
Journal of Inorganic Materials ›› 2017, Vol. 32 ›› Issue (7): 681-690.DOI: 10.15541/jim20160484
• Orginal Article • Previous Articles Next Articles
JIANG Jiu-Xin1,2, WU Yue1, HE Yao1, GAO Song1, ZHANG Chen1, SHEN Tong3, LIU Jia-Ning3,4
Received:2016-08-29
Revised:2016-10-29
Published:2017-07-20
Online:2017-06-23
About author:JIANG Jiu-Xin. E-mail: jiuxinjiang@hotmail.com
Supported by:CLC Number:
JIANG Jiu-Xin, WU Yue, HE Yao, GAO Song, ZHANG Chen, SHEN Tong, LIU Jia-Ning. Progress in Tuning of Metastable Vaterite Calcium Carbonate[J]. Journal of Inorganic Materials, 2017, 32(7): 681-690.
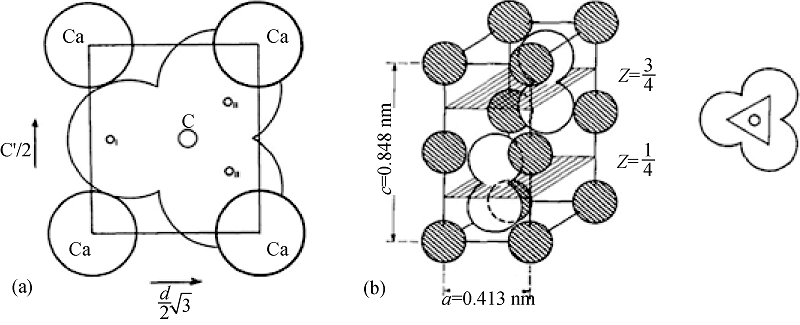
Fig. 1 (a) Vertical projection of vaterite showing orientation of carbonate group relative to calcium atoms[10]; (b) Structure of the vaterite unit cell[11]
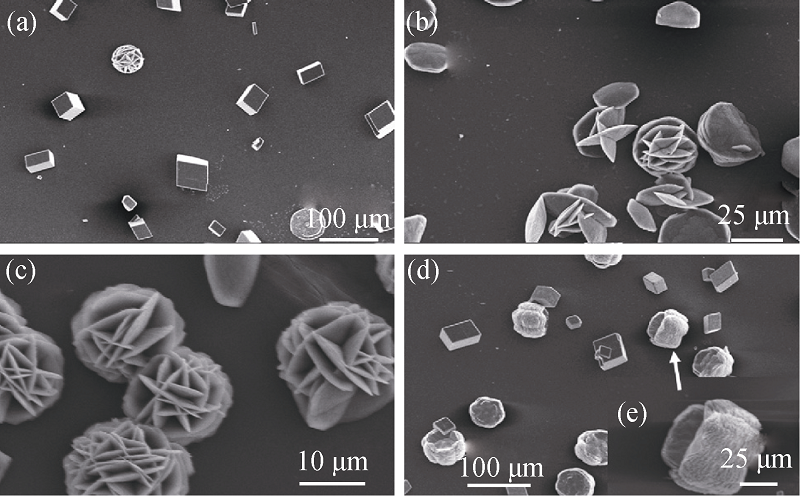
Fig. 3 SEM images of CaCO3 particles in different concentration ratios between p-aminobenzene sulfonic acid anhydrous and l-Lys solutions [29] (a) 0.1 g/L:0.1 g/L; (b) 0.1 g/L:0.3 g/L; (c) 0.1 g/L:0.5 g/L; (d) 0.5 g/L:0.1 g/L; (e) High magnification of selective area of (d)
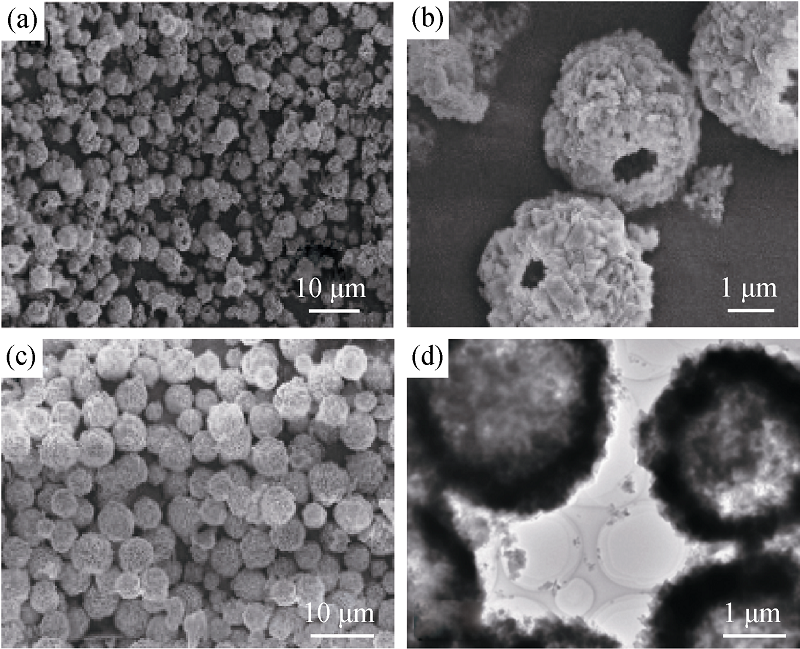
Fig. 4 SEM images of CaCO3 hollow spheres obtained in the presence of PEG10000-SDS[35](a) Low magnification image, (b) high and (c) middle magnification SEM images; and (d) TEM image of CaCO3 hollow spheres obtained in the presence of PEG2000-SDS

Fig. 5 SEM images of CaCO3 prepared at different surfactant concentrations [38](a) 0.1 mol/L; (b) Magnification of (a); (c) 0.07 mol/L; (d) 0.04 mol/L

Fig. 6 SEM images showing sponge-like vaterite spheroids prepared by evaporation[41] from water-in-oil supersaturated microemulsions with compositions of (a) octane︰SDS︰CaHCO3 =71︰4︰25(wt%); (b) octane︰dodecanol︰SDS︰CaHCO3 = 70.8︰0.7︰3.5︰25(wt%); (c) schematic diagram showing the mechanism for the formation of vaterite microsponges in water-in-oil microemulsions
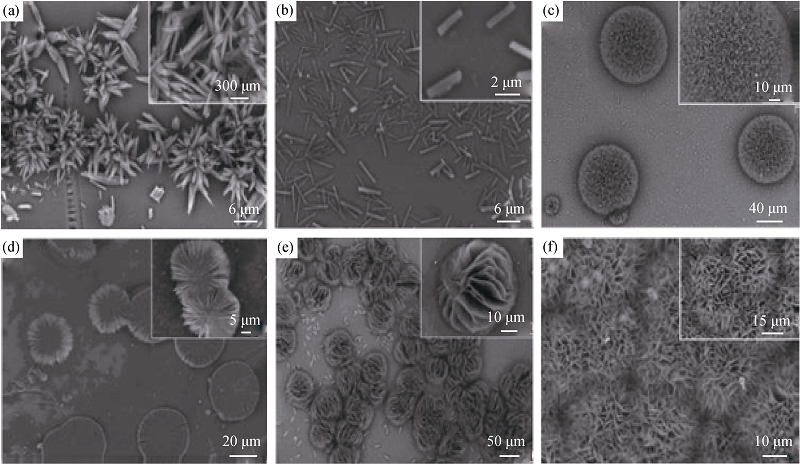
Fig. 7 SEM images of CaCO3 where (a-c) and (d-f) with 10 mL and 20 mL of silk fibroin, respectively, affter being added Mg2+ at the concentration of (a) 10; (b) 20; (c) 50; (d) 10; (e) 20; (f) 50 mmol/L[42]
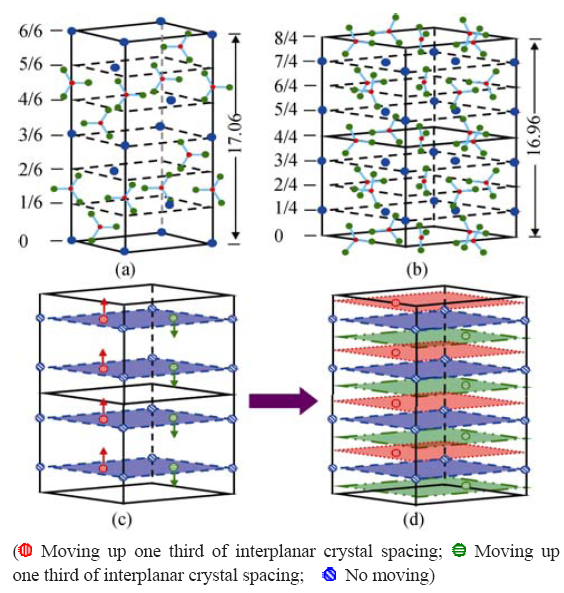
