784-3792.
DOI URL PMID |
| [36] |
KRIEG F, OCHSENBEIN S T, YAKUNIN S , et al. Colloidal CsPbX3(X=Cl, Br, I) nanocrystals 2.0: zwitterionic capping ligands for improved durability and stability. ACS Energy Letters, 2018,3(3):641-646.
DOI URL PMID |
| [37] | YANG D, LI X, ZHOU W , et al. CsPbBr3 quantum dots 2.0: benzenesulfonic acid equivalent ligand awakens complete purification. Advanced Materials, 2019,31(30):1900767. |
| [38] |
WANG C, CHESMAN A S, JASIENIAK J J . Stabilizing the cubic perovskite phase of CsPbI3 nanocrystals by using an alkyl phosphinic acid. Chemical Communications, 2016,53(1):232-235.
DOI URL PMID |
| [39] |
ALMEIDA G, ASHTON O J, GOLDONI L , et al. The phosphine oxide route toward lead halide perovskite nanocrystals. Journal of the American Chemical Society, 2018,140(44):14878-14886.
DOI URL PMID |
| [40] |
XUAN T, YANG X, LOU S , et al. Highly stable CsPbBr3 quantum dots coated with alkyl phosphate for white light-emitting diodes. Nanoscale, 2017,9(40):15286-15290.
DOI URL PMID |
| [41] | YAN D, SHI T, ZANG Z , et al. Ultrastable CsPbBr3 perovskite quantum dot and their enhanced amplified spontaneous emission by surface ligand modification. Small, 2019,15(23):1901173. |
| [42] |
WU L Z, ZHONG Q X, YANG D , et al. Improving the stability and size tunability of cesium lead halide perovskite nanocrystals using trioctylphosphine oxide as the capping ligand. Langmuir, 2017,33(44):12689-12696.
DOI URL PMID |
| [43] |
HOU S, GUO Y, TANG Y , et al. Synthesis and stabilization of colloidal perovskite nanocrystals by multidentate polymer micelles. ACS Applied Materials & Interfaces, 2017,9(22):18417-18422.
DOI URL PMID |
| [44] |
HAI J, LI H, ZHAO Y , et al. Designing of blue, green, and red CsPbX3 perovskite-codoped flexible films with water resistant property and elimination of anion-exchange for tunable white light emission. Chemical Communications, 2017,53(39):5400-5403.
DOI URL PMID |
| [45] |
MEYNS M, PERALVAREZ M, HEUER-JUNGEMAN A , et al. Polymer-enhanced stability of inorganic perovskite nanocrystals and their application in color conversion LEDs. ACS Applied Materials & Interfaces, 2016,8(30):19579-19586.
DOI URL PMID |
| [46] |
RAJA S N, BEKENSTEIN Y, KOC M A , et al. Encapsulation of perovskite nanocrystals into macroscale polymer matrices: enhanced stability and polarization. ACS Applied Materials & Interfaces, 2016,8(51):35523-35533.
DOI URL PMID |
| [47] |
LOIUDICE A, SARIS S, OVEISI E , et al. CsPbBr3 QD/AlOx inorganic nanocomposites with exceptional stability in water, light, and heat. Angewandte Chemie-International Edition, 2017,56(36):10696-10701.
DOI URL PMID |
| [48] | LI Z J, HOFMAN E, LI J , et al. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Advanced Functional Materials, 2018,28(1):1704288. |
| [49] | HU Z, LIU Z, BIAN Y , et al. Enhanced two-photon-pumped emission from in situ synthesized nonblinking CsPbBr3/SiO2 nanocrystals with excellent stability. Advanced Optical Materials, 2018,6(3):1700997. |
| [50] |
LI Z, KONG L, HUANG S , et al. Highly luminescent and ultrastable CsPbBr3 perovskite quantum dots incorporated into a silica/ alumina monolith. Angewandte Chemie International Edition, 2017,56(28):8134-8138.
DOI URL PMID |
| [51] | LI L, ZHANG Z, CHEN Y , et al. Sustainable and self-enhanced electrochemiluminescent ternary suprastructures derived from CsPbBr3 perovskite quantum dots. Advanced Functional Materials, 2019,29(32):1902533. |
| [52] |
WU H, LIN S, WANG R , et al. Water-stable and ion exchange-free inorganic perovskite quantum dots encapsulated in solid paraffin and their application in light emitting diodes. Nanoscale, 2019,11(12):5557-5563.
DOI URL PMID |
| [53] |
WANG B, ZHANG C, HUANG S , et al. Postsynthesis phase transformation for CsPbBr3/Rb4PbBr6 core/shell nanocrystals with exceptional photostability. ACS Applied Materials & Interfaces, 2018,10(27):23303-23310.
DOI URL PMID |
| [54] | WEI Y, XIAO H, XIE Z , et al. Highly luminescent lead halide perovskite quantum dots in hierarchical CaF2 matrices with enhanced stability as phosphors for white light-emitting diodes. Advanced Optical Materials, 2018,6(11):1701343. |
| [55] | VELDHUIS S A, NG Y F, AHMAD R , et al. Crown ethers enable room-temperature synthesis of CsPbBr3 quantum dots for light- emitting diodes. ACS Energy Letters, 2018,3(3):526-531. |
| [56] |
CHEN Y, YU M, YE S , et al. All-inorganic CsPbBr3 perovskite quantum dots embedded in dual-mesoporous silica with moisture resistance for two-photon-pumped plasmonic nanolasers. Nanoscale, 2018,10(14):6704-6711.
