无机材料学报 ›› 2014, Vol. 29 ›› Issue (8): 785-794.DOI: 10.15541/jim20130633 CSTR: 32189.14.10.15541/jim20130633
• • 下一篇
楚增勇,原 博,颜廷楠
收稿日期:2013-12-04
修回日期:2014-01-16
出版日期:2014-08-20
网络出版日期:2014-07-15
基金资助:CHU Zeng-Yong, YUAN Bo, YAN Ting-Nan
Received:2013-12-04
Revised:2014-01-16
Published:2014-08-20
Online:2014-07-15
Supported by:摘要:
利用光催化剂将太阳能转化为人类可以直接利用的能量, 并用其解决地球资源的枯竭和生存环境的恶化是可再生清洁能源研究的一个方向。g-C3N4的独特结构赋予其良好的光催化性能, 使之成为光催化领域的研究热点。目前在光催化领域, g-C3N4主要用于催化污染物分解、水解制氢制氧、有机合成及氧气还原。在实际应用中, 为进一步提高g-C3N4的光催化效果, 科研工作者开发了多种改进方法, 例如物理复合改性、化学掺杂改性、微观结构调整等。本文主要论述了g-C3N4在光催化领域的应用以及光催化性能的改进方法, 简要阐述了光催化和各种改进方法的机理, 分析了目前g-C3N4在光催化领域面临的问题和挑战, 展望了g-C3N4的应用前景。
中图分类号:
楚增勇,原 博,颜廷楠. g-C3N4光催化性能的研究进展[J]. 无机材料学报, 2014, 29(8): 785-794.
CHU Zeng-Yong, YUAN Bo, YAN Ting-Nan. Recent Progress in Photocatalysis of g-C3N4[J]. Journal of Inorganic Materials, 2014, 29(8): 785-794.
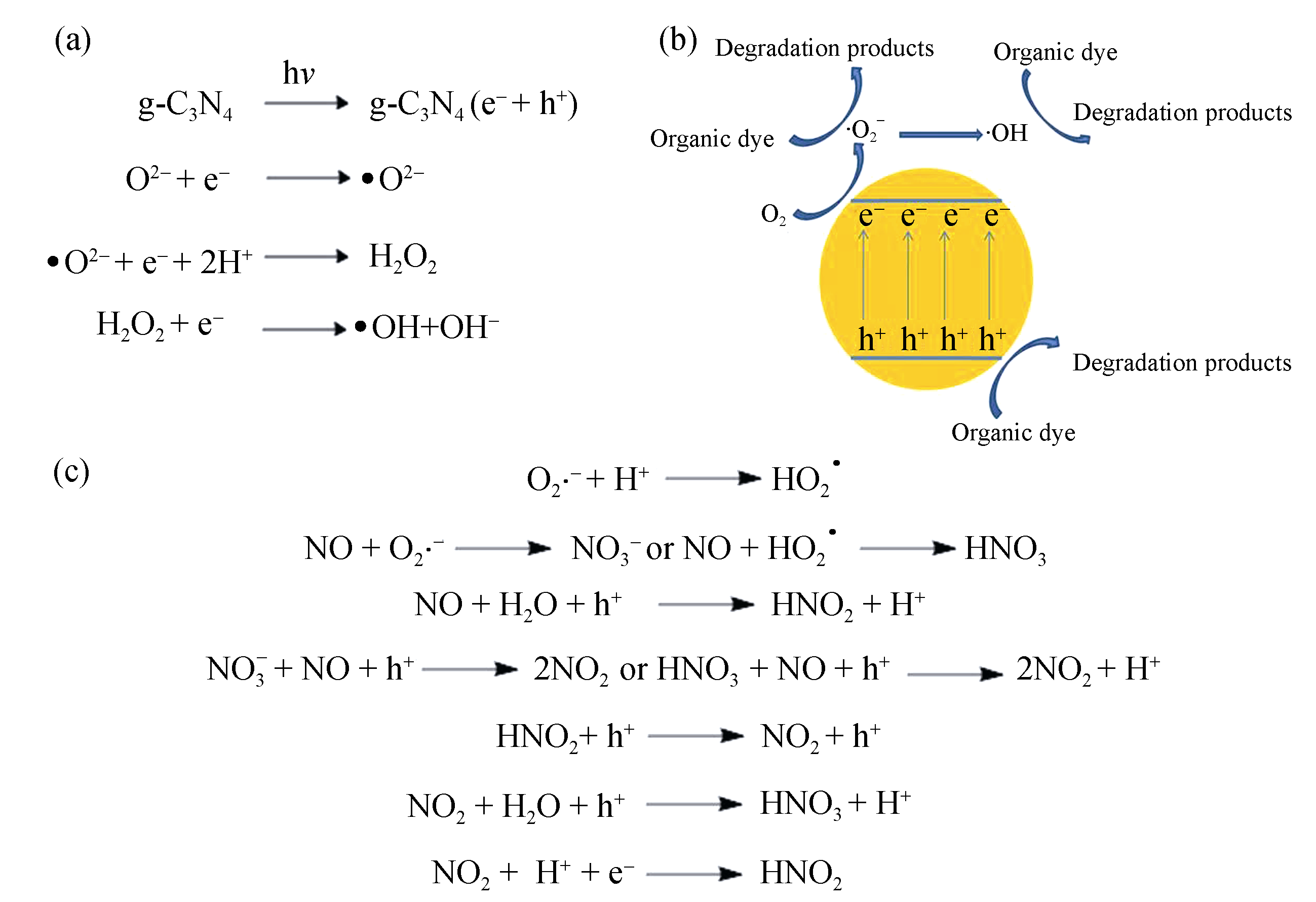
图2 活性粒子的产生(a)[21]与其催化有机染料降解(b)[27-28]和含NO气体净化的机理(c)[38]
Fig. 2 Generation of reactive species (a)[21] and action mechanism for degradation of organic dye (b)[27-28] and purification of gas containing NO (c)[38]
| Photocatalyt | Application | Photocatalytic performance <br/>of pure g-C3N4 [a]/ <br/>(min-1 or μmol•g•h-1) | photocatalytic performance <br/>of modified g-C3N4 [a]/ <br/>(min-1 or μmol•g•h-1) | Reference |
|---|---|---|---|---|
| Fe2O3/g-C3N4 | Degradation of MO | 0.0030 | 0.0163 | [27] |
| AgX/g-C3N4(X=Br, I) | Degradation of MO | 0.0006 | 0.1900 [b]<br/>0.0068 [c] | [70] |
| ZnO/g-C3N4 | Degradation of RhB | 0.0078 | 0.0239 | [28] |
| SmVO4/g-C3N4 | Degradation of RhB | 0.0143 | 0.0338 | [72] |
| GdVO4/g-C3N4 | Degradation of RhB | 0.0142 | 0.0434 | [80] |
| DyVO4/g-C3N4 | Degradation of RhB | 0.0142 | 0.0365 | [81] |
| Formate anion/g-C3N4 | Reduction of Cr(Ⅵ) | 0.0010 | 0.0033 | [37] |
| MoS2/g-C3N4 | Hydrogen generation by hydrolysis | 0.15 | 23.10 | [41] |
| CdS QDs/g-C3N4 | Hydrogen generation by hydrolysis | 38 | 4494 | [40] |
| Gr/g-C3N4 | Hydrogen generation by hydrolysis | 147 | 451 | [74] |
| P3HT/g-C3N4 | Hydrogen generation by hydrolysis | 1.8 | 555.0 | [76] |
| TiO2/g-C3N4 | Degradation of phenol | 0.022 | 0.053 | [30] |
| g-C3N4/rGO/MoS2 | Degradation of MB | 0.0054 | 0.0338 | [82] |
| Reduction of Cr(Ⅵ) | 0.0028 | 0.0157 | ||
| GO/g-C3N4 | Degradation of RhB | 0.0041 | 0.0156 | [32] |
| Degradation of 2,4-DCP | 0.0037 | 0.0077 |
表1 g-C3N4与物质复合后光催化性能的提高
Table 1 Improvement of photocatalytic performance of g-C3N4 physically coupled with other materials
| Photocatalyt | Application | Photocatalytic performance <br/>of pure g-C3N4 [a]/ <br/>(min-1 or μmol•g•h-1) | photocatalytic performance <br/>of modified g-C3N4 [a]/ <br/>(min-1 or μmol•g•h-1) | Reference |
|---|---|---|---|---|
| Fe2O3/g-C3N4 | Degradation of MO | 0.0030 | 0.0163 | [27] |
| AgX/g-C3N4(X=Br, I) | Degradation of MO | 0.0006 | 0.1900 [b]<br/>0.0068 [c] | [70] |
| ZnO/g-C3N4 | Degradation of RhB | 0.0078 | 0.0239 | [28] |
| SmVO4/g-C3N4 | Degradation of RhB | 0.0143 | 0.0338 | [72] |
| GdVO4/g-C3N4 | Degradation of RhB | 0.0142 | 0.0434 | [80] |
| DyVO4/g-C3N4 | Degradation of RhB | 0.0142 | 0.0365 | [81] |
| Formate anion/g-C3N4 | Reduction of Cr(Ⅵ) | 0.0010 | 0.0033 | [37] |
| MoS2/g-C3N4 | Hydrogen generation by hydrolysis | 0.15 | 23.10 | [41] |
| CdS QDs/g-C3N4 | Hydrogen generation by hydrolysis | 38 | 4494 | [40] |
| Gr/g-C3N4 | Hydrogen generation by hydrolysis | 147 | 451 | [74] |
| P3HT/g-C3N4 | Hydrogen generation by hydrolysis | 1.8 | 555.0 | [76] |
| TiO2/g-C3N4 | Degradation of phenol | 0.022 | 0.053 | [30] |
