
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (4): 390-398.DOI: 10.15541/jim20230473
Special Issue: 【能源环境】污染物催化去除(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
WU Guangyu1,2( ), SHU Song1,2, ZHANG Hongwei1,2, LI Jianjun1,3(
), SHU Song1,2, ZHANG Hongwei1,2, LI Jianjun1,3( )
)
Received:2023-10-13
Revised:2023-12-04
Published:2024-04-20
Online:2023-12-25
Contact:
LI Jianjun, professor. E-mail: jjli@scu.edu.cnAbout author:WU Guangyu (1999-), male, Master candidate. E-mail: 1464075183@qq.com
Supported by:CLC Number:
WU Guangyu, SHU Song, ZHANG Hongwei, LI Jianjun. Enhanced Styrene Adsorption by Grafted Lactone-based Activated Carbon[J]. Journal of Inorganic Materials, 2024, 39(4): 390-398.
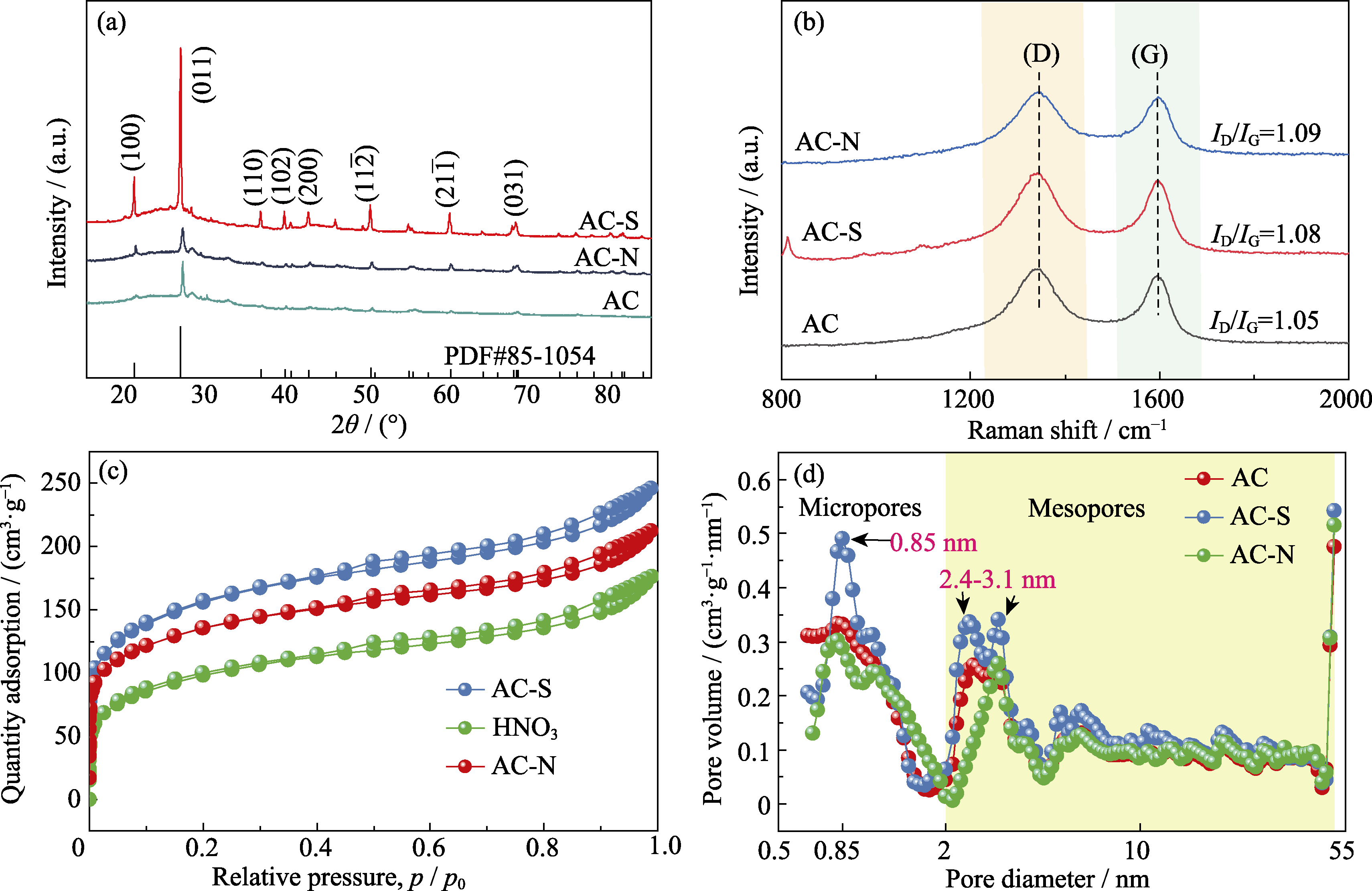
Fig. 1 (a) XRD patterns, (b) Raman spectra, (c) N2 adsorption-desorption isotherms, and (d) pore size distributions of AC, AC-S, and AC-N Colorful figures are available on website
