无机材料学报 ›› 2025, Vol. 40 ›› Issue (4): 348-362.DOI: 10.15541/jim20240368 CSTR: 32189.14.10.15541/jim20240368
所属专题: 【能源环境】储能电池(202506)
收稿日期:2024-08-12
修回日期:2024-11-05
出版日期:2025-04-20
网络出版日期:2024-11-29
通讯作者:
孙士恩, 正高级工程师. E-mail: shiensun@126.com作者简介:张继国(1988-), 男, 工程师. E-mail: zhangjiguo@zjenergy.com.cn
基金资助:
ZHANG Jiguo( ), WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien(
), WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien( )
)
Received:2024-08-12
Revised:2024-11-05
Published:2025-04-20
Online:2024-11-29
Contact:
SUN Shien, professor. E-mail: shiensun@126.comAbout author:ZHANG Jiguo (1988-), male, engineer. E-mail: zhangjiguo@zjenergy.com.cn
Supported by:摘要:
与传统锂离子电池相比, 钠离子电池因其成本优势与可持续的资源供应, 被看作是锂离子电池的理想替代品。现阶段主流钠离子电池正极材料包括过渡金属氧化物、聚阴离子型化合物以及普鲁士蓝化合物。然而, 正极材料存在不可逆相转化、Jahn-Teller效应及界面不稳定等问题, 这严重影响了钠离子电池的循环稳定性。本文系统介绍了钠离子电池正极材料循环稳定性提升策略的研究进展与产业化进程。首先, 详细分析了正极材料的结构、优缺点, 并对比了结构稳定性、成本以及循环性能等。其次, 详细阐述了结构优化与化学元素掺杂策略在提升正极材料循环性能方面的最新研究进展, 探索了结构稳定性、电子电导率、离子迁移速率等与电化学性能之间的相互影响关系。然后, 归纳总结了钠离子电池的发展历程与近年来国内外的产业化进展。最后, 梳理了钠离子电池正极材料及钠离子电池体系仍需关注的问题并展望了其发展前景, 以期推进钠离子电池产业稳步、健康发展。
中图分类号:
张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362.
ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries[J]. Journal of Inorganic Materials, 2025, 40(4): 348-362.
| Charge carrier | Li+ | Na+ |
|---|---|---|