| g-C3N4/rGO/MoS2 | Degradation of MB | 0.0054 | 0.0338 | [82] |
| Reduction of Cr(Ⅵ) | 0.0028 | 0.0157 | ||
| GO/g-C3N4 | Degradation of RhB | 0.0041 | 0.0156 | [32] |
| Degradation of 2,4-DCP | 0.0037 | 0.0077 |
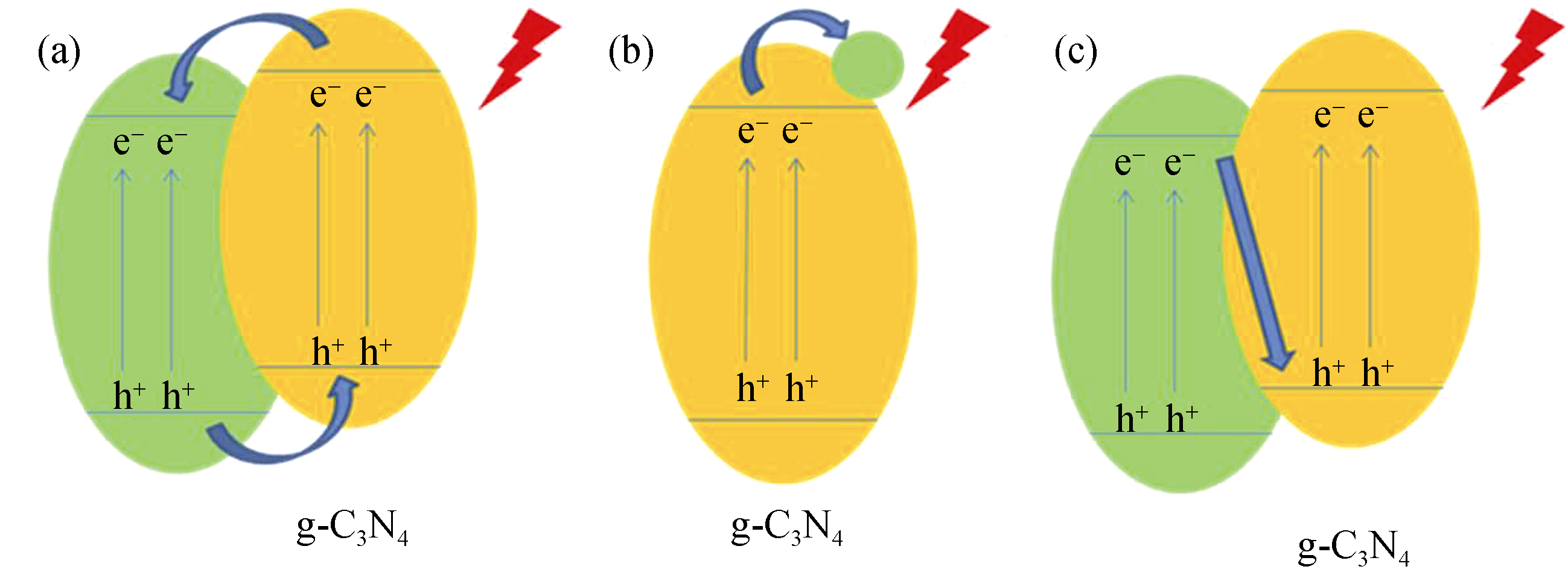
图3 g-C3N4与物质复合后电子和空穴的分离
Fig. 3 Separation of electrons and holes of g-C3N4 physically coupled with other materials (a) Convection-type charge transfer[30, 40] (such as TiO2, CdS); (b) Advection-type charge transfer[27, 74] (such as Fe3O4, graphene); (c) Z-type charge transfer [35, 71]
图4 引入不同的杂环对g-C3N4水解制氢速率的影响[83]
Fig. 4 Influence of different heterocycles introduced into g-C3N4 on the rate of hydrogen production[83] Every figure (μmol/h) below heterocycles means the rate of hydrogen production and the rate of hydrogen production of unmodified g-C3N4 is 18 μmol/h
| Introduced<br/>component | Application | Photocatalytic performance of unmodified g-C3N4[a]<br/>/(μmol•h-1, min-1 or %) | Photocatalytic performance<br/>of modified g-C3N4[a]/<br/>(μmol•h-1, min-1 or %) | Reference |
|---|---|---|---|---|
| PMDA<br/> | Hydrogen generation by hydrolysis | 7.0 | 20.6 | [44] |
| Oxygen generation by hydrolysis | 0.8 | 7.7 | ||
| Degradation of MO | 0.0050 | 0.0557 | ||
| ABN<br/> | Hydrogen generation by hydrolysis | 18[b] | 147[b] | [83] |
| 127[c] | 229[c] | |||
| BA<br/> | Hydrogen generation by hydrolysis | 148.2[d] | 253.1[d] | [84] |
| 6.5[e] | 29.4[e] | |||
| B, F | Oxidation of cyclohexane | 1.6[f] | 5.3[f] | [91] |
| F | Hydrogen generation by hydrolysis | 4.9 | 13.0 | [87] |
| Oxidation of benzene | 0.0001 | 0.0021 | ||
| S | Hydrogen generation by hydrolysis | 20[d] | 160[d] | [29] |
| 10[e] | 75[e] | |||
| B | Degradation of RhB | 0.055 | 0.199 | [86] |
| C | Degradation of RhB | 0.0081 | 0.0362 | [90] |
| Reduction of Cr(Ⅵ) | 0.0010 | 0.0017 | ||
| Hydrogen generation by hydrolysis | 17.8 | 25.3 |
表2 化学掺杂对g-C3N4光催化性能的影响
Table 2 Influence of chemical binding modification on photocatalytic performance of g-C3N4
| Introduced<br/>component | Application | Photocatalytic performance of unmodified g-C3N4[a]<br/>/(μmol•h-1, min-1 or %) | Photocatalytic performance<br/>of modified g-C3N4[a]/<br/>(μmol•h-1, min-1 or %) | Reference |
|---|---|---|---|---|
| PMDA<br/> | Hydrogen generation by hydrolysis | 7.0 | 20.6 | [44] |
| Oxygen generation by hydrolysis | 0.8 | 7.7 | ||
| Degradation of MO | 0.0050 | 0.0557 | ||
| ABN<br/> | Hydrogen generation by hydrolysis | 18[b] | 147[b] | [83] |
| 127[c] | 229[c] | |||
| BA<br/> | Hydrogen generation by hydrolysis | 148.2[d] | 253.1[d] | [84] |
| 6.5[e] | 29.4[e] | |||
| B, F | Oxidation of cyclohexane | 1.6[f] | 5.3[f] | [91] |
| F | Hydrogen generation by hydrolysis | 4.9 | 13.0 | [87] |
| Oxidation of benzene | 0.0001 | 0.0021 | ||
| S | Hydrogen generation by hydrolysis | 20[d] | 160[d] | [29] |
| 10[e] | 75[e] | |||
| B | Degradation of RhB | 0.055 | 0.199 | [86] |
| C | Degradation of RhB | 0.0081 | 0.0362 | [90] |
| Reduction of Cr(Ⅵ) | 0.0010 | 0.0017 | ||
| Hydrogen generation by hydrolysis | 17.8 | 25.3 |
| Microstructure | Application | Photocatalytic performance of bulk g-C3N4[a] <br/>/(μmol•h-1, min-1 or %) | Photocatalytic performance<br/>of modified g-C3N4[a]/<br/>(μmol•h-1, min-1 or %) | Reference |
|---|---|---|---|---|
| Porous structure | Degradation of RhB | 0.014 | 0.131 | [94] |
| Porous structure | Oxidation of toluene | 24[b] | >99[b] | [47] |
| Porous structure | Friedel-Crafts reaction of benzene | 0[c] | 90[c] | [53] |