| Sample | SBET/ (m2•g-1) | Vmicro/ (cm3•g-1) | Vmeso/ (cm3•g-1) | Vtotal/ (cm3•g-1) |
|---|---|---|---|---|
| AC | 485.51 | 0.12 | 0.21 | 0.33 |
| AC-S | 558.10 | 0.14 | 0.24 | 0.38 |
| AC-N | 348.88 | 0.06 | 0.21 | 0.27 |
Table 1 Textural characteristics of AC, AC-S, and AC-N
| Sample | SBET/ (m2•g-1) | Vmicro/ (cm3•g-1) | Vmeso/ (cm3•g-1) | Vtotal/ (cm3•g-1) |
|---|---|---|---|---|
| AC | 485.51 | 0.12 | 0.21 | 0.33 |
| AC-S | 558.10 | 0.14 | 0.24 | 0.38 |
| AC-N | 348.88 | 0.06 | 0.21 | 0.27 |
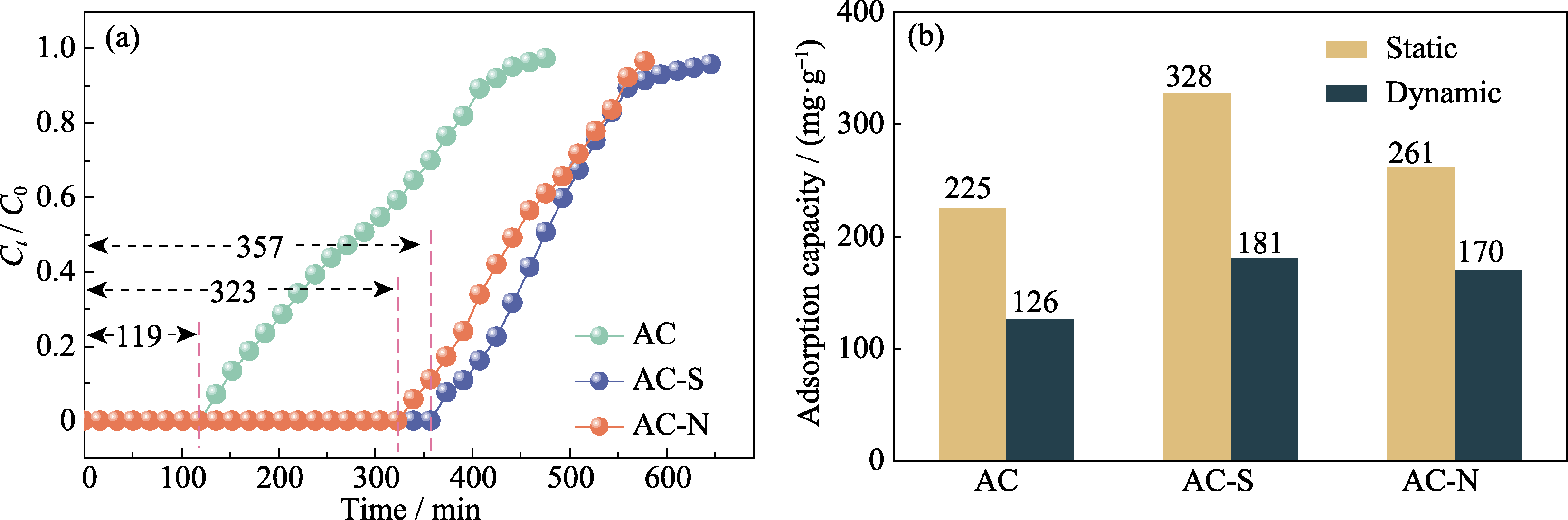
Fig. 3 (a) Breakthrough curves and (b) saturation adsorption capacity for the adsorption of styrene by AC, AC-S, and AC-N Colorful figures are available on website
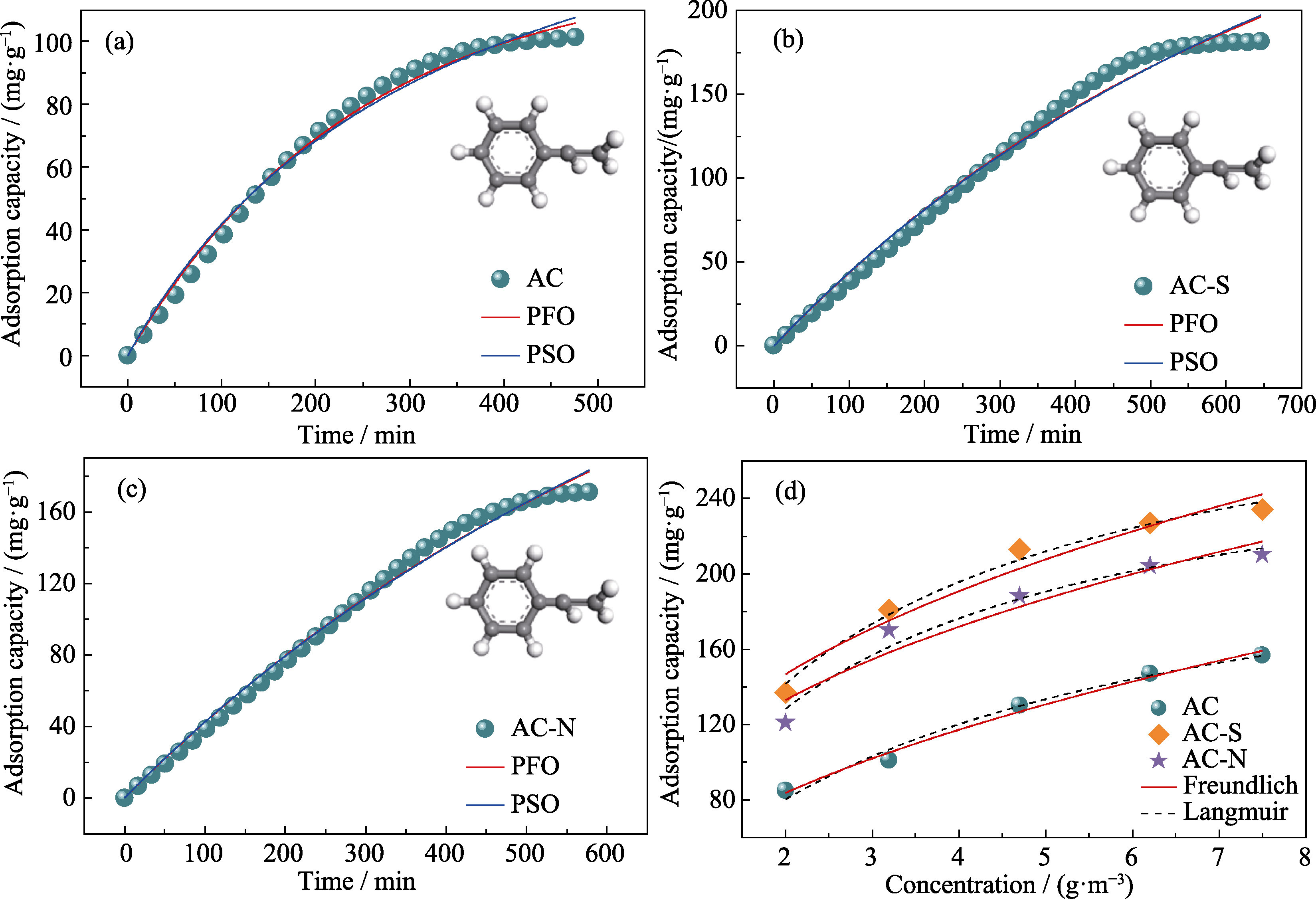
Fig. 4 Adsorption kinetics of (a) AC, (b) AC-S and (c) AC-N, and (d) correspoding adsorption isotherm fitting Colorful figures are available on website
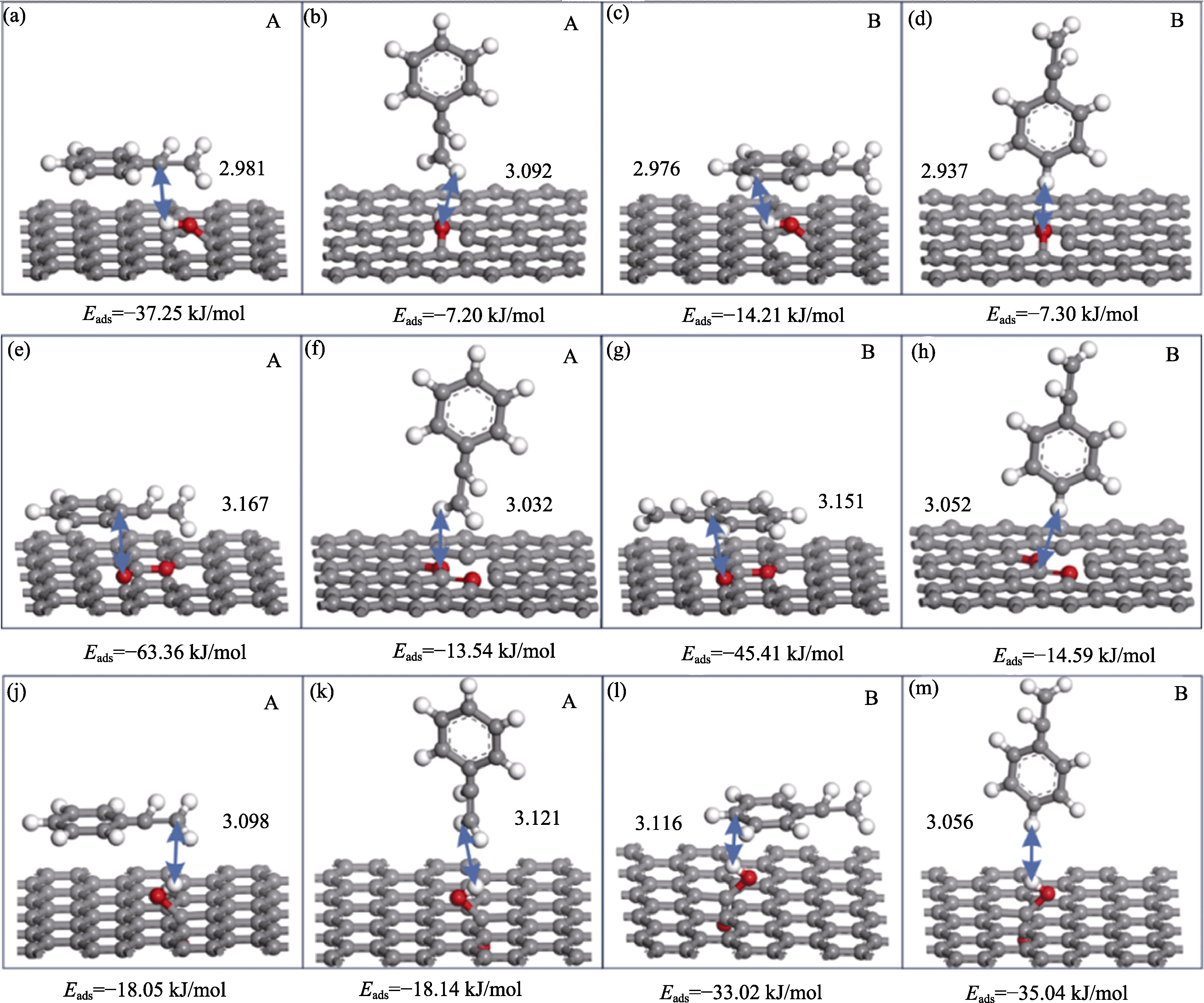