Fig. 11 Atomic structure of (a) calcite and (b) vaterite, (c) Ca2+ ions stacking in vaterite and the motion direction in the (0001) faces under agitation, and (d) Ca2+ ions stacking in the (0001) faces after motion[59]
| [1] | CUI Z, CUI C, ZHU Y, et al.Multiple phase inversion of emulsions stabilized by in situ surface activation of CaCO3 nanoparticles via adsorption of fatty acids.Langmuir, 2011, 28(1): 314-320. |
| [2] | LIU S. Stabilized Vaterite.U.S. Patent Application, US20030717 310. 2003.11.19. |
| [3] | DEVENNEY M, FERNANDEZ M, MORGAN S.Non-cementitious Compositions Comprising Vaterite and Methods Thereof. U.S. Patent Application, US201313804558. 2013.3.14. |
| [4] | TAS A.Use of vaterite and calcite in forming calcium phosphate cement scaffolds.Ceram. Eng. Sci. Proc., 2009, 28(9): 135-150. |
| [5] | OHGUSHI H, OKUMURA M, YOSHIKAWA T, et al.Bone formation processin porous calcium carbonate and hydroxyapatite.J. Biomed. Mater. Res., 1992, 26(7): 885-895. |
| [6] | BUKREEVA T, MARCHENKO I, BORODINA T, et al.Calcium carbonate and titanium dioxide particles as a basis for container fabrication for brain delivery of compounds.Dokl. Phys. Chem., 2011, 440(1): 165-167. |
| [7] | LI Q, DING Y, LI F, et al.Solvothermal growth of vaterite in the presence of ethylene glycol, 1, 2-propanediol and glycerin. J. Cryst. Growth, 2002, 236(1): 357-362. |
| [8] | FLATEN E, SEIERSTEN M, ANDREASSEN J.Polymorphism and morphology of calcium carbonate precipitated in mixed solvents of ethylene glycol and water.J. Cryst. Growth, 2009, 311(13): 3533-3538. |
| [9] | DUPONT L, PORTEMER F.Synthesis and study of a well crystallized CaCO3 vaterite showing a new habitus.J. Mater. Chem., 1997, 7(5): 797-800. |
| [10] | KAMHI S.On the structure of vaterite CaCO3.Acta Crystallogr., 1963, 16(8): 770-772. |
| [11] | MEYER H.Struktur und fehlordnung des vaterits.Z. Kristallogr., 1969, 128(1-6): 183-212. |
| [12] | DEMICHELIS R, RAITERI P, GALE J, et al.A new structural model for disorder in vaterite from first-principles calculations.CrystEngComm, 2012, 14(1): 44-47. |
| [13] | DEMICHELIS R, RAITERI P, GALE J, et al.The multiple structures of vaterite.Cryst. Growth Des., 2013, 13(6): 2247-2251. |