| Nanosheet | Degradation of RhB | 0.0012 | 0.0163 | [20] |
| Nanorod | Hydrogen generation by hydrolysis | 28 | 84 | [23] |
| Hydrogen generation by hydrolysis | 3.9 | 7 | ||
| Nanorod | Degradation of MB | 0.0017[d] | 0.0025[d] | [97] |
| 0.0021[e] | 0.0029[e] | |||
| Nanosheet | Hydrogen generation by hydrolysis | 31.0[f] | 169.7[f] | [51] |
| 10.7[g] | 31.8[g] | |||
| Nanosheet | Hydrogen generation by hydrolysis | 10.4 | 93.1 | [39] |
表3 微观结构对g-C3N4光催化性能的影响
Table 3 Influence of microstructure on the photocatalytic performance of g-C3N4
| Microstructure | Application | Photocatalytic performance of bulk g-C3N4[a] <br/>/(μmol•h-1, min-1 or %) | Photocatalytic performance<br/>of modified g-C3N4[a]/<br/>(μmol•h-1, min-1 or %) | Reference |
|---|---|---|---|---|
| Porous structure | Degradation of RhB | 0.014 | 0.131 | [94] |
| Porous structure | Oxidation of toluene | 24[b] | >99[b] | [47] |
| Porous structure | Friedel-Crafts reaction of benzene | 0[c] | 90[c] | [53] |
| Nanosheet | Degradation of RhB | 0.0012 | 0.0163 | [20] |
| Nanorod | Hydrogen generation by hydrolysis | 28 | 84 | [23] |
| Hydrogen generation by hydrolysis | 3.9 | 7 | ||
| Nanorod | Degradation of MB | 0.0017[d] | 0.0025[d] | [97] |
| 0.0021[e] | 0.0029[e] | |||
| Nanosheet | Hydrogen generation by hydrolysis | 31.0[f] | 169.7[f] | [51] |
| 10.7[g] | 31.8[g] | |||
| Nanosheet | Hydrogen generation by hydrolysis | 10.4 | 93.1 | [39] |
| [1] | TADA H, JIN Q, NISHIJIMA H, et al. Titanium(IV) dioxide surface-modified with iron oxide as a visible light photocatalyst. Angewandte Chemie International Edition, 2011, 50(15): 3501-3505. |
| [2] | XU T, ZHANG L, CHENG H, et al. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Applied Catalysis B: Environmental, 2011, 101(3/4): 382-387. |
| [3] | HE K, LI M, GUO L. Preparation and photocatalytic activity of PANI-CdS composites for hydrogen evolution. International Journal of Hydrogen Energy, 2012, 37(1): 755-759. |
| [4] | MIAO Y, PAN G, HUO Y, et al. Aerosol-spraying preparation of Bi2MoO6: a visible photocatalyst in hollow microspheres with a porous outer shell and enhanced activity. Dyes and Pigments, 2013, 99(2): 382-389. |
| [5] | WANG Y, SHI Z, FAN C, et al. Synthesis, characterization, and photocatalytic properties of BiOBr catalyst . Journal of Solid State Chemistry, 2013, 199: 224-229. |
| [6] | BAI S, SHEN X, LV H, et al. Assembly of Ag3PO4 nanocrystals on graphene-based nanosheets with enhanced photocatalytic performance. Journal of Colloid and Interface Science, 2013, 405: 1-9. |
| [7] | WANG X, BLECHERT S, ANTONIETTI M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catalysis, 2012, 2(8): 1596-1606. |
| [8] | WANG F, NG W K H, YU J C, et al. Red phosphorus: an elemental photocatalyst for hydrogen formation from water. Applied Catalysis B: Environmental, 2012, 111-112: 409-414. |
| [9] | LI C, CAO C B, ZHU H S. Graphitic carbon nitride thin films deposited by electrodeposition. Materials Letters, 2004, 58(12/13): 1903-1906. |
| [10] | LOTSCH B V, SCHNICK W. From triazines to heptazines: novel nonmetal tricyanomelaminates as precursors for graphitic carbon nitride materials. Chemistry of Materials, 2006, 18(7): 1891-1900. |
| [11] | WIRNHIER E, D BLINGER M, GUNZELMANN D, et al. Poly(triazine imide) with intercalation of lithium and chloride ions [(C3N3)2(NHxLi1-x)3•LiCl]: a crystalline 2D carbon nitride network. Chemistry - A European Journal, 2011, 17(11): 3213-3221. |
