
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (10): 1131-1135.DOI: 10.15541/jim20180107
Special Issue: 离子电池材料
• RESEARCH PAPER • Previous Articles Next Articles
GU Feng1,2, WANG You-Wei2, ZHENG Zhi-Hui1,2, LIU Jian-Jun2, LU Wen-Cong1
Received:2018-03-12
Revised:2018-05-02
Published:2018-10-20
Online:2018-09-25
About author:GU Feng. E-mail: fenggu@student.sic.ac.cn
Supported by:CLC Number:
GU Feng, WANG You-Wei, ZHENG Zhi-Hui, LIU Jian-Jun, LU Wen-Cong. Catalytic Mechanism of Palladium Catalyst for the Oxidation Reduction and Evolution Reaction of Lithium-air Battery[J]. Journal of Inorganic Materials, 2018, 33(10): 1131-1135.

Fig. 1 Fcc, Hcp and Top sites on the Pd (111) surface of oxygen atoms from (a) top view and (b) main view, and (c) relationship between three kinds of adsorption sites on the coverage rate
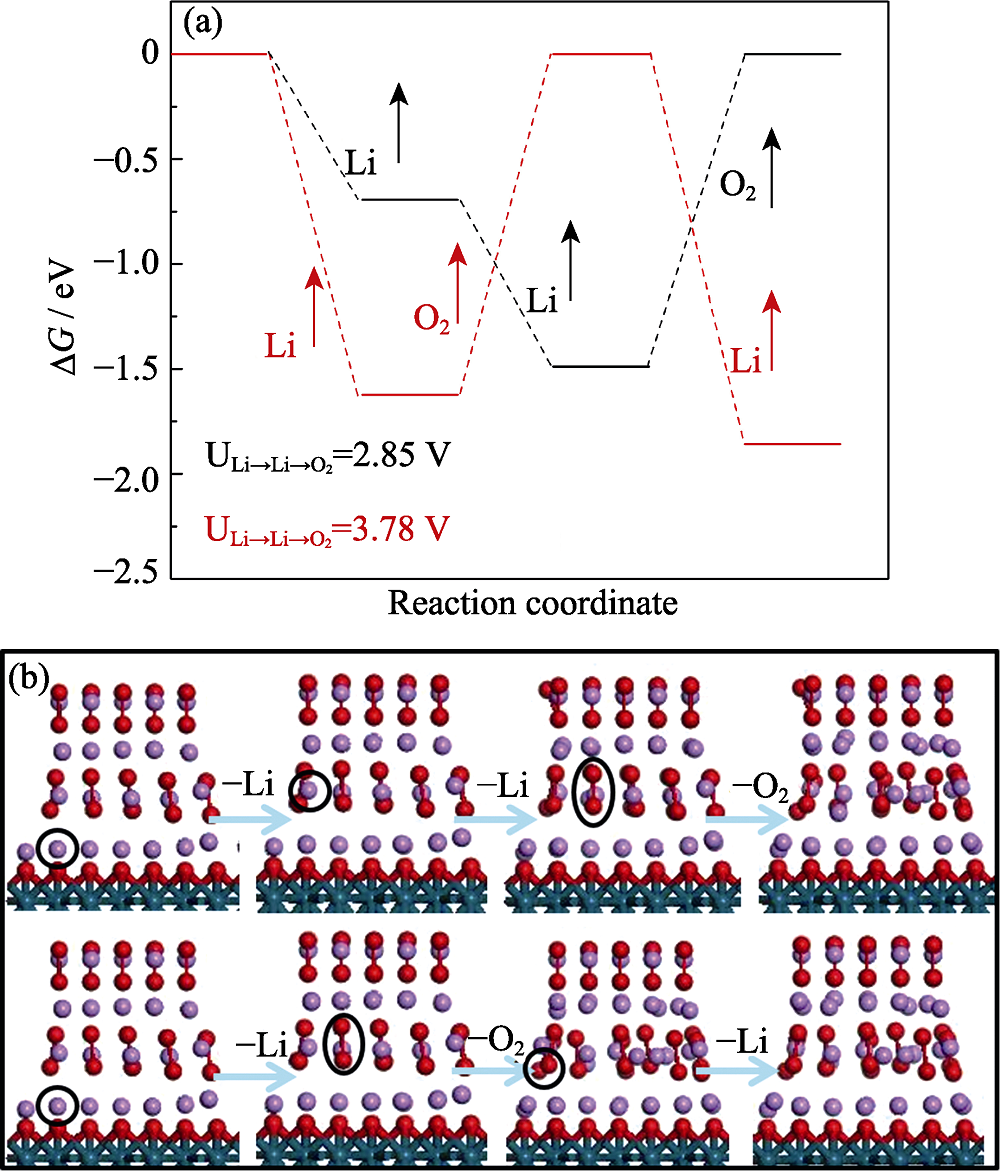
Fig. 2 (a) Energy profiles of two different oxygen evolution reaction paths of Li2O2 for Li+→Li+→O2 with charge voltage of 2.85 V (black line) and Li+→O2→Li+ with charge voltage of 3.78 V (red line); (b) Sketch maps of Li+→Li+→O2 and Li+→O2→ Li+ oxygen evolution reaction paths of Li2O2 with red ball indicating O, blue ball indicating Pd, and purple ball indicating Li
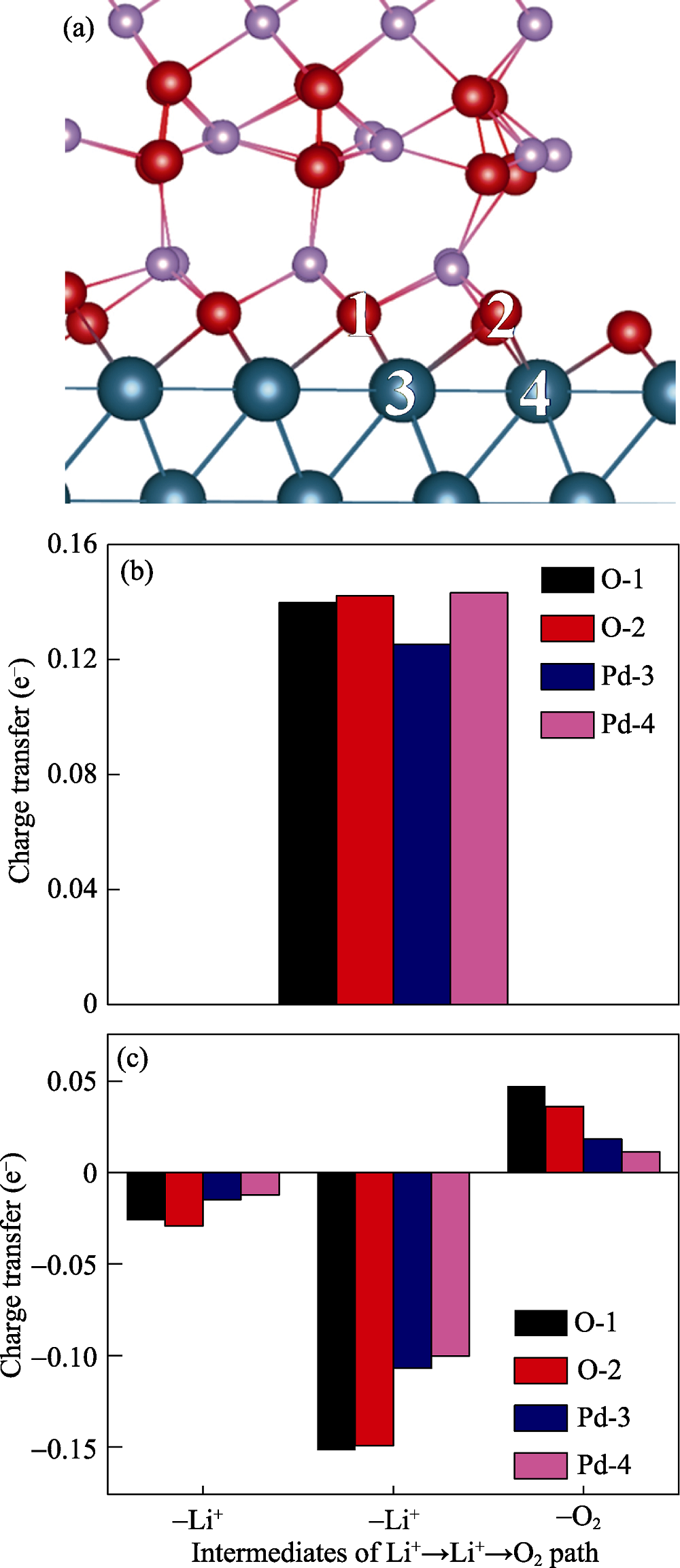
Fig. 3 (a) Calculated structure of Pd(111)/ Li2O2/O2 interface with red ball indicating O, blue ball indicating Pd, and purple ball indicating Li; (b) Bader charge analysis of O and Pd with oxygen reduction reaction of Li2O2, the calculated O atoms and Pd atoms in charge transfer analysis correspond to labeled O atoms and Pd atoms in figure (a); (c) Bader charge analysis of O and Pd with the oxygen evolution reaction of Li2O2, the calculated O atoms and Pd atoms in charge transfer analysis correspond to labeled O atoms and Pd atoms in figure (a)
| [1] | PENG Z Q, FREUNBERGER S A, CHEN Y H, et al. A reversible and higher-rate LiO2 battery. Science, 2012, 337(6094): 563-566. |
| [2] | GIRISHKUMAR G, MCCLOSKEY B, LUNTZ A C, et al. Lithium- air battery: promise and challenges. Journal of Physical Chemistry Letters, 2010, 1(14): 2193-2203. |