Fig. 5 Configurations of (a-d) styrene interacting with hydroxyl group, (e-h) styrene interacting with lactone group, and (j-m) styrene interacting with the carboxyl group
| Sample | PFO | PSO | ||||
|---|---|---|---|---|---|---|
| qe/(mg•g-1) | k1/min-1 | R2 | qe/(mg•g-1) | k2/(g•mg-1•min-1) | R2 | |
| AC | 123 | 0.0041 | 0.99 | 184 | 1.59×10-5 | 0.99 |
| AC-S | 319 | 0.0015 | 0.99 | 548 | 1.59×10-6 | 0.99 |
| AC-N | 338 | 0.0014 | 0.99 | 595 | 1.29×10-6 | 0.99 |
Table S1 Kinetic parameters of styrene adsorption by AC, AC-S, and AC-N
| Sample | PFO | PSO | ||||
|---|---|---|---|---|---|---|
| qe/(mg•g-1) | k1/min-1 | R2 | qe/(mg•g-1) | k2/(g•mg-1•min-1) | R2 | |
| AC | 123 | 0.0041 | 0.99 | 184 | 1.59×10-5 | 0.99 |
| AC-S | 319 | 0.0015 | 0.99 | 548 | 1.59×10-6 | 0.99 |
| AC-N | 338 | 0.0014 | 0.99 | 595 | 1.29×10-6 | 0.99 |
| Isotherm model | Parameter | AC | AC-S | AC-N |
|---|---|---|---|---|
| Langmuir | qe/(mg•g-1) | 239 | 317 | 281 |
| KL/(m3•mg-1) | 0.25 | 0.40 | 0.42 | |