DOI URL PMID |
| [57] | HAO J, QU X, QIU L , et al. One-step loading on natural mineral halloysite nanotube: an effective way to enhance the stability of perovskite CsPbX3(X=Cl, Br, I) quantum dots. Advanced Optical Materials, 2019,7(4):1801323. |
| [58] |
WU L, HU H, XU Y , et al. From nonluminescent Cs4PbX6(X=Cl, Br, I) nanocrystals to highly luminescent CsPbX3 nanocrystals: water-triggered transformation through a CsX-stripping mechanism. Nano Letters, 2017,17(9):5799-5804.
DOI URL PMID |
| [59] |
HU H, WU L, TAN Y , et al. Interfacial synthesis of highly stable CsPbX3/oxide Janus nanoparticles. Journal of the American Chemical Society, 2018,140(1):406-412.
DOI URL PMID |
| [60] |
ZHONG Q, CAO M, HU H , et al. One-pot synthesis of highly stable CsPbBr3@SiO2 core-shell nanoparticles. ACS Nano, 2018,12(8):8579-8587.
DOI URL PMID |
| [61] |
ZHANG X, LU M, ZHANG Y , et al. PbS capped CsPbI3 nanocrystals for efficient and stable light-emitting devices using p-i-n structures. ACS Central Science, 2018,4(10):1352-1359.
DOI URL PMID |
| [62] |
ZHANG D, ZHOU W, LIU Q , et al. CH3NH3PbBr3 perovskite nanocrystals encapsulated in lanthanide metal-organic frameworks as a photoluminescence converter for anti-counterfeiting. ACS Applied Materials & Interfaces, 2018,10(33):27875-27884.
DOI URL PMID |
| [63] |
ZHANG C, WANG B, LI W , et al. Conversion of invisible metal-organic frameworks to luminescent perovskite nanocrystals for confidential information encryption and decryption. Nature Communications, 2017,8(1):1138.
DOI URL PMID |
| [64] |
ZHANG D, XU Y, LIU Q , et al. Encapsulation of CH3NH3PbBr3 perovskite quantum dots in MOF-5 microcrystals as a stable platform for temperature and aqueous heavy metal ion detection. Inorganic Chemistry, 2018,57(8):4613-4619.
DOI URL PMID |
| [65] |
ZHANG Q, YIN Y . All-inorganic metal halide perovskite nanocrystals: opportunities and challenges . ACS Central Science, 2018,4(6):668-679.
DOI URL PMID |
| [66] | CHEN W, HAO J, HU W , et al. Enhanced stability and tunable photoluminescence in perovskite CsPbX3/ZnS quantum dot heterostructure. Small, 2017,13(21):1604085. |
| [67] |
TANG X, YANG J, LI S , et al. Single halide perovskite/semiconductor core/shell quantum dots with ultrastability and nonblinking properties. Advanced Science, 2019,6(18):1900412.
DOI URL PMID |
| [1] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [2] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [3] | 瞿牡静, 张淑兰, 朱梦梦, 丁浩杰, 段嘉欣, 代恒龙, 周国红, 李会利. CsPbBr3@MIL-53纳米复合荧光粉的合成、性能及其白光LEDs应用[J]. 无机材料学报, 2024, 39(9): 1035-1043. |
| [4] | 潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919. |
| [5] | 苗鑫, 闫世强, 韦金豆, 吴超, 樊文浩, 陈少平. Te基热电器件反常界面层生长行为及界面稳定性研究[J]. 无机材料学报, 2024, 39(8): 903-910. |
| [6] | 陈甜, 罗媛, 朱刘, 郭学益, 杨英. 有机-无机共添加增强柔性钙钛矿太阳能电池机械弯曲及环境稳定性能[J]. 无机材料学报, 2024, 39(5): 477-484. |
| [7] | 杨博, 吕功煊, 马建泰. 镍铁氢氧化物-磷化钴复合电极电催化分解水研究[J]. 无机材料学报, 2024, 39(4): 374-382. |
| [8] | 张宇晨, 陆知遥, 赫晓东, 宋广平, 朱春城, 郑永挺, 柏跃磊. 硫族MAX相硼化物的物相稳定性和性能预测[J]. 无机材料学报, 2024, 39(2): 225-232. |
| [9] | 王煜, 熊浩, 黄孝坤, 江琳沁, 吴波, 黎健生, 杨爱军. 低剂量异辛酸亚锡调控两步法制备Sn-Pb混合钙钛矿太阳能电池[J]. 无机材料学报, 2024, 39(12): 1339-1347. |
| [10] | 周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158. |
| [11] | 方万丽, 沈黎丽, 李海艳, 陈薪羽, 陈宗琦, 寿春晖, 赵斌, 杨松旺. NiOx介孔层的成膜过程对碳电极钙钛矿太阳能电池性能的影响[J]. 无机材料学报, 2023, 38(9): 1103-1109. |
| [12] | 陈雨, 林埔安, 蔡冰, 张文华. 钙钛矿太阳能电池无机空穴传输材料的研究进展[J]. 无机材料学报, 2023, 38(9): 991-1004. |
| [13] | 胡忠良, 傅赟天, 蒋蒙, 王连军, 江莞. Nb/Mg3SbBi界面层热稳定性研究[J]. 无机材料学报, 2023, 38(8): 931-937. |
| [14] | 刘建, 王凌坤, 许保亮, 赵倩, 王耀萱, 丁艺, 张胜泰, 段涛. 熔盐法低温合成掺钕ZrSiO4陶瓷的物相演变和化学稳定性[J]. 无机材料学报, 2023, 38(8): 910-916. |
| [15] | 肖娅妮, 吕嘉南, 李振明, 刘铭扬, 刘伟, 任志刚, 刘弘景, 杨东旺, 鄢永高. Bi2Te3基热电材料的湿热稳定性研究[J]. 无机材料学报, 2023, 38(7): 800-806. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||