
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (5): 454-460.DOI: 10.15541/jim20150486
• Orginal Article • Previous Articles Next Articles
WANG Xiao-Ning1,2, MENG Hu1,2, MA Fu-Yin1,2, LI Zheng2, ZHANG Lan2
Received:2015-10-10
Revised:2016-01-14
Published:2016-05-20
Online:2016-04-25
About author:WANG Xiao-Ning. E-mail: wangxiaoning@sinap.ac.cn
Supported by:CLC Number:
WANG Xiao-Ning, MENG Hu, MA Fu-Yin, LI Zheng, ZHANG Lan. Influence of Preparation Method on Oxidation Degree of Graphene Oxide and Adsorption for Th (IV) and U(VI)[J]. Journal of Inorganic Materials, 2016, 31(5): 454-460.
| C=C/% | C-O/% | C=O/% | O=C-O/% | C/O* | |
|---|---|---|---|---|---|
| HGO | 54.8 | 30.8 | 10.4 | 4.0 | 2.36 |
| IGO | 49.3 | 34.1 | 8.7 | 7.9 | 1.91 |
| MGO | 53.2 | 30.4 | 9.4 | 7.0 | 2.14 |
Table1 C1s XPS results of HGO, IGO and MGO
| C=C/% | C-O/% | C=O/% | O=C-O/% | C/O* | |
|---|---|---|---|---|---|
| HGO | 54.8 | 30.8 | 10.4 | 4.0 | 2.36 |
| IGO | 49.3 | 34.1 | 8.7 | 7.9 | 1.91 |
| MGO | 53.2 | 30.4 | 9.4 | 7.0 | 2.14 |
| C/% | H/% | O/% | |
|---|---|---|---|
| HGO | 47.72 | 2.37 | 49.91 |
| IGO | 40.67 | 2.43 | 56.90 |
| MGO | 41.95 | 2.40 | 55.65 |
Table 2 Element analysis results of HGO, IGO and MGO
| C/% | H/% | O/% | |
|---|---|---|---|
| HGO | 47.72 | 2.37 | 49.91 |
| IGO | 40.67 | 2.43 | 56.90 |
| MGO | 41.95 | 2.40 | 55.65 |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| Qmax/(mg·g-1) | KL/(L·mg-1) | R2 | KF/(mg1-n·Ln·g-1) | 1/n | R2 | ||
| U(VI) | HGO | 121.9 | 0.3306 | 0.997 | 48.11 | 0.2207 | 0.972 |
| IGO | 156.2 | 0.1688 | 0.994 | 31.43 | 0.3987 | 0.963 | |
| MGO | 161.2 | 0.1294 | 0.993 | 32.48 | 0.3801 | 0.973 | |
| Th(IV) | HGO | 138.8 | 0.4199 | 0.984 | 53.54 | 0.2279 | 0.973 |
| IGO | 192.3 | 0.6753 | 0.991 | 93.13 | 0.1786 | 0.971 | |
| MGO | 188.6 | 0.5773 | 0.975 | 61.86 | 0.2981 | 0.976 | |
Table 3 Parameters for the Langmuir and Freundlich models of U(VI) and Th(IV) sorption
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| Qmax/(mg·g-1) | KL/(L·mg-1) | R2 | KF/(mg1-n·Ln·g-1) | 1/n | R2 | ||
| U(VI) | HGO | 121.9 | 0.3306 | 0.997 | 48.11 | 0.2207 | 0.972 |
| IGO | 156.2 | 0.1688 | 0.994 | 31.43 | 0.3987 | 0.963 | |
| MGO | 161.2 | 0.1294 | 0.993 | 32.48 | 0.3801 | 0.973 | |
| Th(IV) | HGO | 138.8 | 0.4199 | 0.984 | 53.54 | 0.2279 | 0.973 |
| IGO | 192.3 | 0.6753 | 0.991 | 93.13 | 0.1786 | 0.971 | |
| MGO | 188.6 | 0.5773 | 0.975 | 61.86 | 0.2981 | 0.976 | |
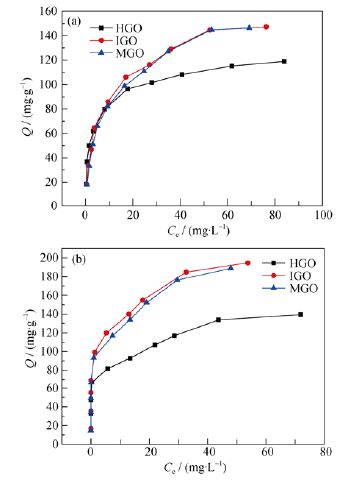
Fig. 4 Adsorption isotherms of U(VI) (a) and Th(IV) (b) on the HGO, IGO and MGO C(GO)=0.3 g/L, C(U)=0.05-0.5 mmol/L, pH=4±0.05 (a) and C (Th)= 0.05-0.5 mmol/L (b), pH=3±0.05, (T=298.15 K, t=12 h)
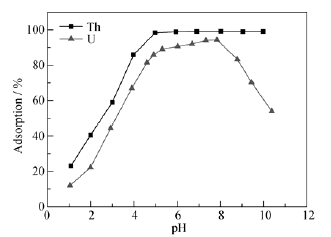
Fig. 5 Adsorption of Th(IV) and U(VI) on IGO at a function of pH C(GO)=0.4 g/L, C(Th)=0.5 mmol/L, T=298.15 K, t=12 h; C(GO)=0.4 g/L, C(U)= 0.5 mmol/L, T=298.15 K, t=12 h
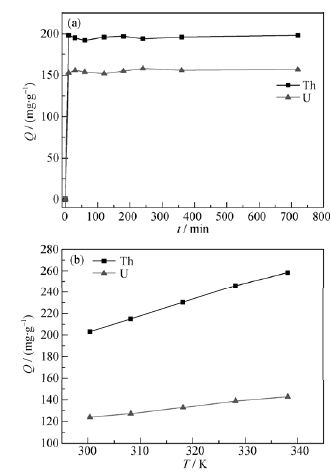
Fig. 7 Effect of contact time (a) and temperature (b) on the Th(IV) and U(VI) adsorption on IGO C(GO)=0.4 g/L,C(Th)=0.5 mmol/L, pH=3±0.05,T=298.15 K; C(GO)= 0.4 g/L,C(U)= 0.5 mmol/L, pH=4±0.05, T=298.15 K