| [14] | NAKA K, TANAKA Y, CHUJO Y.Effect of anionic starburst dendrimers on the crystallization of CaCO3 in aqueous solution: size control of spherical vaterite particles.Langmuir, 2002, 18(9): 3655-3658. |
| [15] | IMAI H, TOCHIMOTO N, NISHINO Y, et al.Oriented nanocrystal mosaic in monodispersed CaCO3 microspheres with functional organic molecules.Cryst. Growth Des., 2012, 12(2): 876-882. |
| [16] | YU S, CÖLFEN H, ANTONIETTI M. Polymer-controlled morphosynthesis and mineralization of metal carbonate superstructures.J. Phys. Chem. B, 2003, 107(30): 7396-7405. |
| [17] | NEHRKE G, VAN CAPPELLEN P.Framboidal vaterite aggregates and their transformation into calcite: a morphological study.J. Cryst. Growth, 2006, 287(2): 528-530. |
| [18] | CÖLFEN H, QI L. A systematic examination of the morphogenesis of calcium carbonate in the presence of a double-hydrophilic block copolymer.Chem.-Eur. J., 2001, 7(1): 106-116. |
| [19] | HU Q, ZHANG J, TENG H, et al.Growth process and crystallographic properties of ammonia-induced vaterite.Am. Mineral., 2012, 97(8/9): 1437-1445. |
| [20] | FRICKE M, VOLKMER D, KRILL C, et al.Vaterite polymorph switching controlled by surface charge density of an amphiphilic dendron-calix [4] arene.Cryst. Growth Des., 2006, 6(5): 1120-1123. |
| [21] | GEHRKE N, CÖLFEN H, PINNA N, et al. Superstructures of calcium carbonate crystals by oriented attachment.Cryst. Growth Des., 2005, 5(4): 1317-1319. |
| [22] | QI L, LI J, MA J.Biomimetic morphogenesis of calcium carbonate in mixed solutions of surfactants and double-hydrophilic block copolymers.Adv. Mater., 2002, 14(4): 300-303. |
| [23] | MUGNAIOLI E, ANDRUSENKO I, SCHÜLER T, et al. Ab initio structure determination of vaterite by automated electron diffraction.Angew. Chem. Int. Ed., 2012, 51(28): 7041-7045. |
| [24] | WEI H, MA N, SONG B, et al.Formation of multilayered vaterite via phase separation, crystalline transformation, and self-assembly of nanoparticles at the air/water interface.J. Phys. Chem. C, 2007, 111(15): 5628-5632. |
| [25] | WEI H, SHEN Q, ZHAO Y, et al.Influence of polyvinylpyrrolidone on the precipitation of calcium carbonate and on the transformation of vaterite to calcite.J. Cryst. Growth, 2003, 250(3): 516-524. |
