
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (11): 1281-1291.DOI: 10.15541/jim20230209
Special Issue: 【能源环境】污染物催化去除(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
TANG Ya1,2( ), SUN Shengrui2, FAN Jia1,2, YANG Qingfeng3, DONG Manjiang2, KOU Jiahui1(
), SUN Shengrui2, FAN Jia1,2, YANG Qingfeng3, DONG Manjiang2, KOU Jiahui1( ), LIU Yangqiao2(
), LIU Yangqiao2( )
)
Received:2023-04-28
Revised:2023-05-29
Published:2023-06-01
Online:2023-06-01
Contact:
LIU Yangqiao, professor. E-mail: yqliu@mail.sic.ac.cn;About author:About author: TANG Ya (1998-), male, Master candidate. E-mail: tangya153@163.com
Supported by:CLC Number:
TANG Ya, SUN Shengrui, FAN Jia, YANG Qingfeng, DONG Manjiang, KOU Jiahui, LIU Yangqiao. PEI Modified Hydrated Calcium Silicate Derived from Fly Ash and Its adsorption for Removal of Cu (II) and Catalytic Degradation of Organic Pollutants[J]. Journal of Inorganic Materials, 2023, 38(11): 1281-1291.

Fig. 3 Adsorption characteristics of samples (a) Variation of adsorption capacity of samples with time; (b) Effect of pH on the adsorption capacity of PCSH; (c) Adsorption isotherms of samples; (d) Variation of adsorption capacity of samples with initial concentration of Zn(II)-Pb(II)
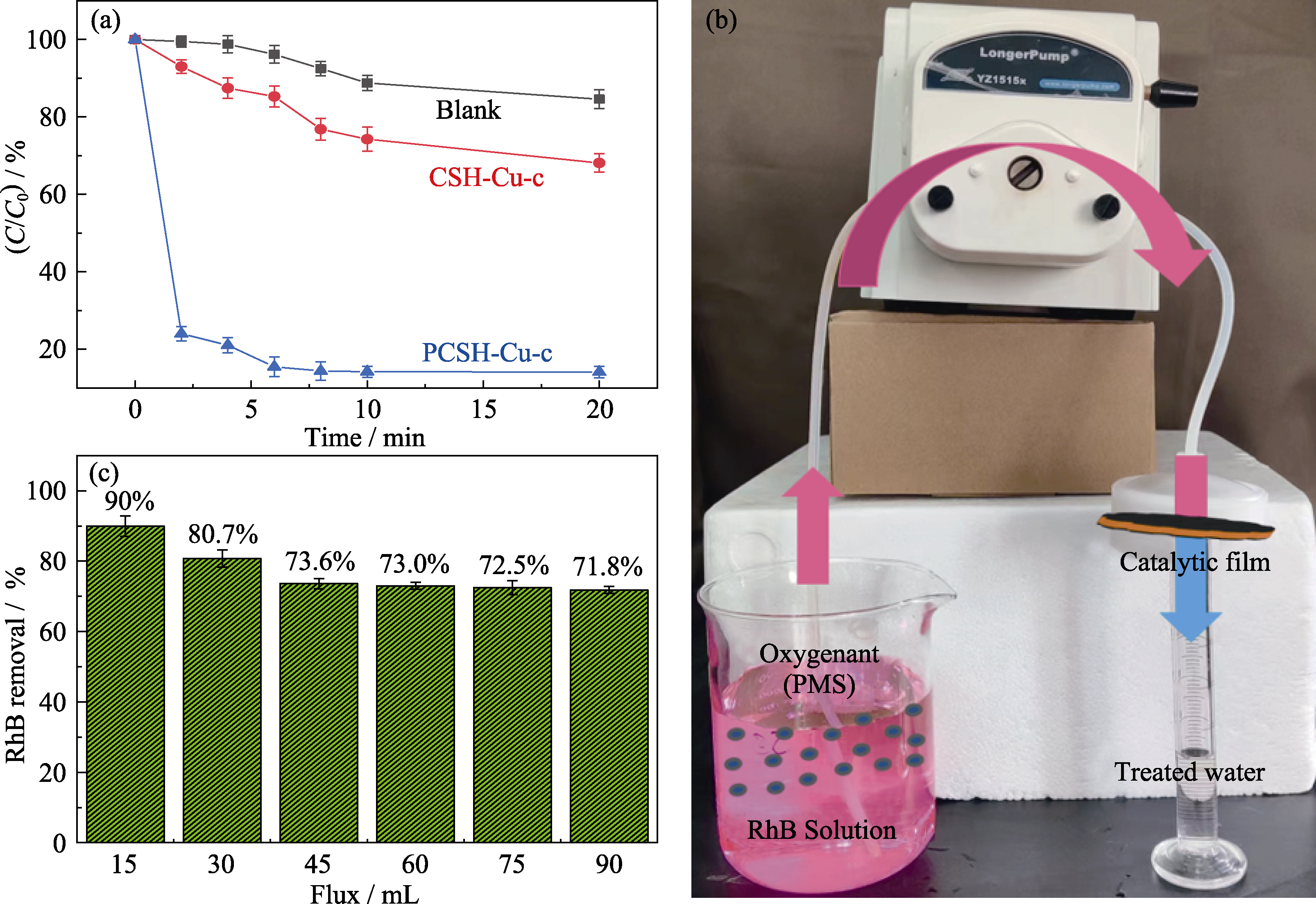
Fig. 7 Degradation of RhB by PMS with CSH-Cu-c and PCSH-Cu-c (a) RhB residue percentage; (b) Photo of catalytic device; (c) Catalytic performance of PCSH-Cu-c-M
| Catalyst | C1/(g·L-1) | CPMS/(g·L-1) | CRhB/(mg·L-1) | k/min-1 | Ref. |
|---|---|---|---|---|---|
| MPC | 1 | 0.616 | 20 | 0.013 | [ |
| α-MnO2/Pal | 0.1 | 0.1 | 20 | 0.0204 | [ |
| Vis/BiVO4 | 0.5 | 0.616 | 10 | 0.04 | [ |
| rGO-CoPc | 0.3 | 0.616 | 10 | 0.288 | [ |
| CSH-Cu-c | 0.8 | 0.12 | 20 | 0.036 | This work |