| [12] | ZHANG X, XIE X, WANG H, et al. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. Journal of the American Chemical Society, 2013, 135(1): 18-21. |
| [13] | BOJDYS M J,M LLER J O, ANTONIETTI M, et al. Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chemistry - A European Journal, 2008, 14(27): 8177-8182. |
| [14] | WU F, LIU Y, YU G, et al. Visible-light-absorption in graphitic C3N4 bilayer: enhanced by interlayer coupling. The Journal of Physical Chemistry Letters, 2012, 3(22): 3330-3334. |
| [15] | HUANG Z, LI F, CHEN B, et al. Well-dispersed g-C3N4 nanophases in mesoporous silica channels and their catalytic activity for carbon dioxide activation and conversion. Applied Catalysis B: Environmental, 2013, 136-137: 269-277. |
| [16] | KROKE E, SCHWARZ M, HORATH-BORDON E, et al. Tri-s-triazine derivatives. Part I. From trichloro-tri-s-triazine to graphitic C3N4 structures. New Journal of Chemistry, 2002, 26(5): 508-512. |
| [17] | MA H A, JIA X P, CHEN L X, et al. High-pressure pyrolysis study of C3NH6: a route to preparing bulk C3N4. Journal of Physics: Condensed Matter, 2002, 14(44): 11269-11273. |
| [18] | GUO Q, XIE Y, WANG X, et al. Characterization of well-crystal-lized graphitic carbon nitride nanocrystallites via a benzene-thermal route at low temperatures. Chemical Physics Letters, 2003, 380(1/2): 84-87. |
| [19] | LI Y, ZHANG J, WANG Q, et al. Nitrogen-rich carbon nitride hollow vessels: synthesis, characterization, and their properties. The Journal of Physical Chemistry B, 2010, 114(29): 9429-9434. |
| [20] | DONG F, WANG Z, SUN Y, et al. Engineering the nanoarchitecture and texture of polymeric carbon nitride semiconductor for enhanced visible light photocatalytic activity. Journal of Colloid and Interface Science, 2013, 401: 70-79. |
| [21] | XIN G, MENG Y. Pyrolysis synthesized g-C3N4 for photocatalytic degradation of methylene blue. Journal of Chemistry, 2013, 2013: 1-5. |
| [22] | JI H, CHANG F, HU X, et al. Photocatalytic degradation of 2,4,6-trichlorophenol over g-C3N4 under visible light irradiation. Chemical Engineering Journal, 2013, 218: 183-190. |
| [23] | LI X H, ZHANG J, CHEN X, et al. Condensed graphitic carbon nitride nanorods by nanoconfinement: promotion of crystallinity on photocatalytic conversion. Chemistry of Materials, 2011, 23(19): 4344-4348. |
| [24] | JORGE A B, MARTIN D J,DHANOA M T S, et al. H2 and O2 evolution from water half-splitting reactions by graphitic carbon nitride materials. The Journal of Physical Chemistry C, 2013, 117(14): 7178-7185. |
| [25] | DING G, WANG W, JIANG T, et al. Highly selective synthesis of phenol from benzene over a vanadium-doped graphitic carbon nitride catalyst. ChemCatChem, 2013, 5(1): 192-200. |
| [26] | ZHAI H S, CAO L, XIA X H. Synthesis of graphitic carbon nitride through pyrolysis of melamine and its electrocatalysis for oxygen reduction reaction. Chinese Chemical Letters, 2013, 24(2): 103-106. |
| [27] | ZHOU X, JIN B, CHEN R, et al. Synthesis of porous Fe3O4/g- C3N4 nanospheres as highly efficient and recyclable photocatalysts. Materials Research Bulletin, 2013, 48(4): 1447-1452. |
| [28] | LIU W, WANG M, XU C, et al. Significantly enhanced visible-light photocatalytic activity of g-C3N4 via ZnO modification and the mechanism study. Journal of Molecular Catalysis A: Chemical, 2013, 368-369: 9-15. |
| [29] | LIU G, NIU P, SUN C, et al. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. Journal of the American Chemical Society, 2010, 132(33): 11642-11648. |