| [26] | POLITI Y, ARAD T, KLEIN E, et al.Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase.Science, 2004, 306(5699): 1161-1164. |
| [27] | 章峻, 包富荣, 戴冬萍, 等. 马来酸酐 (MAH) 表面改性纳米碳酸钙粉体的制备及表面性能. 无机化学学报, 2007, 23(5): 822-826. |
| [28] | KONTREC J, KRALJ D, BREČEVIĆ L, et al. Influence of some polysaccharides on the production of calcium carbonate filler particles.J. Cryst. Growth, 2008, 310(21): 4554-4560. |
| [29] | ZHANG Q, REN L, SHENG Y, et al.Control of morphologies and polymorphs of CaCO3 via multi-additives system.Mater. Chem. Phys., 2010, 122(1): 156-163. |
| [30] | 汪小红, 张群, 董晓庆, 等. 超声辅助的荔枝状球霰石的制备与表征. 人工晶体学报, 2013, 42(10): 2164-2169. |
| [31] | YANG YA-NAN, ZHU XIAO-LI, KONG XIANG-ZHENG.Controls of crystal morphology, size and structure in spontaneous preparation of calcium carbonate.Journal of Inorganic Materials, 2013, 28(12): 1313-1320. |
| [32] | RAMESH T, INCHARA S A, PALLAVI K.Para-amino benzoic acid-mediated synthesis of vaterite phase of calcium carbonate.J. Chem. Sci., 2015, 127(5): 843-848. |
| [33] | 夏宏宇, 张群, 王刚, 等. 球形和橄榄形球霰石的简易制备研究. 人工晶体学报, 2015, 44(6): 1701-1706. |
| [34] | ZOU JIAN-PENG, YANG HONG-ZHI, XIAO-PING, et al.Controllable fabrication of calcium carbonate hollow microspheres with micro-nano hierarchical structure.Journal of Inorganic Materials, 2016, 31(7): 711-718. |
| [35] | JI X, LI G, HUANG X.The synthesis of hollow CaCO3 microspheres in mixed solutions of surfactant and polymer. Mater. Lett., 2008, 62(4): 751-754. |
| [36] | 徐国峰, 王洁欣, 沈志刚, 等. 单分散纳米碳酸钙的制备和表征. 北京化工大学学报(自然科学版), 2009, 36(5): 27-30. |
| [37] | KANG S, HIRASAWA I, KIM W, et al.Morphological control of calcium carbonate crystallized in reverse micelle system with anionic surfactants SDS and AOT.J. Colloid. Interf. Sci. 2005, 288(2): 496-502. |
| [38] | JIANG J, MA Y, ZHANG T, et al.Morphology and size control of calcium carbonate crystallized in a reverse micelle system with switchable surfactants.RSC Adv., 2015, 5: 80216-80219. |
| [39] | 陈银霞, 纪献兵, 赵改青, 等. 低温溶剂热法合成圆饼状球霰石碳酸钙. 材料导报, 2010, 24(12): 99-102. |
| [40] | 陈先勇, 唐琴, 刘代俊. 独特形貌碳酸钙的微波水热合成与表征. 功能材料, 2012, 43(9): 1109-1112. |