| [3] | SHAO Y Y, DING F, XIAO J et al. Making Li-air batteries rechargeable: material challenges. Advanced Functional Materials, 2013, 23(8): 987-1004. |
| [4] | LI F J, ZHANG T, ZHOU H S.Challenges of non-aqueous Li-O2 batteries: electrolytes, catalysts, and anodes.Energy & Environmental Science, 2013, 6(4): 1125-1141. |
| [5] | CHRISTENSEN J, ALBERTUS P, SANCHEZ-CARRERA R S, et a. A critical review of Li/Air batteries. Journal of the Electrochemical Society, 2012, 159(2): R1-R30. |
| [6] | JUNG H G, HASSOUN J, PARK J B, et al. An improved high-performance lithium-air battery. Nature Chemistry, 2012, 4(7): 579-585. |
| [7] | CHENG H, SCOTT K.Selection of oxygen reduction catalysts for rechargeable lithium-air batteries-metal or oxide?Applied Catalysis B-Environmental, 2011, 108(1/2): 140-151. |
| [8] | WANG L, ZHAO X, LU Y H, et al. CoMn2O4 spinel nanoparticles grown on graphene as bifunctional catalyst for lithium-air batteries. Journal of the Electrochemical Society, 2011, 158(12): A1379-A1382. |
| [9] | YANG W, SALIM J, LI S A, et al. Perovskite Sr0.95Ce0.05CoO3-δ loaded with copper nanoparticles as a bifunctional catalyst for lithium-air batteries. Journal of Materials Chemistry, 2012, 22(36): 18902-18907. |
| [10] | DEBART A, BAO J, ARMSTRONG G, et al. An O2 cathode for rechargeable lithium batteries: the effect of a catalyst. Journal of Power Sources, 2007, 174(2): 1177-1182. |
| [11] | DEBART A, PATERSON A J, BAO J, et al. α-MnO2 nanowires: a catalyst for the O2 electrode in rechargeable lithium batteries. Angewandte Chemie-International Edition, 2008, 47(24): 4521-4524. |
| [12] | THAPA A K, SAIMEN K, ISHIHARA T.Pd/MnO2 air electrode catalyst for rechargeable lithium/air battery.Electrochemical and Solid State Letters, 2010, 13(11): A165-A167. |
| [13] | LU Y C, XU Z C, GASTEIGER H A,et al. Platinum-gold nanoparticles: a highly active bifunctional electrocatalyst for rechargeable lithium-air batteries. Journal of the American Chemical Society, 2010, 132(35): 12170-12171. |
| [14] | LI P F, ZHANG J K, YU Q L, et al. One-dimensional porous La0.5Sr0.5CoO2. 91 nanotubes as a highly efficient electrocatalyst for rechargeable lithium-oxygen batteries. Electrochimica Acta, 2015, 165: 78-84. |
