
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (12): 1365-1370.DOI: 10.15541/jim20180160
• RESEARCH LETTER • Previous Articles
CHEN Meng-Qiu1, CHEN Yun2, SHU Zhu2, WANG Yu1, WU Hong-Juan1, GUO Li-Min1
Received:2018-04-13
Published:2018-12-20
Online:2018-11-27
About author:CHEN Meng-Qiu (1984-), male, candidate of PhD. E-mail: mqchen@hust.edu.cn
Supported by:CLC Number:
CHEN Meng-Qiu, CHEN Yun, SHU Zhu, WANG Yu, WU Hong-Juan, GUO Li-Min. Template-free Synthesis of Mesoporous Silica with High Specific Surface Area from Natural Halloysite and Its Application in Methylene Blue Adsorption[J]. Journal of Inorganic Materials, 2018, 33(12): 1365-1370.
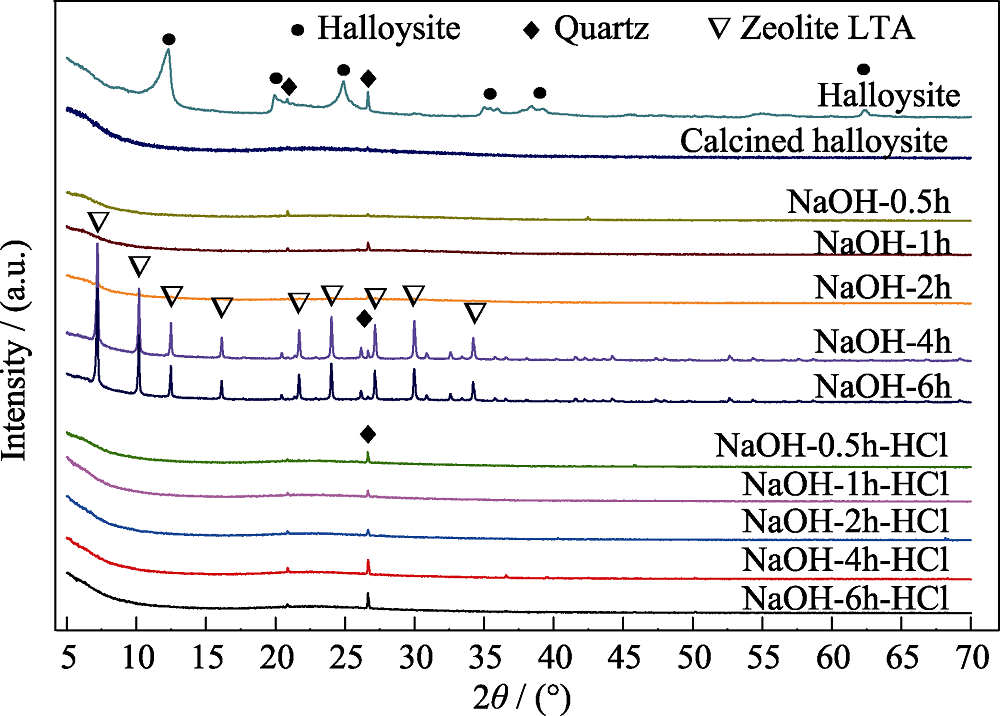
Fig. 2 XRD patterns of halloysite, calcined halloyiste, alkali- treated pre-calcinedhalloysite (NaOH-0.5/1/2/4/6 h), and final acid-etched samples (NaOH-0.5/1/2/4/6 h-HCl)
| Si/Ala | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | Others | LOIb | |
|---|---|---|---|---|---|---|---|---|---|---|
| Halloysite | 1.03 | 45.2 | 37.2 | 0.305 | 0.008 | 0.738 | 0.022 | 0.057 | 1.35 | 15.1 |
| Calcined halloysite | 1.03 | 52.8 | 43.5 | 0.356 | 0.009 | 0.862 | 0.026 | 0.067 | 1.58 | 0.8 |
Table 1 XRF results of halloysite and calcined halloysite (wt%)
| Si/Ala | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | Others | LOIb | |
|---|---|---|---|---|---|---|---|---|---|---|
| Halloysite | 1.03 | 45.2 | 37.2 | 0.305 | 0.008 | 0.738 | 0.022 | 0.057 | 1.35 | 15.1 |
| Calcined halloysite | 1.03 | 52.8 | 43.5 | 0.356 | 0.009 | 0.862 | 0.026 | 0.067 | 1.58 | 0.8 |

Fig. 3 SEM images of the alkali-treated pre-calcined halloysite and the final acid-etched samples Alkali-treated pre-calcined halloysite: (a1) NaOH-0.5h; (b1) NaOH-1h; (c1) NaOH-2h; (d1) NaOH-4h; (e1) NaOH-6h Final acid-etched samples: (a2) NaOH-0.5h-HCl; (b2) NaOH-1h-HCl; (c2) NaOH-2h-HCl; (d2) NaOH-4h-HCl; (e2) NaOH-6h-HCl
| Si | Al | Na | O | |
|---|---|---|---|---|
| NaOH-0.5h | 18.39 | 16.79 | 1.93 | 62.91 |
| NaOH-1h | 20.92 | 15.16 | 6.50 | 57.41 |
| NaOH-2h | 20.06 | 15.25 | 7.94 | 56.71 |
| NaOH-4h | 19.23 | 15.08 | 9.76 | 55.88 |
| NaOH-6h | 20.34 | 13.70 | 9.85 | 56.05 |
| NaOH-0.5h-HCl | 32.37 | 0.81 | 0.39 | 66.43 |
| NaOH-1h-HCl | 32.43 | 0.83 | 0.43 | 66.31 |
| NaOH-2h-HCl | 32.71 | 0.67 | 0.14 | 66.49 |
| NaOH-4h-HCl | 32.37 | 0.90 | 0.42 | 66.31 |
| NaOH-6h-HCl | 32.61 | 0.59 | 0.48 | 66.33 |
Table 2 EDS data of the alkali-treated samples and their acid-treated counterparts.