| [30] | ZHAO S, CHEN S, YU H, et al. g-C3N4/TiO2 hybrid photocatalyst with wide absorption wavelength range and effective photogenerated charge separation. Separation and Purification Technology, 2012, 99: 50-54. |
| [31] | MIRANDA C, MANSILLA H, Yan E Z J, et al. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. Journal of Photochemistry and Photobiology A: Chemistry, 2013, 253: 16-21. |
| [32] | LIAO G, CHEN S, QUAN X, et al. Graphene oxide modified g-C3N4 hybrid with enhanced photocatalytic capability under visible light irradiation. Journal of Materials Chemistry, 2012, 22(6): 2721-2726. |
| [33] | SUN C, CHEN C, MA W, et al. Photocatalytic debromination of decabromodiphenyl ether by graphitic carbon nitride . Science ChinaChemistry, 2012, 55(12): 2532-2536. |
| [34] | KATSUMATA K I, MOTOYOSHI R, MATSUSHITA N, et al. Preparation of graphitic carbon nitride (g-C3N4)/WO3 composites and enhanced visible-light-driven photodegradation of acetaldehyde gas. Journal of Hazardous Materials, 2013, 260: 475-482. |
| [35] | KONDO K, MURAKAMI N, YE C, et al. Development of highly efficient sulfur-doped TiO2 photocatalysts hybridized with graphitic carbon nitride. Applied Catalysis B: Environmental, 2013, 142-143: 362-367. |
| [36] | LIU W, WANG M, XU C, et al. Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chemical Engineering Journal, 2012, 209: 386-393. |
| [37] | DONG G, ZHANG L. Synthesis and enhanced Cr(VI) photoreduction property of formate anion containing graphitic carbon nitride. The Journal of Physical Chemistry C, 2013, 117(8): 4062-4068. |
| [38] | SANO T, TSUTSUI S, KOIKE K, et al. Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. Journal of Materials Chemistry A, 2013, 1(21): 6489-6496. |
| [39] | YANG S, GONG Y, ZHANG J, et al. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Advanced Materials, 2013, 25(17): 2452-2456. |
| [40] | SHAO-WEN C, YU-PENG Y, JUN F, et al. In-situ growth of CdS quantum dots on g-C3N4 nanosheets for highly efficient photocatalytic hydrogen generation under visible light irradiation .International Journal of Hydrogen Energy, 2013, 38(3): 1258-1266. |
| [41] | GE L, HAN C, XIAO X, et al. Synthesis and characterization of composite visible light active photocatalysts MoS2-g-C3N4 with enhanced hydrogen evolution activity. International Journal of Hydrogen Energy, 2013, 38(17): 6960-6969. |
| [42] | WANG X, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater., 2009, 8(1): 76-80. |
| [43] | HONG J, WANG Y, WANG Y, et al. Noble-metal-free NiS/C3N4 for efficient photocatalytic hydrogen evolution from water. ChemSusChem, 2013: 1-7. |
| [44] | CHU S, WANG Y, GUO Y, et al. Band structure engineering of carbon nitride: in search of a polymer photocatalyst with high photooxidation property. ACS Catalysis, 2013, 3(5): 912-919. |
| [45] | SU F, MATHEW S C, LIPNER G, et al. mpg-C3N4-catalyzed selective oxidation of alcohols using O2 and visible light. Journal of the American Chemical Society, 2010, 132(46): 16299-16301. |
| [46] | LI X H, CHEN J S, WANG X, et al. Metal-free activation of dioxygen by graphene/g-C3N4 nanocomposites: functional dyads for selective oxidation of saturated hydrocarbons. Journal of the American Chemical Society, 2011, 133(21): 8074-8077. |
| [47] | LI X H, WANG X, ANTONIETTI M. Solvent-free and metal-free oxidation of toluene using O2 and g-C3N4 with nanopores: nanostructure boosts the catalytic selectivity. ACS Catalysis, 2012, 2(10): 2082-2086. |
| [48] | ZHANG P, WANG Y, YAO J, et al. Visible-light-induced metal-free allylic oxidation utilizing a coupled photocatalytic system of g-C3N4 and N-Hydroxy compounds. Advanced Synthesis & Catalysis, 2011, 353(9): 1447-1451. |
