
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (10): 1117-1122.DOI: 10.15541/jim20190588
Special Issue: 计算材料论文精选(2020)
Previous Articles Next Articles
LIN Qimin1( ),CUI Jiangong2,YAN Xin1,YUAN Xueguang1(
),CUI Jiangong2,YAN Xin1,YUAN Xueguang1( ),CHEN Xiaoyu1,LU Qichao1,LUO Yanbin1,HUANG Xue3,ZHANG Xia1(
),CHEN Xiaoyu1,LU Qichao1,LUO Yanbin1,HUANG Xue3,ZHANG Xia1( ),REN Xiaomin1
),REN Xiaomin1
Received:2019-11-20
Revised:2019-12-09
Published:2020-10-20
Online:2020-01-20
About author:LIN Qimin, male, PhD candidate. E-mail: lqm@bupt.edu.cn
Supported by:CLC Number:
LIN Qimin, CUI Jiangong, YAN Xin, YUAN Xueguang, CHEN Xiaoyu, LU Qichao, LUO Yanbin, HUANG Xue, ZHANG Xia, REN Xiaomin. First-principles Study on Electronic Structure and Optical Properties of Single Point Defect Graphene Oxide[J]. Journal of Inorganic Materials, 2020, 35(10): 1117-1122.
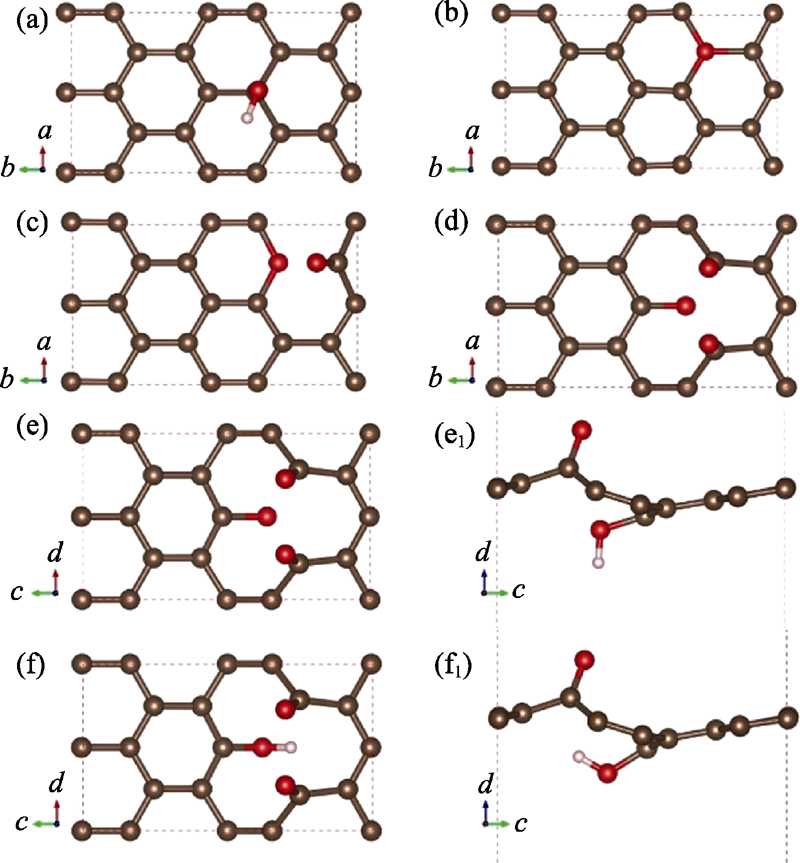
Fig. 1 Different types of graphene oxide structures (a) Graphene oxide adsorbed with hydroxyl; (b) Graphene oxide with single substitution epoxy bond; (c) Graphene oxide with carbon oxygen double bond and sp3 hybrid epoxy bond; (d) Graphene oxide with two carbon-oxygen double bonds and one carbon-oxygen single bond; (e, f) and (e1, f1) Top and side views of the structure in (d) adsorbed with hydrogen on the upper and lower side of the suspended oxygen atom, respectively
| * | O1-C2 | O2-C6 | O3-C12 | |
|---|---|---|---|---|
| LDA | d1 | 0.128 | 0.128 | 0.137 |
| d2 | 0.128 | 0.128 | 0.136 | |
| e | 0.124 | 0.124 | 0.138 | |
| f | 0.124 | 0.124 | 0.137 | |
| GGA | d | 0.124 | 0.124 | 0.133 |
| e | 0.122 | 0.122 | 0.139 | |