| [15] | KALUBARME R S, PARK G E, JUNG K N, et al. LaNixCo1-xO3-δ perovskites as catalyst material for non-aqueous lithium-oxygen batteries. Journal of the Electrochemical Society, 2014, 161(6): A880-A889. |
| [16] | SUN N, LIU H X, YU Z Y, et al. The La0.6Sr0.4CoO3 perovskite catalyst for Li-O2 battery. Solid State Ionics, 2014, 268: 125-130. |
| [17] | ZHONG L, MITCHELL R R, LIU Y, et al. In situ transmission electron microscopy observations of electrochemical oxidation of Li2O2. Nano Letters, 2013, 13(5): 2209-2214. |
| [18] | LEI Y, LU J, LUO X, et al. Synthesis of porous carbon supported palladium nanoparticle catalysts by atomic layer deposition: application for rechargeable lithium-O2 battery. Nano Letters, 2013, 13(9): 4182-4189. |
| [19] | MA S, WU Y, WANG J, et al. Reversibility of noble metal- catalyzed aprotic Li-O2 batteries. Nano Letters, 2015, 15(12): 8084-8090. |
| [20] | KRESSE G, FURTHMULLER J.Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science, 1996, 6(1): 15-50. |
| [21] | KRESSE G, FURTHMüLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B, 1996, 54(16): 11169-11186. |
| [22] | SETYAWAN W, CURTAROLO S.High-throughput electronic band structure calculations: challenges and tools.Computational Materials Science, 2010, 49(2): 299-312. |
| [23] | REUTER K, SCHEFFLER M.Composition, structure,stability of RuO2 (110) as a function of oxygen pressure. Physical Review B, 2001, 65(3): 035406-1-11. |
| [24] | REUTER K, SCHEFFLER M.Composition and structure of the RuO2 (110) surface in an O2 and CO environment: implications for the catalytic formation of CO2. Physical Review B, 2003, 68(4): 045407-1-11. |
| [25] | REUTER K, SCHEFFLER M. First-principles atomistic thermodynamics for oxidation catalysis: surface phase diagrams and catalytically interesting regions. Physical Review Letters, 2003, 90(4): 046103-1-4. |
| [26] | ZHANG W, SMITH J R, WANG X G. Thermodynamics from ab initio computations. Physical Review B, 2004, 70(2): 024103-1-8. |
| [27] | MO Y, ONG S P, CEDER G. First-principles study of the oxygen evolution reaction of lithium peroxide in the lithium-air battery. Physical Review B, 2011, 84(20): 205446-1-9. |