| [41] | WALSH D, LEBEAU B, MANN S.Morphosynthesis of calcium carbonate (vaterite) microsponges.Adv. Mater., 1999, 11(4): 324-328. |
| [42] | AN X, CAO C.Biomineralization of CaCO3 through the cooperative interactions between multiple additives and self-assembled monolayers.J. Phys. Chem. C, 2008, 112(16): 6526-6530. |
| [43] | YANG B, NAN Z.Abnormal polymorph conversion of calcium carbonate from calcite to vaterite.Mater. Res. Bull., 2012, 47(3): 521-526. |
| [44] | YANG D, YU K, AI Y, et al.The mineralization of electrospun chitosan/poly (vinyl alcohol) nanofibrous membranes.Carbohydr. Polym., 2011, 84(3): 990-996. |
| [45] | RAUTARAY D, AHMAD A, SASTRY M.Biosynthesis of CaCO3 crystals of complex morphology using a fungus and an actinomycete.J. Am. Chem. Soc., 2003, 125(48): 14656-14657. |
| [46] | BEUVIER T, CALVIGNAC B, DELCROIX G, et al.Synthesis of hollow vaterite CaCO3 microspheres in supercritical carbon dioxide medium. J. Mater. Chem., 2011, 21(26): 9757-9761. |
| [47] | 潘晓芳, 王海水. 乙醇/水混合溶剂体系中碳酸钙晶体晶型和取向的控制. 无机化学学报, 2014, 30(6): 1312-1316. |
| [48] | 韩金鑫, 连宾, 唐源, 等. 恶臭假单胞菌对碳酸钙的诱导矿化作用. 南京大学学报 (自然科学版), 2013, 49(6): 681-688. |
| [49] | 马晓明, 杨媛媛, 张晓婷, 等. 大豆胰岛素指导下具有分级结构碳酸钙的仿生合成. 河南师范大学学报(自然科学版), 2014, 42(2): 64-68. |
| [50] | 黄玉刚, 褚日环, 何明辉, 等. 富含羧基的多肽基双亲水杂化共聚物控制碳酸钙的形成. 中山大学学报(自然科学版), 2014, 53(3): 73-79. |
| [51] | GUO Y, WANG F, ZHANG J, et al.Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids.Res. Chem. Intermed., 2013, 39(6): 2407-2415. |
| [52] | 任丽英, 张群, 朱万华, 等. 仿生合成碳酸钙微环. 人工晶体学报, 2015, 44(1): 250-255. |
| [53] | LIU L, JIANG J, YU S.Polymorph selection and structure evolution of CaCO3 mesocrystals under control of poly (sodium 4-styrenesulfonate): synergetic effect of temperature and mixed solvent.Cryst. Growth Des., 2014, 14(11): 6048-6056. |
| [54] | 陈晓东, 辛梅华, 李明春, 等. N-琥珀酰基-O-羟丙基磺酸壳聚糖仿生合成球霰石碳酸钙. 材料研究学报, 2016, 30(1): 31-37. |
| [55] | 朱文杰, 蔡春华, 林嘉平. 碳酸钙在聚合物胶束控制下的仿生合成. 高分子学报, 2011, 4: 335-339. |
| [56] | 汪玉瑛. 生物成因碳酸钙矿化机制的仿生实验研究. 合肥: 中国科学技术大学博士学位论文, 2015. |
| [57] | 张群, 张清. 不同晶型碳酸钙的仿生矿化研究. 硅酸盐通报, 2014, 33(5): 1236-1240. |
| [58] | JIANG J, YE J, ZHANG G, et al.Polymorph and morphology control of CaCO3 via temperature and PEG during the decomposition of Ca(HCO3)2.J. Am. Ceram. Soc., 2012, 95(12): 3735-3738. |