| f | 0.122 | 0.122 | 0.138 |
Table 1 Bond length in different structures calculated by different pseudopotential functions (nm)
| * | O1-C2 | O2-C6 | O3-C12 | |
|---|---|---|---|---|
| LDA | d1 | 0.128 | 0.128 | 0.137 |
| d2 | 0.128 | 0.128 | 0.136 | |
| e | 0.124 | 0.124 | 0.138 | |
| f | 0.124 | 0.124 | 0.137 | |
| GGA | d | 0.124 | 0.124 | 0.133 |
| e | 0.122 | 0.122 | 0.139 | |
| f | 0.122 | 0.122 | 0.138 |
| * | (a) | (b) | (c) | (d) | (e) | (f) |
|---|---|---|---|---|---|---|
| LDA | -12.7 | -6.1 | -6.5 | -13.1 | -19.6 | -18.6 |
Table 2 Formation energy with different structures (eV)
| * | (a) | (b) | (c) | (d) | (e) | (f) |
|---|---|---|---|---|---|---|
| LDA | -12.7 | -6.1 | -6.5 | -13.1 | -19.6 | -18.6 |
| * | C11 | C22 | C12 | C66 |
|---|---|---|---|---|
| a | 1738.99 | 1618.45 | 282.75 | 1.03 |
| b | 1789.57 | 1761.01 | 309.15 | 1.63 |
| c | 1607.47 | 958.73 | 163.94 | 0.99 |
| d | 2015.11 | 1405.41 | 147.00 | 1.39 |
| e | 1968.65 | 825.43 | 148.48 | -11.90 |
| f | 1846.90 | 710.38 | 240.54 | -8.83 |
Table 3 Elastic coefficients of graphene oxide with different structures
| * | C11 | C22 | C12 | C66 |
|---|---|---|---|---|
| a | 1738.99 | 1618.45 | 282.75 | 1.03 |
| b | 1789.57 | 1761.01 | 309.15 | 1.63 |
| c | 1607.47 | 958.73 | 163.94 | 0.99 |
| d | 2015.11 | 1405.41 | 147.00 | 1.39 |
| e | 1968.65 | 825.43 | 148.48 | -11.90 |
| f | 1846.90 | 710.38 | 240.54 | -8.83 |
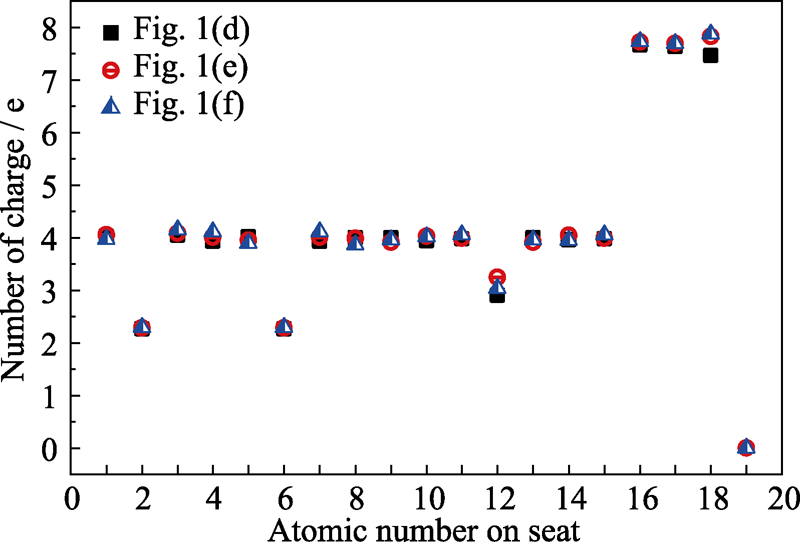
Fig. 2 The charge number of three kinds of different atoms in three structures showing in Fig. 1(d), (e) and (f) (d) Graphene oxide with two carbon-oxygen double bonds and one carbon-oxygen single bond; (e, f) Structure (d) adsorbed with hydrogen on the upper and lower side of the suspended oxygen atom, respectively