| R2 | 0.98 | 0.99 | 0.97 | |
| RMSE | 3.53 | 3.86 | 5.39 | |
| Freundlich | KF/((mg•g-1)·(m3•mg-1)1/n) | 59.82 | 122.75 | 102.76 |
| n | 2.06 | 2.63 | 2.69 | |
| R2 | 0.99 | 0.95 | 0.93 | |
| RMSE | 2.73 | 7.74 | 8.52 |
Table S2 Fitting parameters of the Langmuir and Freundlich models for styrene adsorption
| Isotherm model | Parameter | AC | AC-S | AC-N |
|---|---|---|---|---|
| Langmuir | qe/(mg•g-1) | 239 | 317 | 281 |
| KL/(m3•mg-1) | 0.25 | 0.40 | 0.42 | |
| R2 | 0.98 | 0.99 | 0.97 | |
| RMSE | 3.53 | 3.86 | 5.39 | |
| Freundlich | KF/((mg•g-1)·(m3•mg-1)1/n) | 59.82 | 122.75 | 102.76 |
| n | 2.06 | 2.63 | 2.69 | |
| R2 | 0.99 | 0.95 | 0.93 | |
| RMSE | 2.73 | 7.74 | 8.52 |
| Sample | Hydroxyl group/(mmol•g-1) | Lactone group/(mmol•g-1) | Carboxyl group/(mmol•g-1) | Total/(mmol•g-1) |
|---|---|---|---|---|
| AC | 0.10 | 0.01 | 0.02 | 0.13 |
| AC-S | 0.21 | 0.82 | 0.15 | 1.18 |
| AC-N | 0.14 | 0.63 | 0.37 | 1.14 |
Table S3 Amounts of surface functional groups of AC, AC-S, and AC-N