| [49] | YUAN Y P, CAO S W, LIAO Y S, et al. Red phosphor/g-C3N4 heterojunction with enhanced photocatalytic activities for solar fuels production. Applied Catalysis B: Environmental, 2013, 140-141: 164-168. |
| [50] | TAN B, XU J, XUE B, et al. Mesoporous graphitic carbon nitride: synthesis and application towards knoevenagel condensation reactions. Chemistry, 2013, 76(2): 150-156. |
| [51] | NIU P, ZHANG L, LIU G, et al. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Advanced Functional Materials, 2012, 22(22): 4763-4770. |
| [52] | JIN X, BALASUBRAMANIAN V V, SELVAN S T, et al. Highly ordered mesoporous carbon nitride nanoparticles with high nitrogen content: a metal-free basic catalyst. Angewandte Chemie, 2009, 121(42): 8024-8027. |
| [53] | GOETTMANN F, FISCHER A, ANTONIETTI M, et al. Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for friedel-crafts reaction of benzene. Angewandte Chemie International Edition, 2006, 45(27): 4467-4471. |
| [54] | SONG L, ZHANG S, WU X, et al. Graphitic C3N4 photocatalyst for esterification of benzaldehyde and alcohol under visible light radiation. Industrial & Engineering Chemistry Research, 2012, 51(28): 9510-9514. |
| [55] | SU F, MATHEW S C, M HLMANN L, et al. Aerobic oxidative coupling of amines by carbon nitride photocatalysis with visible light. Angewandte Chemie International Edition, 2011, 50(3): 657-660. |
| [56] | IKEDA T, BOERO M, HUANG S F, et al. Carbon alloy catalysts: active sites for oxygen reduction reaction. The Journal of Physical Chemistry C, 2008, 112(38): 14706-14709. |
| [57] | ZHENG Y, LIU J, LIANG J, et al. Graphitic carbon nitride materials: controllable synthesis and applications in fuel cells and photocatalysis. Energy & Environmental Science, 2012, 5(5): 6717-6731. |
| [58] | ASPERA S M, KASAI H, KAWAI H. Density functional theory-based analysis on O2 molecular interaction with the tri-s- triazine-based graphitic carbon nitride. Surface Science, 2012, 606(11-12): 892-901. |
| [59] | ZHENG Y, JIAO Y, JARONIEC M, et al. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small, 2012, 8(23): 3550-3566. |
| [60] | JIN J, FU X, LIU Q, et al. A highly active and stable electrocatalyst for the oxygen reduction reaction based on a graphene-supported g-C3N4@cobalt oxide core-shell hybrid in alkaline solution. Journal of Materials Chemistry A, 2013, 1(35): 10538-10545. |
| [61] | LIU Q, ZHANG J. Graphene supported Co-g-C3N4 as a novel metal-macrocyclic electrocatalyst for the oxygen reduction reaction in fuel cells. Langmuir, 2013, 29(11): 3821-3828. |
| [62] | ZHENG Y, JIAO Y, CHEN J, et al. Nanoporous graphitic- C3 N4@carbon metal-free electrocatalysts for highly efficient oxygen reduction. Journal of the American Chemical Society, 2011, 133(50): 20116-20119. |
| [63] | LI S, WANG J T, CHEN X Y, et al. Catalytic performance of heat-treated Fe-melamine/C and Fe-g-C3N4/C electrocatalysts for oxygen reduction reaction. Acta Phys. -Chim. Sin., 2013, 29(4): 792-798. |
| [64] | BU Y, CHEN Z, YU J, et al. A novel application of g-C3N4 thin film in photoelectrochemical anticorrosion. Electrochimica Acta, 2013, 88: 294-300. |
| [65] | LI X H, KURASCH S, KAISER U, et al. Synthesis of monolayer- patched graphene from glucose. Angewandte Chemie International Edition, 2012, 51(38): 9689-9692. |
| [66] | ZHANG Y, ANTONIETTI M. Phosphorus-doped carbon nitride solid enhanced electrical conductivity and photocurrent generation . Chemistry - An Asian Journal, 2010, 132(18): 6294-6295. |