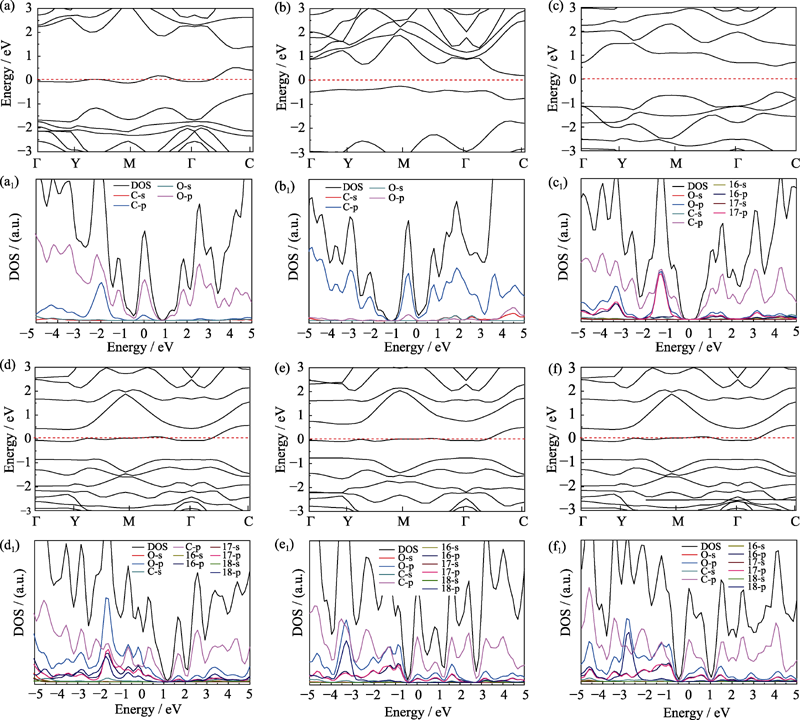
Fig. 3 Band structures and density of states (DOS) of different structure models (a) Graphene oxide adsorbed with hydroxyl; (b) Graphene oxide with single substitution epoxy bond; (c) Graphene oxide with carbon oxygen double bond and sp3 hybrid epoxy bond; (d) Graphene oxide with two carbon-oxygen double bonds and one carbon-oxygen single bond; (e,f) Structure (d) adsorbed with hydrogen on the upper and lower side of the suspended oxygen atom, respectively
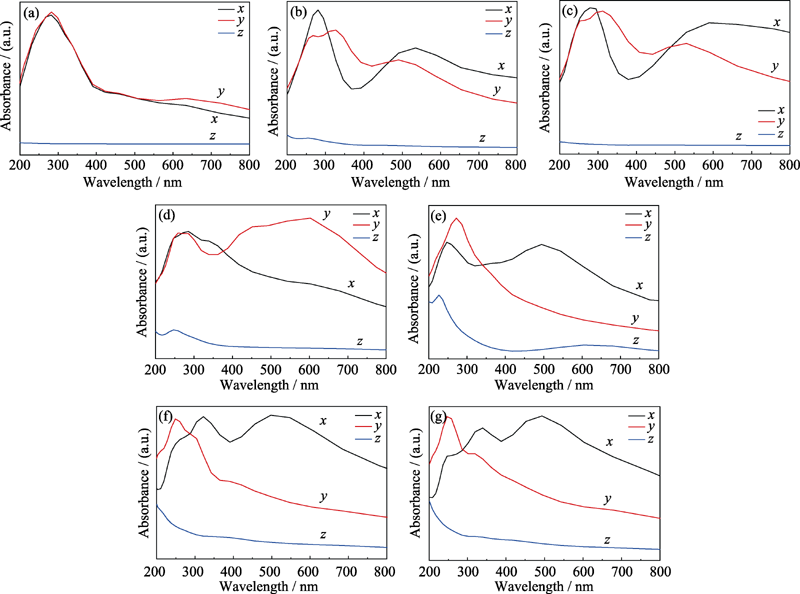
Fig. 4 Absorption coefficient of different structure models in which (b-g) the absorption coefficient of graphene and structures in Fig. 1(a-f) (a) Graphene; (b) Graphene oxide adsorbed with hydroxyl; (c) Graphene oxide with single substitution epoxy bond; (d) Graphene oxide with carbon oxygen double bond and sp3 hybrid epoxy bond; (e) Graphene oxide with two carbon-oxygen double bonds and one carbon-oxygen single bond; (f, g) Structure (d) adsorbed with hydrogen on the upper and lower side of the suspended oxygen atom, respectively
| [1] | YANG K, SHUAI X R, YANG H C , et al. Electrochemical performance of activated graphene powder supercapacitors using a room temperature ionic lLiquid electrolyte. Acta Phys. -Chim. Sin., 2019,35(7):755-765. |
| [2] |
PERES N M R . The Transport properties of graphene. J. Phys. Condens. Matter., 2009,21(32):323201-323210.
DOI URL PMID |
| [3] | CHEN D M . Variation of graphene Raman G peak splitting with strain. Acta. Phys. Sin., 2010,59(9):6399-6404. |