| Sample | Hydroxyl group/(mmol•g-1) | Lactone group/(mmol•g-1) | Carboxyl group/(mmol•g-1) | Total/(mmol•g-1) |
|---|---|---|---|---|
| AC | 0.10 | 0.01 | 0.02 | 0.13 |
| AC-S | 0.21 | 0.82 | 0.15 | 1.18 |
| AC-N | 0.14 | 0.63 | 0.37 | 1.14 |
| Sample | Relative content/% | |||
|---|---|---|---|---|
| C-C | C-O | C=O/COOH | O-C=O | |
| AC | 70.67 | 20.99 | 2.91 | 5.43 |
| AC-S | 65.14 | 16.56 | 4.99 | 13.32 |
| AC-N | 72.94 | 6.70 | 14.57 | 5.79 |
Table S4 XPS results of C1s on the surface of AC, AC-S, and AC-N
| Sample | Relative content/% | |||
|---|---|---|---|---|
| C-C | C-O | C=O/COOH | O-C=O | |
| AC | 70.67 | 20.99 | 2.91 | 5.43 |
| AC-S | 65.14 | 16.56 | 4.99 | 13.32 |
| AC-N | 72.94 | 6.70 | 14.57 | 5.79 |
| [1] |
HE C, CHENG J, ZHANG X, et al. Recent advances in the catalytic oxidation of volatile organic compounds: a review based on pollutant sorts and sources. Chemical Reviews, 2019, 119(7): 4471.