| Si | Al | Na | O | |
|---|---|---|---|---|
| NaOH-0.5h | 18.39 | 16.79 | 1.93 | 62.91 |
| NaOH-1h | 20.92 | 15.16 | 6.50 | 57.41 |
| NaOH-2h | 20.06 | 15.25 | 7.94 | 56.71 |
| NaOH-4h | 19.23 | 15.08 | 9.76 | 55.88 |
| NaOH-6h | 20.34 | 13.70 | 9.85 | 56.05 |
| NaOH-0.5h-HCl | 32.37 | 0.81 | 0.39 | 66.43 |
| NaOH-1h-HCl | 32.43 | 0.83 | 0.43 | 66.31 |
| NaOH-2h-HCl | 32.71 | 0.67 | 0.14 | 66.49 |
| NaOH-4h-HCl | 32.37 | 0.90 | 0.42 | 66.31 |
| NaOH-6h-HCl | 32.61 | 0.59 | 0.48 | 66.33 |
| Samples | SSA/(m2·g-1) | Smicro/(m2·g-1) | Sexternal/(m2·g-1) | Vt/(cm3·g-1) | Vmicro/(cm3·g-1) | Vmeso/(cm3·g-1) |
|---|---|---|---|---|---|---|
| NaOH-0.5h-HCl | 547 | 17.2 | 530 | 0.762 | 0.002 | 0.760 |
| NaOH-1h-HCl | 573 | 33.8 | 539 | 0.826 | 0.007 | 0.819 |
| NaOH-2h-HCl | 709 | 12.7 | 696 | 0.725 | 0 | 0.725 |
| NaOH-4h-HCl | 749 | 13.4 | 736 | 0.703 | 0 | 0.703 |
| NaOH-6h-HCl | 767 | 8.64 | 759 | 0.669 | 0 | 0.669 |
Table 3 Porosity parameters of the acid-etched samples
| Samples | SSA/(m2·g-1) | Smicro/(m2·g-1) | Sexternal/(m2·g-1) | Vt/(cm3·g-1) | Vmicro/(cm3·g-1) | Vmeso/(cm3·g-1) |
|---|---|---|---|---|---|---|
| NaOH-0.5h-HCl | 547 | 17.2 | 530 | 0.762 | 0.002 | 0.760 |
| NaOH-1h-HCl | 573 | 33.8 | 539 | 0.826 | 0.007 | 0.819 |
| NaOH-2h-HCl | 709 | 12.7 | 696 | 0.725 | 0 | 0.725 |
| NaOH-4h-HCl | 749 | 13.4 | 736 | 0.703 | 0 | 0.703 |
| NaOH-6h-HCl | 767 | 8.64 | 759 | 0.669 | 0 | 0.669 |
| Adsorbent | Qm/(mg·g-1) | SSA/(m2·g-1) | (Qm/SSA)/(mg·m-2) | Ref. |
|---|---|---|---|---|
| NaOH-6h-HCl | 741 | 767 | 0.966 | This work |
| Mesoporous silica nanotubes | 618 | 608 | 1.020 | [10] |
| Mesoporous silica nanotubes | 427 | 415 | 1.030 | [9] |
| Bamboo activated-carbon | 454 | 1896 | 0.240 | [18] |
| Mesoporous silica SBA-15 | 280 | 668 | 0.419 | [19] |
| Graphene | 154 | 295 | 0.522 | [20] |
Table 4 MB adsorption capacities of NaOH-6h-HCl and other excellent adsorbents
| Adsorbent | Qm/(mg·g-1) | SSA/(m2·g-1) | (Qm/SSA)/(mg·m-2) | Ref. |
|---|---|---|---|---|
| NaOH-6h-HCl | 741 | 767 | 0.966 | This work |
| Mesoporous silica nanotubes | 618 | 608 | 1.020 | [10] |
| Mesoporous silica nanotubes | 427 | 415 | 1.030 | [9] |
| Bamboo activated-carbon | 454 | 1896 | 0.240 | [18] |
| Mesoporous silica SBA-15 | 280 | 668 | 0.419 | [19] |
| Graphene | 154 | 295 | 0.522 | [20] |
| Isotherm models | Parameters | Value |
|---|---|---|
| Langmuir | Qm/(mg·g-1) | 741 |
| b/(mg·L-1) | 0.053 | |
| R2 | 0.986 | |
| Freundlich | KF | 139 |
| 1/n | 0.300 | |
| R2 | 0.936 | |
| Redlich-Peterson | g | 0.912 |
| A/(L·g-1) | 51.2 | |
| B/(L·mg-1) | 0.111 | |
| R2 | 0.991 |
Table S1 Parameters of three adsorption isotherm models for MB adsorption on NaOH-6 h-HCl
| Isotherm models | Parameters | Value |
|---|---|---|
| Langmuir | Qm/(mg·g-1) | 741 |
| b/(mg·L-1) | 0.053 | |
| R2 | 0.986 | |
| Freundlich | KF | 139 |
| 1/n | 0.300 | |
| R2 | 0.936 | |
| Redlich-Peterson | g | 0.912 |
| A/(L·g-1) | 51.2 | |
| B/(L·mg-1) | 0.111 | |
| R2 | 0.991 |
| [1] | WAN Y, ZHAO D Y.On the controllable soft-templating approach to mesoporous silicates.Chem. Rev., 2007, 107(7): 2821-2860. |