| [4] | WANG Y F, LI X W . First-principle calculation on electronic structures and optical properties of hybrid graphene and BiOI nanosheets. Acta. Phys. Sin., 2018,67(11):168-175. |
| [5] | WANG J J, WANG F, YUAN P F , et al. First-principles study of nanoscale friction between graphenes. Acta Phys. Sin., 2012,61(10):337-343. |
| [6] | JOSHI R K, ALWARAPPAN S, YOSHIMURA M , et al. Graphene oxide: the new membrane material. Applied Materials Today, 2015,1:1-12. |
| [7] |
GAO W, SINGH N, SONG L , et al. Direct laser writing of micro- supercapacitors on hydrated graphite oxide films. Nature Nanotechnology, 6:496-500.
DOI URL PMID |
| [8] | WANG G X, PEI Z B, YE C H , et al. Inkjet-printing and performance investigation of self-powered flexible graphene oxide humidity sensors. Journal of Inorganic Materials , 2019,34(1):114-120. |
| [9] | HUANG J R, WANG L Y, SHI C C , et al. Selective detection of picric acid using functionalized reduced graphene oxide sensor device. Sensors & Actuators B Chemical, 196:567-573. |
| [10] | LI C, CAI L, LI W W , et al. Adsorption of NO2 by hydrazine hydrate-reduced graphene oxide. Acta Phys. Sin., 2019,68(11):257-262. |
| [11] | PENG P, LIU H T, WU B , et al. Nitrogen doped graphene with a p-type field-effect and its fine modulation. Acta Phys. Chim. Sin., 2019,35(11):1282-1290. |
| [12] | CHU C, ZHANG J, BEI Z , et al. Hydrogen adsorption of Mg- doped Graphene oxide: afirst-principles study. Journal of Physical Chemistry C , 2013,117:4337-4344. |
| [13] |
ROGERS G W, LIU J Z . High-performance graphene oxide electromechanical actuators. Journal of the American Chemical Society, 2012,134:1250-1255.