DOI PMID |
| [2] |
BHAT A, VENKAT M, CHEN X, et al. Chemical surface modification of beaded activated carbon: a strategy to inhibit heel accumulation from VOC. Journal of Industrial and Engineering Chemistry, 2021, 103: 205.
DOI URL |
| [3] |
ZHU L, SHEN D, LUO K H. A critical review on VOCs adsorption by different porous materials: species, mechanisms and modification methods. Journal of Hazardous Materials, 2020, 389: 122102.
DOI URL |
| [4] |
ZHU J, LI Y, XU L, et al. Removal of toluene from waste gas by adsorption-desorption process using corncob-based activated carbons as adsorbents. Ecotoxicology and Environmental Safety, 2018, 165: 115.
DOI PMID |
| [5] |
CANDIA-LOMELÍ M, COVARRUBIAS-GARCIA I, AIZPURU A, et al. Preparation and physicochemical characterization of deep eutectic solvents and ionic liquids for the potential absorption and biodegradation of styrene vapors. Journal of Hazardous Materials, 2023, 441: 129835.
DOI URL |
| [6] |
PAN H, HE Z, LIN Q, et al. The effect of copper valence on catalytic combustion of styrene over the copper based catalysts in the absence and presence of water vapor. Chinese Journal of Chemical Engineering, 2016, 24(4): 468.
DOI |
| [7] |
ZHANG Y, ZHANG L, LU J, et al. Investigation of defect-rich CeO2 catalysts for super low-temperature catalytic oxidation and durable styrene removal. Chemosphere, 2022, 303: 134863.
DOI URL |
| [8] |
WANG Y, DU X, LONG Y, et al. Real-time detection of styrene using SAW sensors based on hexafluoroisopropanol group functionalized hydrogen-bond acidic polymers. Sensors and Actuators B: Chemical, 2015, 206: 252.
DOI URL |
| [9] |
LI D, SU R, MA X, et al. Porous carbon for oxygenated and aromatic VOCs adsorption by molecular simulation and experimental study: effect pore structure and functional groups. Applied Surface Science, 2022, 605: 154708.
DOI URL |
| [10] |
WANG H, HUANG L, QING J, et al. Mesoporous organic- inorganic hybrid siliceous hollow spheres: synthesis and VOCs adsorption. Journal of Inorganic Materials, 2022, 37(9): 991.
DOI URL |
| [11] |
HOU B, ZHAO Y, SUN W, et al. Glycine based modification of activated carbons for VOCs adsorption. Chemical Engineering Journal Advances, 2021, 7: 100126.
DOI URL |
| [12] |
MATUSIK J, KOTEJA-KUNECKA A, MAZIARZ P, et al. Styrene removal by surfactant-modified smectite group minerals: efficiency and factors affecting adsorption/desorption. Chemical Engineering Journal, 2022, 428: 130848.
DOI URL |
| [13] |
HOU S, HUANG Z H, ZHU T, et al. Adsorption removal of styrene on C-Cl grafted silica gel adsorbents. Chemosphere, 2023, 315: 137679.
DOI URL |
| [14] |
LI Z, JIN Y, CHEN T, et al. Trimethylchlorosilane modified activated carbon for the adsorption of VOCs at high humidity. Separation and Purification Technology, 2021, 272: 118659.
DOI URL |
| [15] |
DIZBAY-ONAT M, FLOYD E, VAIDYA U K, et al. Applicability of industrial sisal fiber waste derived activated carbon for the adsorption of volatile organic compounds (VOCs). Fibers and Polymers, 2018, 19(4): 805.
DOI |
| [16] | 刘俊岭. 用于吸附VOCs的碳基材料和MOFs研究. 北京: 北京石油化工学院硕士学位论文, 2021. |
| [17] |
YU H, LI T, YANG X, et al. Hydrophobic carbon-based coating on metal tube with efficient and stable adsorption-desorption of CO2 from wet flue gas. Separation and Purification Technology, 2023, 307: 122798.
DOI URL |
| [18] |
YU X, LIU S, LIN G, et al. Insight into the significant roles of microstructures and functional groups on carbonaceous surfaces for acetone adsorption. RSC Advances, 2018, 8(38): 21541.
DOI URL |
| [19] |
BECKER P, GLENK F, KORMANN M, et al. Chlorination of titanium carbide for the processing of nanoporous carbon: a kinetic study. Chemical Engineering Journal, 2010, 159(1-3): 236.