| [2] | WANG Y, YANG D, LI S,et al. Ru/hierarchical HZSM-5 zeolite as efficient bi-functional adsorbent/catalyst for bulky aromatic VOCs elimination. Microporous Mesoporous Mater., 2018, 258: 17-25. |
| [3] | REN Y, MA Z, BRUCE P G.Ordered mesoporous metal oxides: synthesis and applications.Chem. Soc. Rev., 2012, 41(14): 4909-4927. |
| [4] | LI W, ZHAO D Y.An overview of the synthesis of ordered mesoporous materials.Chem. Commun., 2013, 49(10): 943-946. |
| [5] | YUAN P, TAN D Y, ANNABI-BERGAYA F.Properties and applications of halloysite nanotubes: recent research advances and future prospects. Appl. Clay Sci., 2015, 112-113: 75-93. |
| [6] | LUN H L, OUYANG J, YANG H M.Enhancing dispersion of halloysite nanotubes via chemical modification. Phys. Chem. Miner., 2014, 41(4): 281-288. |
| [7] | ABDULLAYEV E, JOSHI A, WEI W,et al. Enlargement of halloysite clay nanotube lumen by selective etching of aluminum oxide. ACS Nano, 2012, 6(8): 7216-7226. |
| [8] | ZHANG A B, PAN L, ZHANG H Y,et al. Effects of acid treatment on the physico-chemical and pore characteristics of halloysite. Colloid Surf. A-Physicochem. Eng. Asp., 2012, 396: 182-188. |
| [9] | SHU Z, CHEN Y, ZHOU J, et al. Nanoporous-walled silica and alumina nanotubes derived from halloysite: controllable preparation and their dye adsorption applications. Appl. Clay Sci., 2015, 112-113: 17-24. |
| [10] | SHU Z, CHEN Y, ZHOU J,et al. Preparation of halloysite-derived mesoporous silica nanotube with enlarged specific surface area for enhanced dye adsorption. Appl. Clay Sci., 2016, 132: 114-121. |
| [11] | DA SILVA L C C, INFANTE C M C, URIO R D,et al. Adsorption/ desorption of Hg(II) on FDU-1 silica and FDU-1 silica modified with humic acid. Sep. Sci. Technol., 2015, 50(7): 984-992. |
| [12] | CHANDRASEKAR G, SON W J, AHN W S.Synthesis of mesoporous materials SBA-15 and CMK-3 from fly ash and their application for CO2 adsorption.J. Porous Mat., 2009, 16(5): 545-551. |
| [13] | LI T T, SHU Z, ZHOU J,et al. Template-free synthesis of kaolin-based mesoporous silica with improved specific surface area by a novel approach. Appl. Clay Sci., 2015, 107: 182-187. |
| [14] | OUYANG J, ZHOU Z, ZHANG Y,et al. High morphological stability and structural transition of halloysite (Hunan, China) in heat treatment. Appl. Clay Sci., 2014, 101: 16-22. |
| [15] | JOO Y, SIM J H, JEON Y,et al. Opening and blocking the inner- pores of halloysite. Chem. Commun., 2013, 49(40): 4519-4521. |
| [16] | HARRIS R G, JOHNSON B B, WELLS J D.Studies on the adsorption of dyes to kaolinite.Clay Clay Min., 2006, 54(4): 435-448. |
| [17] | BALATHANIGAIMANI M S, SHIM W G, PARK K H,et al. Effects of structural and surface energetic heterogeneity properties of novel corn grain-based activated carbons on dye adsorption. Micropor. Mesopor. Mat., 2009, 118(1/2/3): 232-238. |
| [18] | HAMEED B H, DIN A T M, AHMAD A L. Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies.J. Hazard. Mater., 2007, 141(3): 819-825. |