DOI URL PMID |
| [14] | ZHU Y, MURALI S, CAI W , et al. Graphene and graphene oxide: synthesis, properties, and applications. Cheminform, 2010,22:3906-3924. |
| [15] |
KIM S, ZHOU S, HU, Y, et al. Room-temperature metastability of multilayer graphene oxide films. Nature Materials , 2012,11(6):544-549.
DOI URL PMID |
| [16] | ZHAO H, ZHOU L, WEI D , et al. Effects of external electric field on hydrogen storage performance of Li-decorated graphene oxide. Chemical Journal of Chinese Universities, 37(1):100-107. |
| [17] |
LOH K P, BAO Q, EDA G , et al. Graphene oxide as a chemically tunable platform for optical applications. Nature Chemistry, 2:1015-1024.
DOI URL PMID |
| [18] | ZHANG Q, ZHANG H, CHENG X L . Highly stable two-dimensional graphene oxide: electronic properties of its periodic structure and optical properties of its nanostructures. Chinese Physics B, 27(2): 027301-1-7. |
| [19] |
BAE S, KIM H, LEE Y , et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol, 2010,5:574-578.
DOI URL PMID |
| [20] | COMPTON, O C, NGUYEN, S B T. Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small, 2010,6:711-723. |
| [21] | HONG F, ZHOU L Q, HUANG Y , et al. Synthesis and characterization of graphene by improved hummers method. Chemistry & Bioengineering, 2012,29:31-33. |
| [22] |
CAI W W, PINER R D, STADERMANN F J , et al. Synthesis and solid-state NMR structural characterization of 13c-labeled graphite oxide. Science, 2008,321:1815-1817
DOI URL PMID |
| [23] | MO J W, QIU Y W, YI R B , et al. Temperature-dependent properties of metastable graphene oxide. Acta Phys. Sin. , 2019,68(15):284-292. |
| [24] |
YAN J A, XIAN L, CHOU M Y. Structural and electronic properties of oxidized graphene. Phys. Rev. Lett., 2009,103: 086802-1-4.
DOI URL PMID |
| [25] | WANG L, SUN Y Y, LEE K , et al. Stability of graphene oxide phases from firstp calculations. Physical Review B 2010, 82: 161406-1-4. |
| [26] | PENG Y, LI J . Ammonia adsorption on graphene and graphene oxide: a first-principles study. Frontiers of Environmental Science & Engineering, 2013,7:403-411. |
| [27] | ZHANG Y, SHI Y M, BAO Y Z , et al. Effect of surface passivation on the electronic properties of GaAs nanowire: A first-principle study. Acta Phys. Sin. , 2017,66(19):295-301. |
| [28] | YI W C, HU T, SU T , et al. A CNH monolayer: a direct gap 2d semiconductor with anisotropic electronic and optical properties. Journal of Materials Chemistry C , 2017,5:8498-8503. |
| [29] | LIN Q M, ZHANG X, LU Q C , , et al. First-principles study on structural stability of graphene oxide. First-principles study on structural stability of graphene oxide and catalytic activity of nitric acid. Acta Phys. Sin., 2019, 68(24): 247302-1-6. |
| [1] | GAO Chenguang, SUN Xiaoliang, CHEN Jun, LI Daxin, CHEN Qingqing, JIA Dechang, ZHOU Yu. SiBCN-rGO Ceramic Fibers Based on Wet Spinning Technology: Microstructure, Mechanical and Microwave-absorbing Properties [J]. Journal of Inorganic Materials, 2025, 40(3): 290-296. |