DOI URL |
| [20] |
LIU T, ZHANG R, ZHANG X, et al. One-step room-temperature preparation of expanded graphite. Carbon, 2017, 119: 544.
DOI URL |
| [21] |
THOMMES M, CYCHOSZ K A. Physical adsorption characterization of nanoporous materials: progress and challenges. Adsorption, 2014, 20(2/3): 233.
DOI URL |
| [22] |
KAN Y, ZHANG R, XU X, et al. Comparative study of raw and HNO3-modified porous carbon from waste printed circuit boards for sulfadiazine adsorption: experiment and DFT study. Chinese Chemical Letters, 2023, 34(7): 108272.
DOI URL |
| [23] |
LI L, QUINLIVAN P A, KNAPPE D R U. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon, 2002, 40(12): 2085.
DOI URL |
| [24] |
ZEYNALI M E. Evaluation of the effect of catalyst pore-size distribution on the effectiveness factor in ethylbenzene dehydrogenation by orthogonal collocation. Defect and Diffusion Forum, 2011, 316-317: 155.
DOI URL |
| [25] |
TANG L, LI L, CHEN R, et al. Adsorption of acetone and isopropanol on organic acid modified activated carbons. Journal of Environmental Chemical Engineering, 2016, 4(2): 2045.
DOI URL |
| [26] |
EL-HENDAWY A. Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon, 2003, 41(4): 713.
DOI URL |
| [27] |
FU Y, SHEN Y, ZHANG Z, et al. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Science of the Total Environment, 2019, 646: 1567.
DOI URL |
| [28] |
MA X, LV H, YANG L, et al. Removal characteristics of organic pollutants by the adsorbent injection coupled with bag filtering system. Journal of Hazardous Materials, 2021, 405: 124193.
DOI URL |
| [29] | 杨颖, 李磊, 孙振亚, 等. 活性炭表面官能团的氧化改性及其吸附机理的研究. 科学技术与工程, 2012, 24(12): 1671. |
| [30] |
MA X, LI L, CHEN R, et al. Porous carbon materials based on biomass for acetone adsorption: effect of surface chemistry and porous structure. Applied Surface Science, 2018, 459: 657.
DOI URL |
| [31] |
WANG X, YAO F, ZHU W, et al. Experimental and computational investigation on the organic acid modification of porous carbon for toluene adsorption under humid conditions. Chemical Engineering Journal, 2022, 450: 138070.
DOI URL |
| [32] |
KUTLUAY S. Excellent adsorptive performance of novel magnetic nano-adsorbent functionalized with 8-hydroxyquinoline-5-sulfonic acid for the removal of volatile organic compounds (BTX) vapors. Fuel, 2021, 287: 119691.
DOI URL |
| [33] | 梁鑫. 有机酸改性活性炭及其VOCs吸附行为研究. 长沙: 中南大学能源科学与工程学院硕士学位论文, 2014. |
| [34] |
LAWAL A A, HASSAN M A, AHMAD FARID M A, et al. Adsorption mechanism and effectiveness of phenol and tannic acid removal by biochar produced from oil palm frond using steam pyrolysis. Environmental Pollution, 2021, 269: 116197.
DOI URL |
| [35] | KOMNITSAS K A, ZAHARAKI D. Morphology of modified biochar and its potential for phenol removal from aqueous solutions. Frontiers in Environmental Science, 2016, 4: 26. |
| [36] |
YIN Q, SI L, WANG R, et al. DFT study on the effect of functional groups of carbonaceous surface on ammonium adsorption from water. Chemosphere, 2022, 287: 132294.
DOI URL |
| [37] |
CHEN L, YUAN J, LI T, et al. A regenerable N-rich hierarchical porous carbon synthesized from waste biomass for H2S removal at room temperature. Science of the Total Environment, 2021, 768: 144452.
DOI URL |
| [38] |
KRASNENKO V, KIKAS J, BRIK M G. Modification of the structural and electronic properties of graphene by the benzene molecule adsorption. Physica B: Condensed Matter, 2012, 407(23): 4557.
DOI URL |
| [39] |
BHUVANESWARI R, NAGARAJAN V, CHANDIRAMOULI R. First-principles research on adsorption properties of o-xylene and styrene on 5-8 phosphorene sheets. Chemical Physics Letters, 2021, 765: 138244.