| PCSH-Cu-c | 0.8 | 0.12 | 20 | 0.7135 | This work |
Table 1 Rate constants (k) for the degradation of RhB by PMS with different catalysts
| Catalyst | C1/(g·L-1) | CPMS/(g·L-1) | CRhB/(mg·L-1) | k/min-1 | Ref. |
|---|---|---|---|---|---|
| MPC | 1 | 0.616 | 20 | 0.013 | [ |
| α-MnO2/Pal | 0.1 | 0.1 | 20 | 0.0204 | [ |
| Vis/BiVO4 | 0.5 | 0.616 | 10 | 0.04 | [ |
| rGO-CoPc | 0.3 | 0.616 | 10 | 0.288 | [ |
| CSH-Cu-c | 0.8 | 0.12 | 20 | 0.036 | This work |
| PCSH-Cu-c | 0.8 | 0.12 | 20 | 0.7135 | This work |
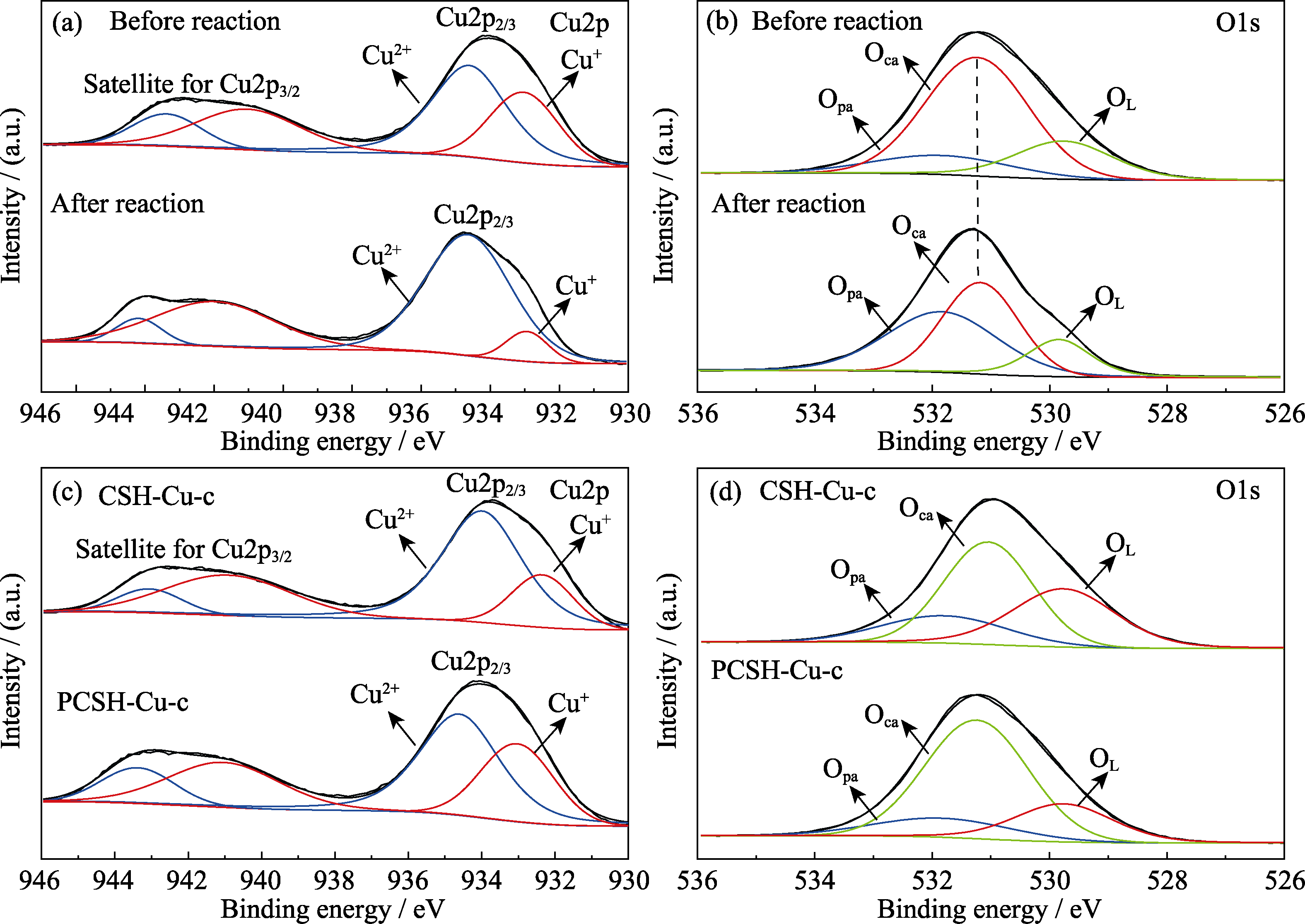
Fig. 8 Suface elements and chemical status of samples (a) Cu2p and (b) O1s XPS spectra of PCSH-Cu-c before and after reaction; (c) Cu2p and (d) O1s XPS spectra of CSH-Cu-c and PCSH-Cu-c before reaction
| Catalyst | C1/ (g·L-1) | CNaBH4/ (mmol·L-1) | C4-NP/ (mmol·L-1) | k/(×10-3, s-1) | Ref. |
|---|---|---|---|---|---|
| CuO NLs | 0.307 | 10 | 0.12 | 0.36 | [ |
| Ag-NP/C | 0.333 | 6.667 | 4.7×10-2 | 1.69 | [ |
| Cu2−xSe/ rGO/PVP | 2.5 | 62.5 | 0.125 | 2.3 | [ |
| Pd-FG | 0.5 | 10 | 0.1 | 2.35 | [ |
| CSH-Cu-c | 0.167 | 5 | 0.1 | 0.61 | This work |
| PCSH-Cu-c | 0.167 | 5 | 0.1 | 11.47 | This work |
Table 2 Rate constants (k) for the degradation of 4-NP by NaBH4 with different catalysts
| Catalyst | C1/ (g·L-1) | CNaBH4/ (mmol·L-1) | C4-NP/ (mmol·L-1) | k/(×10-3, s-1) | Ref. |
|---|---|---|---|---|---|
| CuO NLs | 0.307 | 10 | 0.12 | 0.36 | [ |
| Ag-NP/C | 0.333 | 6.667 | 4.7×10-2 | 1.69 | [ |
| Cu2−xSe/ rGO/PVP | 2.5 | 62.5 | 0.125 | 2.3 | [ |
| Pd-FG | 0.5 | 10 | 0.1 | 2.35 | [ |
| CSH-Cu-c | 0.167 | 5 | 0.1 | 0.61 | This work |
| PCSH-Cu-c | 0.167 | 5 | 0.1 | 11.47 | This work |
| Sample | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | n | KF | R2 | |