| [59] | JIANG J, ZHANG Y, XU D, et al.Can agitation determine the polymorphs of calcium carbonate during the decomposition of calcium bicarbonate?CrystEngComm, 2014, 16(24): 5221-5226. |
| [60] | ZENG H, YAN Z, JIAO M, et al.A novel method for preparing calcium carbonate particles: thermal decomposition from calcium hydrogen carbonate solution.Key Eng. Mater., 2016, 697: 113-118. |
| [61] | TRUSHINA D, BUKREEVA T, KOVALCHUK M, et al.CaCO3 vaterite microparticles for biomedical and personal care applications.Mater. Sci. Eng., C, 2014, 45: 644-658. |
| [62] | RODRIGUEZ-NAVARRO C, JIMENEZ-LOPEZ C, RODRIGUEZ-NAVARRO A, et al.Bacterially mediated mineralization of vaterite.Geochim. Cosmochim. Acta, 2007, 71(5): 1197-1213. |
| [63] | WANG X, WU C, TAO K, et al.Influence of ovalbumin on CaCO3 precipitation during in vitro biomineralization.J. Phys. Chem. B, 2010, 114(16): 5301-5308. |
| [64] | DONNERS J, HEYWOOD B, MEIJER E, et al.Control over calcium carbonate phase formation by dendrimer/surfactant templates.Chem.-Eur. J., 2002, 8(11): 2561-2567. |
| [65] | PARAKHONSKIY B, HAASE A, ANTOLINI R.Sub-micrometer vaterite containers: synthesis, substance loading, and release.Angew. Chem., Int. Edit., 2012, 51(5): 1195-1197. |
| [66] | SAND K, RODRIGUEZ-BLANCO J, MAKOVICKY E, et al.Crystallization of CaCO3 in water-alcohol mixtures: spherulitic growth, polymorph stabilization, and morphology change.Cryst. Growth Des., 2012, 12(2): 842-853. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [8] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [9] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [10] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [11] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [12] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [13] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [14] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| [15] | ZHOU Fan, TIAN Zhilin, LI Bin. Research Progress on Carbide Ultra-high Temperature Ceramic Anti-ablation Coatings for Thermal Protection System [J]. Journal of Inorganic Materials, 2025, 40(1): 1-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||