| [28] | WEAVER J F, CHEN J J, GERRARD A L.Oxidation of Pt(111) by gas-phase oxygen atoms.Surface Science, 2005, 592(1/2/3): 83-103. |
| [29] | PHATAK A A, DELGASS W N, RIBEIRO F H, et al Density functional theory comparison of water dissociation steps on Cu, Au, Ni, Pd, and Pt. The Journal of Physical Chemistry C, 2009, 113(17): 7269-7276. |
| [30] | WU C, SCHMIDT D J, WOLVERTON C,et al. Accurate coverage-dependence incorporated into first-principles kinetic models: catalytic NO oxidation on Pt(111). Journal of Catalysis, 2012, 286: 88-94. |
| [31] | TODOROVA M, REUTER K, SCHEFFLER M. Density- functional theory study of the initial oxygen incorporation in Pd (111). Physical Review B, 2005, 71(19): 195403-1-8. |
| [32] | REN X, ZHU J, DU F, et al. B-doped graphene as catalyst to improve charge rate of lithium air battery. Journal of Physical Chemistry C, 2014, 118(39): 22412-22418. |
| [1] | WANG Xiaobo, ZHU Yuliang, XUE Wenchao, SHI Ruchuan, LUO Bofeng, LUO Chengtao. Effect of PbTiO3 Content Variation on High-power Performance of PMN-PT Single Crystal [J]. Journal of Inorganic Materials, 2025, 40(7): 840-846. |
| [2] | TANG Xinli, DING Ziyou, CHEN Junrui, ZHAO Gang, HAN Yingchao. In vivo Distribution and Metabolism of Calcium Phosphate Nanomaterials Based on Fluorescent Labeling with Rare Earth Europium Ions [J]. Journal of Inorganic Materials, 2025, 40(7): 754-764. |
| [3] | YU Leyangyang, ZHAO Fangxia, ZHANG Shuxin, XU Yixiang, NIU Yaran, ZHANG Zhenzhong, ZHENG Xuebin. Preparation of High-entropy Boride Powders for Plasma Spraying by Inductive Plasma Spheroidization [J]. Journal of Inorganic Materials, 2025, 40(7): 808-816. |
| [4] | YANG Guang, ZHANG Nan, CHEN Shujin, WANG Yi, XIE An, YAN Yujie. WO3 Films Based on Porous ITO Electrodes: Preparation and Electrochromic Property [J]. Journal of Inorganic Materials, 2025, 40(7): 781-789. |
| [5] | SUN Jing, LI Xiang, MAO Xiaojian, ZHANG Jian, WANG Shiwei. Effect of Lauric Acid Modifier on the Hydrolysis Resistance of Aluminum Nitride Powders [J]. Journal of Inorganic Materials, 2025, 40(7): 826-832. |
| [6] | CHAI Runyu, ZHANG Zhen, WANG Menglong, XIA Changrong. Preparation of Ceria Based Metal-supported Solid Oxide Fuel Cells by Direct Assembly Method [J]. Journal of Inorganic Materials, 2025, 40(7): 765-771. |