| [67] | XU L, XIA J, XU H, et al. Reactable ionic liquid assisted solvothermal synthesis of graphite-like C3N4 hybridized α-Fe2O3 hollow microspheres with enhanced supercapacitive performance. Journal of Power Sources, 2014, 245: 866-874. |
| [68] | TIAN J, LIU Q, ASIRI A M, et al. Ultrathin graphitic carbon nitride nanosheet: a highly efficient fluorosensor for rapid, ultrasensitive detection of Cu2+. Analytical Chemistry, 2013, 85(11): 5595-5599. |
| [69] | CHENG C, HUANG Y, TIAN X, et al. Electrogenerated chemiluminescence behavior of graphite-like carbon nitride and its application in selective sensing Cu2+. Analytical Chemistry, 2012, 84(11): 4754-4759. |
| [70] | XU H, YAN J, XU Y, et al. Novel visible-light-driven AgX/graphite-like C3N4 (X=Br, I) hybrid materials with synergistic photocatalytic activity. Applied Catalysis B: Environmental, 2013, 129: 182-193. |
| [71] | YU J, WANG S, LOW J, et al. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Physical Chemistry Chemical Physics, 2013, 15(39): 16883-16890. |
| [72] | LI T, ZHAO L, HE Y, et al. Synthesis of g-C3N4/SmVO4 composite photocatalyst with improved visible light photocatalytic activities in RhB degradation. Applied Catalysis B: Environmental, 2013, 129: 255-263. |
| [73] | GUI M S, WANG P F, YUAN D, et al. Synthesis and visible-light photocatalytic activity of Bi2WO6/g-C3N4 composite photocatalysts. Chinese Journal of Inorganic Chemistry, 2013, 29(10): 2057-2064. |
| [74] | XIANG Q, YU J, JARONIEC M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene /C3N4 composites. The Journal of Physical Chemistry C, 2011, 115(15): 7355-7363. |
| [75] | SURYAWANSHI A, DHANASEKARAN P, MHAMANE D, et al. Doubling of photocatalytic H2 evolution from g-C3N4 via its nanocomposite formation with multiwall carbon nanotubes: electronic and morphological effects. International Journal of Hydrogen Energy, 2012, 37(12): 9584-9589. |
| [76] | YAN H, HUANG Y. Polymer composites of carbon nitride and poly(3-hexylthiophene) to achieve enhanced hydrogen production from water under visible light. Chemical Communications, 2011, 47(14): 4168-4170. |
| [77] | GE L, HAN C, LIU J. In situ synthesis and enhanced visible light photocatalytic activities of novel PANI-g-C3N4 composite photocatalysts. Journal of Materials Chemistry, 2012, 22(23): 11843-11850. |
| [78] | MIN S, LU G. Enhanced electron transfer from the excited eosin Y to mpg-C3N4 for highly efficient hydrogen evolution under 550 nm irradiation. The Journal of Physical Chemistry C, 2012, 116(37): 19644-19652. |
| [79] | SUN A W, CHEN H, SONG C Y, et al. Synthesis of Bi25FeO40- g-C3N4 magnetic catalyst and its photocatalytic performance.Environmental Chemistry, 2013, 32(5): 748-754. |
| [80] | HE Y, CAI J, LI T, et al. Efficient degradation of RhB over GdVO4/g-C3N4 composites under visible-light irradiation. Chemical Engineering Journal, 2013, 215-216: 721-730. |
| [81] | HE Y, CAI J, LI T, et al. Synthesis, characterization, and activity evaluation of DyVO4/g-C3N4 composites under visible-light irradiation. Industrial & Engineering Chemistry Research, 2012, 51(45): 14729-14737. |
| [82] | HOU Y, WEN Z, CUI S, et al. Constructing 2D porous graphitic C3N4 nanosheets/nitrogen-doped graphene/layered MoS2 ternary nanojunction with enhanced photoelectrochemical activity. Advanced Materials, 2013, 25(43): 1-7. |
| [83] | ZHANG J, ZHANG G, CHEN X, et al. Co-monomer control of carbon nitride semiconductors to optimize hydrogen evolution with visible light. Angewandte Chemie International Edition, 2012, 51(13): 3183-3187. |