| CSH | 294.10 | 0.0200 | 0.9945 | 3.810 | 54.00 | 0.8670 |
| PCSH | 588.23 | 0.0563 | 0.9982 | 5.848 | 198.86 | 0.9450 |
Table S1 Langmuir and Freundlich isotherm fitting parameters
| Sample | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | n | KF | R2 | |
| CSH | 294.10 | 0.0200 | 0.9945 | 3.810 | 54.00 | 0.8670 |
| PCSH | 588.23 | 0.0563 | 0.9982 | 5.848 | 198.86 | 0.9450 |
| Sample | q / (mg·g-1) | SBET/ (m2·g-1) | Ref. |
|---|---|---|---|
| Activated carbon | 10 | 921 | [S1] |
| Modified SBA-15 mesoporous silica | 46 | 317 | [S2] |
| MCM-48 | 126 | 511 | [S3] |
| Citrate-LDH | 137 | 8 | [S4] |
| Mesoporous silica | 153 | 462 | [S5] |
| Humulus scandens-derived biochars | 221 | 450 | [S6] |
| Steel slag-derived CSH | 244 | 77 | [S7] |
| Amorphous molybdenum sulphide | 259 | 28 | [S8] |
| NPCS-PEI | 276 | 491 | [S9] |
| CSH | 294 | 240 | This work |
| PCSH | 588 | 371 | This work |
Table S2 Comparison of maximum adsorption capacities of various sorbents as reported in the literature for Cu(II)
| Sample | q / (mg·g-1) | SBET/ (m2·g-1) | Ref. |
|---|---|---|---|
| Activated carbon | 10 | 921 | [S1] |
| Modified SBA-15 mesoporous silica | 46 | 317 | [S2] |
| MCM-48 | 126 | 511 | [S3] |
| Citrate-LDH | 137 | 8 | [S4] |
| Mesoporous silica | 153 | 462 | [S5] |
| Humulus scandens-derived biochars | 221 | 450 | [S6] |
| Steel slag-derived CSH | 244 | 77 | [S7] |
| Amorphous molybdenum sulphide | 259 | 28 | [S8] |
| NPCS-PEI | 276 | 491 | [S9] |
| CSH | 294 | 240 | This work |
| PCSH | 588 | 371 | This work |
| [1] |
CHEN H, WANG X, LI J, et al. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. Journal of Materials Chemistry A, 2015, 3(11): 6073.
DOI URL |
| [2] |