DOI URL |
| [40] |
CUI H, ZHANG X, CHEN D, et al. Adsorption mechanism of SF6 decomposed species on pyridine-like PtN3 embedded CNT: a DFT study. Applied Surface Science, 2018, 447: 594.
DOI URL |
| [41] |
PÉREZ E M, MARTÍN N. π-π interactions in carbon nanostructures. Chemical Society Reviews, 2015, 44(18): 6425.
DOI PMID |
| [42] |
CHOI H J. Assessment of sulfonation in lignocellulosic derived material for adsorption of methylene blue. Environmental Engineering Research, 2022, 27(3): 210034.
DOI URL |
| [43] |
MA X, YANG L, HOU Y, et al. Adsorption/desorption characteristics of low-concentration semi-volatile organic compounds in vapor phase on activated carbon. Journal of Environmental Management, 2022, 305: 114360.
DOI URL |
| [1] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [2] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [3] | HONG Peiping, LIANG Long, WU Lian, MA Yingkang, PANG Hao. Structure Regulation of ZIF-67 and Adsorption Properties for Chlortetracycline Hydrochloride [J]. Journal of Inorganic Materials, 2025, 40(4): 388-396. |
| [4] | XIE Tian, SONG Erhong. Effect of Elastic Strains on Adsorption Energies of C, H and O on Transition Metal Oxides [J]. Journal of Inorganic Materials, 2024, 39(11): 1292-1300. |
| [5] | CHAO Shaofei, XUE Yanhui, WU Qiong, WU Fufa, MUHAMMAD Sufyan Javed, ZHANG Wei. Efficient Potassium Storage through Ti-O-H-O Electron Fast Track of MXene Heterojunction [J]. Journal of Inorganic Materials, 2024, 39(11): 1212-1220. |
| [6] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [7] | GUO Chunxia, CHEN Weidong, YAN Shufang, ZHAO Xueping, YANG Ao, MA Wen. Adsorption of Arsenate in Water by Zirconia-halloysite Nanotube Material [J]. Journal of Inorganic Materials, 2023, 38(5): 529-536. |
| [8] | WANG Shiyi, FENG Aihu, LI Xiaoyan, YU Yun. Pb (II) Adsorption Process of Fe3O4 Supported Ti3C2Tx [J]. Journal of Inorganic Materials, 2023, 38(5): 521-528. |
| [9] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [10] | LING Jie, ZHOU Anning, WANG Wenzhen, JIA Xinyu, MA Mengdan. Effect of Cu/Mg Ratio on CO2 Adsorption Performance of Cu/Mg-MOF-74 [J]. Journal of Inorganic Materials, 2023, 38(12): 1379-1386. |
| [11] | TANG Ya, SUN Shengrui, FAN Jia, YANG Qingfeng, DONG Manjiang, KOU Jiahui, LIU Yangqiao. PEI Modified Hydrated Calcium Silicate Derived from Fly Ash and Its adsorption for Removal of Cu (II) and Catalytic Degradation of Organic Pollutants [J]. Journal of Inorganic Materials, 2023, 38(11): 1281-1291. |
| [12] | DAI Jieyan, FENG Aihu, MI Le, YU Yang, CUI Yuanyuan, YU Yun. Adsorption Mechanism of NaY Zeolite Molecular Adsorber Coating on Typical Space Contaminations [J]. Journal of Inorganic Materials, 2023, 38(10): 1237-1244. |
| [13] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, HUANG Weiqiu, CHEN Ruoyu. Mesoporous Organic-inorganic Hybrid Siliceous Hollow Spheres: Synthesis and VOCs Adsorption [J]. Journal of Inorganic Materials, 2022, 37(9): 991-1000. |
| [14] | LIU Cheng, ZHAO Qian, MOU Zhiwei, LEI Jiehong, DUAN Tao. Adsorption Properties of Novel Bismuth-based SiOCNF Composite Membrane for Radioactive Gaseous Iodine [J]. Journal of Inorganic Materials, 2022, 37(10): 1043-1050. |
| [15] | ZHOU Fan, BI Hui, HUANG Fuqiang. Ultra-large Specific Surface Area Activated Carbon Synthesized from Rice Husk with High Adsorption Capacity for Methylene Blue [J]. Journal of Inorganic Materials, 2021, 36(8): 893-903. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||