| [84] | ZHANG J, CHEN X, TAKANABE K, et al. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angewandte Chemie International Edition, 2010, 49(2): 441-444. |
| [85] | ZHENG H R, ZHANG J S, WANG X C, et al. Modification of carbon nitride phtocatalysts by copolymerization with diaminomaleonitrile. Acta Phys. Chim. Sin., 2012, 28(10): 2336-2342. |
| [86] | YAN S C, LI Z S, ZOU Z G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir, 2010, 26(6): 3894-3901. |
| [87] | WANG Y, DI Y, ANTONIETTI M, et al. Excellent visible-light photocatalysis of fluorinated polymeric carbon nitride solids. Chemistry of Materials, 2010, 22(18): 5119-5121. |
| [88] | GE L, HAN C, XIAO X, et al. Enhanced visible light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4 photocatalysts. Materials Research Bulletin, 2013, 48(10): 3919-3925. |
| [89] | CHEN G, GAO S P. Structure and electronic structure of S-doped graphitic C3N4 investigated by density functional theory. Chinese Physics B, 2012, 21(10): 384-390. |
| [90] | DONG G, ZHAO K, ZHANG L. Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4. Chemical Communications, 2012, 48(49): 6178-6180. |
| [91] | WANG Y, ZHANG J, WANG X, et al. Boron- and fluorine- containing mesoporous carbon nitride polymers: metal-free catalysts for cyclohexane oxidation. Angewandte Chemie International Edition, 2010, 49(19): 3356-3359. |
| [92] | VINU A, ARIGA K, MORI T, et al. Preparation and characterization of well-ordered hexagonal mesoporous carbon nitride. Advanced Materials, 2005, 17(13): 1648-1652. |
| [93] | CHEN X, JUN Y S, TAKANABE K, et al. Ordered mesoporous SBA-15 type graphitic carbon nitride: a semiconductor host structure for photocatalytic hydrogen evolution with visible light. Chemistry of Materials, 2009, 21(18): 4093-4095. |
| [94] | DONG G, ZHANG L. Porous structure dependent photoreactivity of graphitic carbon nitride under visible light. Journal of Materials Chemistry, 2012, 22(3): 1160-1166. |
| [95] | XU J, WANG Y, ZHU Y. Nanoporous graphitic carbon nitride with enhanced photocatalytic performance. Langmuir, 2013, 29(33): 10566-10572. |
| [96] | GROENEWOLT M, ANTONIETTI M. Synthesis of g-C3N4 nanoparticles in mesoporous silica host matrices. Advanced Materials, 2005, 17(14): 1789-1792. |
| [97] | BAI X, WANG L, ZONG R, et al. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. The Journal of Physical Chemistry C, 2013, 117(19): 9952-9961. |
| [98] | CHANG F, XIE Y, LI C, et al. A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Applied Surface Science, 2013, 280: 967-974. |
| [99] | CHENG N, TIAN J, LIU Q, et al. Au-nanoparticle-loaded graphitic carbon nitride nanosheets: green photocatalytic synthesis and application toward the degradation of organic pollutants. ACS Applied Materials & Interfaces, 2013, 5(15): 6815-6819. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [8] | 范小暄, 郑永炅, 徐丽荣, 姚子敏, 曹硕, 王可心, 王绩伟. 基于富氧空位LiYScGeO4: Bi3+长余辉光催化剂的自激活余辉驱动有机污染物芬顿降解[J]. 无机材料学报, 2025, 40(5): 481-488. |
| [9] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [10] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [11] | 贾相华, 张辉霞, 刘艳凤, 左桂鸿. 湿化学法制备Cu2O/Cu空心球异质结光催化剂[J]. 无机材料学报, 2025, 40(4): 397-404. |
| [12] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [13] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [14] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [15] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||