GAWANDE M B, GOSWAMI A, FELPIN F X, et al. Cu and Cu-based nanoparticles: synthesis and applications in review catalysis. Chemical Reviews, 2016, 116(6): 3722.
DOI URL |
| [3] |
KOHANTORABI M, MOUSSAVI G, GIANNAKIS S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chemical Engineering Journal, 2021, 411: 127957.
DOI URL |
| [4] |
ADITYA T, JANA J, SINGH N K, et al. Remarkable facet selective reduction of 4-nitrophenol by morphologically tailored (111) faceted Cu2O nanocatalyst. ACS Omega, 2017, 2(5): 1968.
DOI URL |
| [5] |
TONG T, ZHAO S, BOO C, et al. Relating silica scaling in reverse osmosis to membrane surface properties. Environmental Science & Technology, 2017, 51(8): 4396.
DOI URL |
| [6] |
BERA A, TRIVEDI J S, KUMAR S B, et al. Anti-organic fouling and anti-biofouling poly(piperazineamide) thin film nanocomposite membranes for low pressure removal of heavy metal ions. Journal of Hazardous Materials, 2018, 343: 86.
DOI PMID |
| [7] |
FU F, WANG Q. Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management, 2011, 92(3): 407.
DOI PMID |
| [8] |
SUN L, WU J, WANG J, et al. CO2-assisted ‘Weathering’ of steel slag-derived calcium silicate hydrate: A generalized strategy for recycling noble metals and constructing SiO2-based nanocomposites. Journal of Colloid and Interface Science, 2022, 622: 1008.
DOI URL |
| [9] | SHAO N, TANG S, LIU Z, et al. Hierarchically structured calcium silicate hydrate-based nanocomposites derived from steel slag for highly efficient heavy metal removal from wastewater. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 14926. |
| [10] |
CHEN L, WANG X, CHEN Y, et al. Recycling heavy metals from wastewater for photocatalytic CO2 reduction. Chemical Engineering Journal, 2020, 402: 125922.