| Temp./K | ΔH0/ (kJ•mol-1) | ΔS0/ (J•mol-1•K-1) | ΔG0/ (kJ•mol-1) | |
|---|---|---|---|---|
| Th(IV) | 300 | 5.43 | 92.05 | -22.18 |
| 308 | -22.92 | |||
| 318 | -23.84 | |||
| 328 | -24.76 | |||
| 338 | -25.68 | |||
| U(VI) | 300 | 3.23 | 80.72 | -20.98 |
| 308 | -21.62 | |||
| 318 | -22.43 | |||
| 328 | -23.24 | |||
| 338 | -24.05 | |||
Table 4 Thermodynamic parameters of Th(IV) and U(VI) adsorption on IGO
| Temp./K | ΔH0/ (kJ•mol-1) | ΔS0/ (J•mol-1•K-1) | ΔG0/ (kJ•mol-1) | |
|---|---|---|---|---|
| Th(IV) | 300 | 5.43 | 92.05 | -22.18 |
| 308 | -22.92 | |||
| 318 | -23.84 | |||
| 328 | -24.76 | |||
| 338 | -25.68 | |||
| U(VI) | 300 | 3.23 | 80.72 | -20.98 |
| 308 | -21.62 | |||
| 318 | -22.43 | |||
| 328 | -23.24 | |||
| 338 | -24.05 | |||
| [1] | CHAPMAN N, HOOPER A.The disposal of radioactive wastes underground.Proceedings of the Geologists Association, 2012, 123(1): 46-63. |
| [2] | EJNIK J W, TODOROV T I, MULLICK F G, et al.Uranium analysis in urine by inductively coupled plasma dynamic reaction cell mass spectrometry.Analytical &Bioanalytical Chemistry, 2005, 382(1): 73-79. |
| [3] | LIAO XUE-PIN, LU ZHONG-BI, DU XIAO, et al.Collagen fiber immobilized Myricarubra tannin and its adsorption to UO22+.Environmental Science & Technology, 2004, 38(1): 324-328. |
| [4] | YAKOUT S M, METWALLY S S, EL-ZAKLA T.Uranium sorption onto activated carbon prepared from rice straw: competition with humic acids.Applied Surface Science, 2013, 280(8): 745-750. |
| [5] | FASFOUS I I, DAWOUD J N.Uranium (VI) sorption by multiwalled carbon nanotubes from aqueous solution.Applied Surface Science, 2012, 259(2): 433-440. |
| [6] | ZHAO GUI-XIA, LIJIA-XING, RENXUE-MEI, et al.Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management.Environmental Science & Technology, 2011, 45(24): 10454-10462. |
| [7] | ZHAO GUI-XIA, REN XUE-MEI, GAO XING, et al.Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets.Dalton Transactions, 2011, 40(41): 10945-10952. |
| [8] | LIAN PEI-CHAO, ZHU XUE-FENG, LIANG SHU-ZHAO, et al.Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries.Electrochimica Acta, 2010, 55(12): 3909-3914. |
| [9] | LI YAN, WANG CHUN-LI, GUOZHI-JUN, et al.Sorption of thorium(IV) from aqueous solutions by graphene oxide.Journal of Radio Analytical& Nuclear Chemistry, 2014, 299. |
| [10] | ZHAO GUI-XIA, WEN TAO, YANG XIN, et al.Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions.Dalton Transactions, 2012, 41(20): 6182-6188. |
| [11] | YANG SHUANG, LI LING-YUN, PEI ZHI-GUO, et al.Effects of humic acid on copper adsorption onto few-layer reduced graphene oxide and few-layer graphene oxide.Carbon, 2014, 75(10): 227-235. |
| [12] | WANG XIANG-XUE, CHENZHONG-SHAN, YANGSHU-BING.Application of graphene oxides for the removal of Pb(II) ions from aqueous solutions: Experimental and DFT calculation. Journal of Molecular Liquids, 2015, 211: 957-964. |