| [2] | WANG Yue, WANG Xin, YU Xianli. Room-temperature Ferromagnetic All-carbon Films Based on Reduced Graphene Oxide [J]. Journal of Inorganic Materials, 2025, 40(3): 305-313. |
| [3] | YE Junhao, ZHOU Zhenzhen, HU Chen, WANG Yanbin, JING Yanqiu, LI Tingsong, CHENG Ziqiu, WU Junlin, IVANOV Maxim, HRENIAK Dariusz, LI Jiang. Yb:Sc2O3 Transparent Ceramics Fabricated from Co-precipitated Nano-powders: Microstructure and Optical Property [J]. Journal of Inorganic Materials, 2025, 40(2): 215-224. |
| [4] | LÜ Zhaoyang, XU Yong, YANG Jiuyan, TU Guangsheng, TU Bingtian, WANG Hao. Effect of MgF2 Additive on Preparation and Optical Properties of MgAl1.9Ga0.1O4 Transparent Ceramics [J]. Journal of Inorganic Materials, 2024, 39(5): 531-538. |
| [5] | CHEN Hao, FAN Wenhao, AN Decheng, CHEN Shaoping. Improvement of Thermoelectric Performance of SnTe by Energy Band Optimization and Carrier Regulation [J]. Journal of Inorganic Materials, 2024, 39(3): 306-312. |
| [6] | LI La, SHEN Guozhen. 2D MXenes Based Flexible Photodetectors: Progress and Prospects [J]. Journal of Inorganic Materials, 2024, 39(2): 186-194. |
| [7] | MENG Yuting, WANG Xuemei, ZHANG Shuxian, CHEN Zhiwei, PEI Yanzhong. Single- and Two-band Transport Properties Crossover in Bi2Te3 Based Thermoelectrics [J]. Journal of Inorganic Materials, 2024, 39(11): 1283-1291. |
| [8] | ZHOU Yunkai, DIAO Yaqi, WANG Minglei, ZHANG Yanhui, WANG Limin. First-principles Calculation Study of the Oxidation Resistance of PANI Modified Ti3C2(OH)2 [J]. Journal of Inorganic Materials, 2024, 39(10): 1151-1158. |
| [9] | WU Xiaowei, ZHANG Han, ZENG Biao, MING Chen, SUN Yiyang. Comparison of Hybrid Functionals HSE and PBE0 in Calculating the Defect Properties of CsPbI3 [J]. Journal of Inorganic Materials, 2023, 38(9): 1110-1116. |
| [10] | DONG Yiman, TAN Zhan’ao. Research Progress of Recombination Layers in Two-terminal Tandem Solar Cells Based on Wide Bandgap Perovskite [J]. Journal of Inorganic Materials, 2023, 38(9): 1031-1043. |
| [11] | GU Junyi, FAN Wugang, ZHANG Zhaoquan, YAO Qin, ZHAN Hongquan. Structure and Optical Property of Pr2O3 Powder Prepared by Reduction [J]. Journal of Inorganic Materials, 2023, 38(7): 771-777. |
| [12] | ZHANG Shouchao, CHEN Hongyu, LIU Hongfei, YANG Yu, LI Xin, LIU Defeng. High Temperature Recovery of Neutron Irradiation-induced Swelling and Optical Property of 6H-SiC [J]. Journal of Inorganic Materials, 2023, 38(6): 678-686. |
| [13] | LI Yue, ZHANG Xuliang, JING Fangli, HU Zhanggui, WU Yicheng. Growth and Property of Ce3+-doped La2CaB10O19 Crystal [J]. Journal of Inorganic Materials, 2023, 38(5): 583-588. |
| [14] | YU Ruixian, WANG Guodong, WANG Shouzhi, HU Xiaobo, XU Xiangang, ZHANG Lei. Effect of High-temperature Annealing on AlN Crystal Grown by PVT Method [J]. Journal of Inorganic Materials, 2023, 38(3): 343-349. |
| [15] | LI Wenjun, WANG Hao, TU Bingtian, CHEN Qiangguo, ZHENG Kaiping, WANG Weiming, FU Zhengyi. Preparation and Property of Mg0.9Al2.08O3.97N0.03 Transparent Ceramic with Broad Optical Transmission Range [J]. Journal of Inorganic Materials, 2022, 37(9): 969-975. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||