DOI URL |
| [11] |
CHEN Z, LI Y, CAI Y, et al. Application of covalent organic frameworks and metal-organic frameworks nanomaterials in organic/inorganic pollutants removal from solutions through sorption-catalysis strategies. Carbon Research, 2023, 2(1): 8.
DOI |
| [12] |
TAUSTER S J, FUNG S C. Strong metal-support interactions- occurrence among binary oxides of groups IIA-VB. Journal of Catalysis, 1978, 55(1): 29.
DOI URL |
| [13] |
GU H, LIU X, WANG S, et al. COF-based composites: extraordinary removal performance for heavy metals and radionuclides from aqueous solutions. Reviews of Environmental Contamination and Toxicology, 2022, 260(1): 23.
DOI |
| [14] |
WANG X, LI X, WANG J, et al. Recent advances in carbon nitride-based nanomaterials for the removal of heavy metal ions from aqueous solution. Journal of Inorganic Materials, 2020, 35(3): 260.
DOI |
| [15] |
GUO H, SUN P, LIANG Y, et al. In-situ fabrication of polyelectrolyte-CSH superhydrophilic coatings via layer-by-layer assembly. Chemical Engineering Journal, 2014, 253: 198.
DOI URL |
| [16] |
MEISZTERICS A, ROSTA L, PETERLIK H, et al. Structural characterization of gel-derived calcium silicate systems. Journal of Physical Chemistry A, 2010, 114(38): 10403.
DOI PMID |
| [17] |
KLONKOWSKI A M, GROBENLA B, WIDERNIK T, et al. The coordination state of copper(II) complexes anchored and grafted onto the surface of organically modified silicates. Langmuir, 1999, 15(18): 5814.
DOI URL |
| [18] |
GODIYA C B, REVADEKAR C, KIM J, et al. Amine- bilayer-functionalized cellulose-chitosan composite hydrogel for the efficient uptake of hazardous metal cations and catalysis in polluted water. Journal of Hazardous Materials, 2022, 436: 129112.
DOI URL |
| [19] |
VICENNATI P, GIULIANO A, ORTAGGI G, et al. Polyethylenimine in medicinal chemistry. Current Medicinal Chemistry, 2008, 15(27): 2826.
PMID |
| [20] |
NOSRATI A, LARSSON M, LINDEN J B, et al. Polyethyleneimine functionalized mesoporous diatomite particles for selective copper recovery from aqueous media. International Journal of Mineral Processing, 2017, 166: 29.
DOI URL |
| [21] |
HUANG Y, WU H, SHAO T, et al. Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater. Chemical Engineering Journal, 2018, 339: 322.
DOI URL |
| [22] |
CHADWICK D, KAROLEWSKI M A. Calibration of XPS core-level binding-energies-influence of the surface chemical-shift. Journal of Electron Spectroscopy and Related Phenomena, 1981, 24(2): 181.
DOI URL |
| [23] |
SUTIRMAN Z A, SANAGI M M, ABD KARIM K J, et al. Equilibrium, kinetic and mechanism studies of Cu(II) and Cd(II) ions adsorption by modified chitosan beads. International Journal of Biological Macromolecules, 2018, 116: 255.
DOI PMID |
| [24] |
PENG B, SONG T, WANG T, et al. Facile synthesis of Fe3O4@Cu(OH)2 composites and their arsenic adsorption application. Chemical Engineering Journal, 2016, 299: 15.
DOI URL |
| [25] |
ZHAO C M, WANG G C, LI S L, et al. Reaction pathway led by silicate structure transformation on decomposition of CaSiO3 in alkali fusion process using NaOH. Transactions of Nonferrous Metals Society of China, 2015, 25(11): 3827.
DOI URL |
| [26] |
ZHANG S, ZHANG X, BAI C, et al. Effect of TiO2 content on the structure of CaO-SiO2-TiO2 system by molecular dynamics simulation. ISIJ International, 2013, 53(7): 1131.
DOI URL |
| [27] |
ZHU K, LIU C, XIA W, et al. Non-radical pathway dominated degradation of organic pollutants by nitrogen-doped microtube porous graphitic carbon derived from biomass for activating peroxymonosulfate: performance, mechanism and environmental application. Journal of Colloid and Interface Science, 2022, 625: 890.
DOI PMID |
| [28] |
HUANG C, WANG Y, GONG M, et al. α-MnO2/palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate PMS for the degradation of Rhodamine B. Separation and Purification Technology, 2020, 230: 115877.