| [13] | DANIELAC MARCANO, DMITRYV KOSYNKIN, JACOB M.BERLIN, et al. Improved synthesis of grapheneoxide.ACS Nano, 2010(4): 4806-4814. |
| [14] | PENG CHENG, HU WEN-BIN, ZHOU YUN-TAO, et al.Intracellular imaging with a graphene-based fluorescent probe.Small, 2010, 6(15): 1686-1692. |
| [15] | SHEN JIAN-FENG, HU YI-ZHE, SHI MIN, et al. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets.Chemistry of Materials, 2009(15): 3514-3520. |
| [16] | MOO J G S, KHEZRI B, WEBSTER R D, et al. Graphene oxides prepared by Hummers’, Hofmann’s, and Staudenmaier’s methods:Dramatic Influences on Heavy-Metal-Ion Adsorption. Chemphyschem, 2014, 15(14): 2922-2929. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [3] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [4] | WEI Zhifan, CHEN Guoqing, ZU Yufei, LIU Yuan, LI Minghao, FU Xuesong, ZHOU Wenlong. ZrB2-HfSi2 Ceramics: Microstructure and Formation Mechanism of Core-rim Structure [J]. Journal of Inorganic Materials, 2025, 40(7): 817-825. |
| [5] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [6] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [7] | ZHOU Yangyang, ZHANG Yanyan, YU Ziyi, FU Zhengqian, XU Fangfang, LIANG Ruihong, ZHOU Zhiyong. Enhancement of Piezoelectric Properties in CaBi4Ti4O15-based Ceramics through Bi3+ Self-doping Strategy [J]. Journal of Inorganic Materials, 2025, 40(6): 719-728. |
| [8] | HUANG Zipeng, JIA Wenxiao, LI Lingxia. Crystal Structure and Terahertz Dielectric Properties of (Ti0.5W0.5)5+ Doped MgNb2O6 Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 647-655. |
| [9] | ZHAO Kaixuan, LIU Wenpeng, DING Shoujun, DOU Renqin, LUO Jianqiao, GAO Jinyun, SUN Guihua, REN Hao, ZHANG Qingli. Nd:YLF Crystal Growth: Raw Materials Preparation by Melting Method and Property [J]. Journal of Inorganic Materials, 2025, 40(5): 529-535. |
| [10] | GUO Ziyu, ZHU Yunzhou, WANG Li, CHEN Jian, LI Hong, HUANG Zhengren. Effect of Zn2+ Catalyst on Microporous Structure of Porous Carbon Prepared from Phenolic Resin/Ethylene Glycol [J]. Journal of Inorganic Materials, 2025, 40(5): 466-472. |
| [11] | HONG Peiping, LIANG Long, WU Lian, MA Yingkang, PANG Hao. Structure Regulation of ZIF-67 and Adsorption Properties for Chlortetracycline Hydrochloride [J]. Journal of Inorganic Materials, 2025, 40(4): 388-396. |
| [12] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [13] | MU Haojie, ZHANG Yuanjiang, YU Bin, FU Xiumei, ZHOU Shibin, LI Xiaodong. Preparation and Properties of ZrO2 Doped Y2O3-MgO Nanocomposite Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 281-289. |
| [14] | BAO Weichao, GUO Xiaojie, XIN Xiaoting, PENG Pai, WANG Xingang, LIU Jixuan, ZHANG Guojun, XU Fangfang. Establishment of Symbiotic Structure with Metal Atomic-layer Phase-separation in Carbide Ceramics [J]. Journal of Inorganic Materials, 2025, 40(1): 17-22. |
| [15] | ZHOU Fan, TIAN Zhilin, LI Bin. Research Progress on Carbide Ultra-high Temperature Ceramic Anti-ablation Coatings for Thermal Protection System [J]. Journal of Inorganic Materials, 2025, 40(1): 1-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||