| [7] | WANG Lujie, ZHANG Yuxin, LI Tongyang, YU Yuan, REN Pengwei, WANG Jianzhang, TANG Huaguo, YAO Xiumin, HUANG Yihua, LIU Xuejian, QIAO Zhuhui. Corrosion and Wear Behavior of Silicon Carbide Ceramic in Deep-sea Service Environment [J]. Journal of Inorganic Materials, 2025, 40(7): 799-807. |
| [8] | LI Wenyuan, XU Jianan, DENG Han'ao, CHANG Aimin, ZHANG Bo. Effect of V5+ Substitution on Microstructure and Microwave Dielectric Properties of LaTaO4 Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 697-703. |
| [9] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [10] | DONG Chenyu, ZHENG Weijie, MA Yifan, ZHENG Chunyan, WEN Zheng. Characterizations by Piezoresponse Force Microscopy on Relaxor Properties of Pb(Mg,Nb)O3-PbTiO3 Ultra-thin Films [J]. Journal of Inorganic Materials, 2025, 40(6): 675-682. |
| [11] | HE Guoqiang, ZHANG Kaiheng, WANG Zhentao, BAO Jian, XI Zhaochen, FANG Zhen, WANG Changhao, WANG Wei, WANG Xin, JIANG Jiapei, LI Xiangkun, ZHOU Di. Ba(Nd1/2Nb1/2)O3: Au Underrated K40 Microwave Dielectric Ceramic [J]. Journal of Inorganic Materials, 2025, 40(6): 639-646. |
| [12] | ZHANG Jiawei, CHEN Ning, CHENG Yuan, WANG Bo, ZHU Jianguo, JIN Cheng. Electrical Properties of Bismuth Layered Piezoelectric Bi4Ti3O12 Ceramics with A/B-site Doping [J]. Journal of Inorganic Materials, 2025, 40(6): 690-696. |
| [13] | CUI Ning, ZHANG Yuxin, WANG Lujie, LI Tongyang, YU Yuan, TANG Huaguo, QIAO Zhuhui. Single-phase Formation Process and Carbon Vacancy Regulation of (TiVNbMoW)Cx High-entropy Ceramics [J]. Journal of Inorganic Materials, 2025, 40(5): 511-520. |
| [14] | XIONG Siyu, MO Chen, ZHU Xiaowei, ZHU Guobin, CHEN Deqin, LIU Laijun, SHI Xiaodong, LI Chunchun. Low-temperature Sintering of LiBxAl1-xSi2O6 Microwave Dielectric Ceramics with Ultra-low Permittivity [J]. Journal of Inorganic Materials, 2025, 40(5): 536-544. |
| [15] | AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao. Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance [J]. Journal of Inorganic Materials, 2025, 40(5): 457-465. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||