DOI URL |
| [29] |
LIU Y, GUO H, ZHANG Y, et al. Activation of peroxymonosulfate by BiVO4 under visible light for degradation of Rhodamine B. Chemical Physics Letters, 2016, 653: 101.
DOI URL |
| [30] |
MARINESCU C, BEN ALI M, HAMDI A, et al. Cobalt phthalocyanine-supported reduced graphene oxide: a highly efficient catalyst for heterogeneous activation of peroxymonosulfate for Rhodamine B and pentachlorophenol degradation. Chemical Engineering Journal, 2018, 336: 465.
DOI URL |
| [31] |
ZHAO Y, AN H, FENG J, et al. Impact of crystal types of AgFeO2 nanoparticles on the peroxymonosulfate activation in the water. Environmental Science & Technology, 2019, 53(8): 4500.
DOI URL |
| [32] |
QIN Q, QIAO N, LIU Y, et al. Spongelike porous CuO as an efficient peroxymonosulfate activator for degradation of Acid Orange 7. Applied Surface Science, 2020, 521: 146479.
DOI URL |
| [33] |
LAN Q, SUN S R, WU P, et al. Co-doped CuO/visible light synergistic activation of PMS for degradation of Rhodamine B and its mechanism. Journal of Inorganic Materials, 2021, 36(11): 1171.
DOI |
| [34] |
XIAO R, LUO Z, WEI Z, et al. Activation of peroxymonosulfate/ persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Current Opinion in Chemical Engineering, 2018, 19: 51.
DOI URL |
| [35] |
DUAN X, SU C, MIAO J, et al. Insights into perovskite-catalyzed peroxymonosulfate activation: maneuverable cobalt sites for promoted evolution of sulfate radicals. Applied Catalysis B-Environmental, 2018, 220: 626.
DOI URL |
| [36] |
ZHAO Y, WANG H, LI X, et al. Recovery of CuO/C catalyst from spent anode material in battery to activate peroxymonosulfate for refractory organic contaminants degradation. Journal of Hazardous Materials, 2021, 420: 126552.
DOI URL |
| [37] |
SINGH C, GOYAL A, SINGHAL S. Nickel-doped cobalt ferrite nanoparticles: efficient catalysts for the reduction of nitroaromatic compounds and photo-oxidative degradation of toxic dyes. Nanoscale, 2014, 6(14): 7959.
DOI PMID |
| [38] |
BHATTACHARJEE A, AHMARUZZAMAN M. Green synthesis of 2D CuO nanoleaves (NLs) and its application for the reduction of p-nitrophenol. Materials Letters, 2015, 161: 79.
DOI URL |
| [39] |
TANG S, VONGEHR S, MENG X. Carbon spheres with controllable silver nanoparticle doping. Journal of Physical Chemistry C, 2010, 114(2): 977.
DOI URL |
| [40] | YANG T, ZOU H Y, HUANG C Z. Synergetic catalytic effect of Cu2-xSe nanoparticles and reduced graphene oxide coembedded in electrospu n nanofibers for the reduction of a typical refractory organic compound. ACS Applied Materials & Interfaces, 2015, 7(28): 15447. |
| [41] |
WANG Z, XU C, GAO G, et al. Facile synthesis of well-dispersed Pd-graphene nanohybrids and their catalytic properties in 4-nitrophenol reduction. RSC Advances, 2014, 4(26): 13644.
DOI URL |
| [1] | LAN Qing, SUN Shengrui, WU Ping, YANG Qingfeng, LIU Yangqiao. Co-doped CuO/Visible Light Synergistic Activation of PMS for Degradation of Rhodamine B and Its Mechanism [J]. Journal of Inorganic Materials, 2021, 36(11): 1171-1177. |
| [2] | FANG Mei-Rong,QIN Li-Mei,JIA Xiao-Bo,LI Yong-Sheng,NIU De-Chao,HU Ze-Lan. Construction of Polyethyleneimine-modified Dual-mesoporous Silica Gene Carrier [J]. Journal of Inorganic Materials, 2020, 35(2): 187-192. |
| [3] | WU De-Yi,KONG Hai-Nan,ZHAO Tong-Gang,WANG Chong,YE Chun. Effects of Synthesis Conditions on the Formation and Quality of Zeolite During the Hydrothermal Zeolitization Processes of Fly Ash [J]. Journal of Inorganic Materials, 2005, 20(5): 1153-1158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||