| [19] | DONG Y L, LU B, ZANG S Y,et al. Removal of methylene blue from coloured effluents by adsorption onto SBA-15. J. Chem. Technol. Biotechnol., 2011, 86(4): 616-619. |
| [20] | LIU T, LI Y, DU Q,et al. Adsorption of methylene blue from aqueous solution by graphene. Colloid Surf. B-Biointerfaces, 2012, 90: 197-203. |
| [1] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [2] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [3] | HONG Peiping, LIANG Long, WU Lian, MA Yingkang, PANG Hao. Structure Regulation of ZIF-67 and Adsorption Properties for Chlortetracycline Hydrochloride [J]. Journal of Inorganic Materials, 2025, 40(4): 388-396. |
| [4] | WANG Yueyue, HUANG Jiahui, KONG Hongxing, LI Huaizhu, YAO Xiaohong. Silver Loaded Radial Mesoporous Silica: Preparation and Application in Dental Resins [J]. Journal of Inorganic Materials, 2025, 40(1): 77-83. |
| [5] | WU Guangyu, SHU Song, ZHANG Hongwei, LI Jianjun. Enhanced Styrene Adsorption by Grafted Lactone-based Activated Carbon [J]. Journal of Inorganic Materials, 2024, 39(4): 390-398. |
| [6] | XIE Tian, SONG Erhong. Effect of Elastic Strains on Adsorption Energies of C, H and O on Transition Metal Oxides [J]. Journal of Inorganic Materials, 2024, 39(11): 1292-1300. |
| [7] | CHAO Shaofei, XUE Yanhui, WU Qiong, WU Fufa, MUHAMMAD Sufyan Javed, ZHANG Wei. Efficient Potassium Storage through Ti-O-H-O Electron Fast Track of MXene Heterojunction [J]. Journal of Inorganic Materials, 2024, 39(11): 1212-1220. |
| [8] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [9] | GUO Chunxia, CHEN Weidong, YAN Shufang, ZHAO Xueping, YANG Ao, MA Wen. Adsorption of Arsenate in Water by Zirconia-halloysite Nanotube Material [J]. Journal of Inorganic Materials, 2023, 38(5): 529-536. |
| [10] | WANG Shiyi, FENG Aihu, LI Xiaoyan, YU Yun. Pb (II) Adsorption Process of Fe3O4 Supported Ti3C2Tx [J]. Journal of Inorganic Materials, 2023, 38(5): 521-528. |
| [11] | WANG Lei, LI Jianjun, NING Jun, HU Tianyu, WANG Hongyang, ZHANG Zhanqun, WU Linxin. Enhanced Degradation of Methyl Orange with CoFe2O4@Zeolite Catalyst as Peroxymonosulfate Activator: Performance and Mechanism [J]. Journal of Inorganic Materials, 2023, 38(4): 469-476. |
| [12] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [13] | LING Jie, ZHOU Anning, WANG Wenzhen, JIA Xinyu, MA Mengdan. Effect of Cu/Mg Ratio on CO2 Adsorption Performance of Cu/Mg-MOF-74 [J]. Journal of Inorganic Materials, 2023, 38(12): 1379-1386. |
| [14] | TANG Ya, SUN Shengrui, FAN Jia, YANG Qingfeng, DONG Manjiang, KOU Jiahui, LIU Yangqiao. PEI Modified Hydrated Calcium Silicate Derived from Fly Ash and Its adsorption for Removal of Cu (II) and Catalytic Degradation of Organic Pollutants [J]. Journal of Inorganic Materials, 2023, 38(11): 1281-1291. |
| [15] | DAI Jieyan, FENG Aihu, MI Le, YU Yang, CUI Yuanyuan, YU Yun. Adsorption Mechanism of NaY Zeolite Molecular Adsorber Coating on Typical Space Contaminations [J]. Journal of Inorganic Materials, 2023, 38(10): 1237-1244. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||