| Ionic radius/Å | 0.76 | 1.02 |
| Electronic polarizability of ion/Å3 | 0.03 | 0.2-0.3 |
| Relative atomic mass | 6.94 | 23.00 |
| Ionization energy/eV | 5.39 | 5.14 |
| Melting point/℃ | 180.5 | 97.7 |
| Desolvation-energy in propylene carbonate/ (kJ·mol-1) | 215.8 | 158.2 |
| E0/V(vs. SHE) | -3.04 | -2.71 |
| Electronegativity | 0.98 | 0.93 |
| Molar mass/(g·mol-1) | 6.9 | 23.0 |
表1 锂离子与钠离子作为电荷载体的性能对比[6-7]
Table 1 Comparison of characteristics of Li+ and Na+ as charge carriers[6-7]
| Charge carrier | Li+ | Na+ |
|---|---|---|
| Ionic radius/Å | 0.76 | 1.02 |
| Electronic polarizability of ion/Å3 | 0.03 | 0.2-0.3 |
| Relative atomic mass | 6.94 | 23.00 |
| Ionization energy/eV | 5.39 | 5.14 |
| Melting point/℃ | 180.5 | 97.7 |
| Desolvation-energy in propylene carbonate/ (kJ·mol-1) | 215.8 | 158.2 |
| E0/V(vs. SHE) | -3.04 | -2.71 |
| Electronegativity | 0.98 | 0.93 |
| Molar mass/(g·mol-1) | 6.9 | 23.0 |
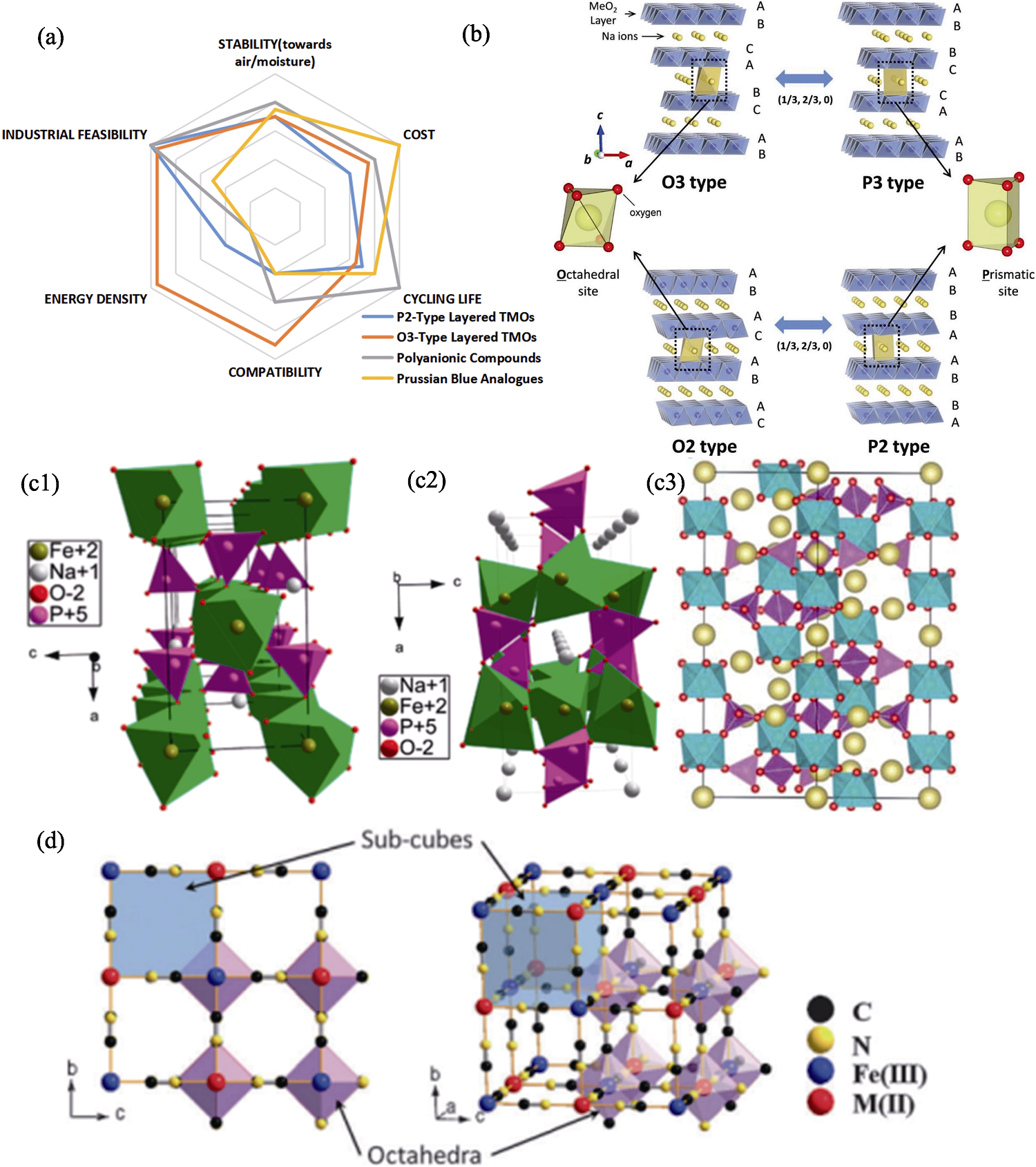
图2 钠离子电池正极材料的性能及结构[10,13⇓⇓⇓ -17]
Fig. 2 Properties and structures of cathode materials for Na+ battery[10,13⇓⇓⇓ -17] (a) Properties of cathode materials[13-14]; (b) Classification and phase transition processes of layered materials[10]; (c) Structures of (c1) maricite NaFePO4[15], (c2) olivine NaFePO4[15] and (c3) NASICON-Na3V2(PO4)3[16]; (d) Framework of Prussian blue analogues[17]
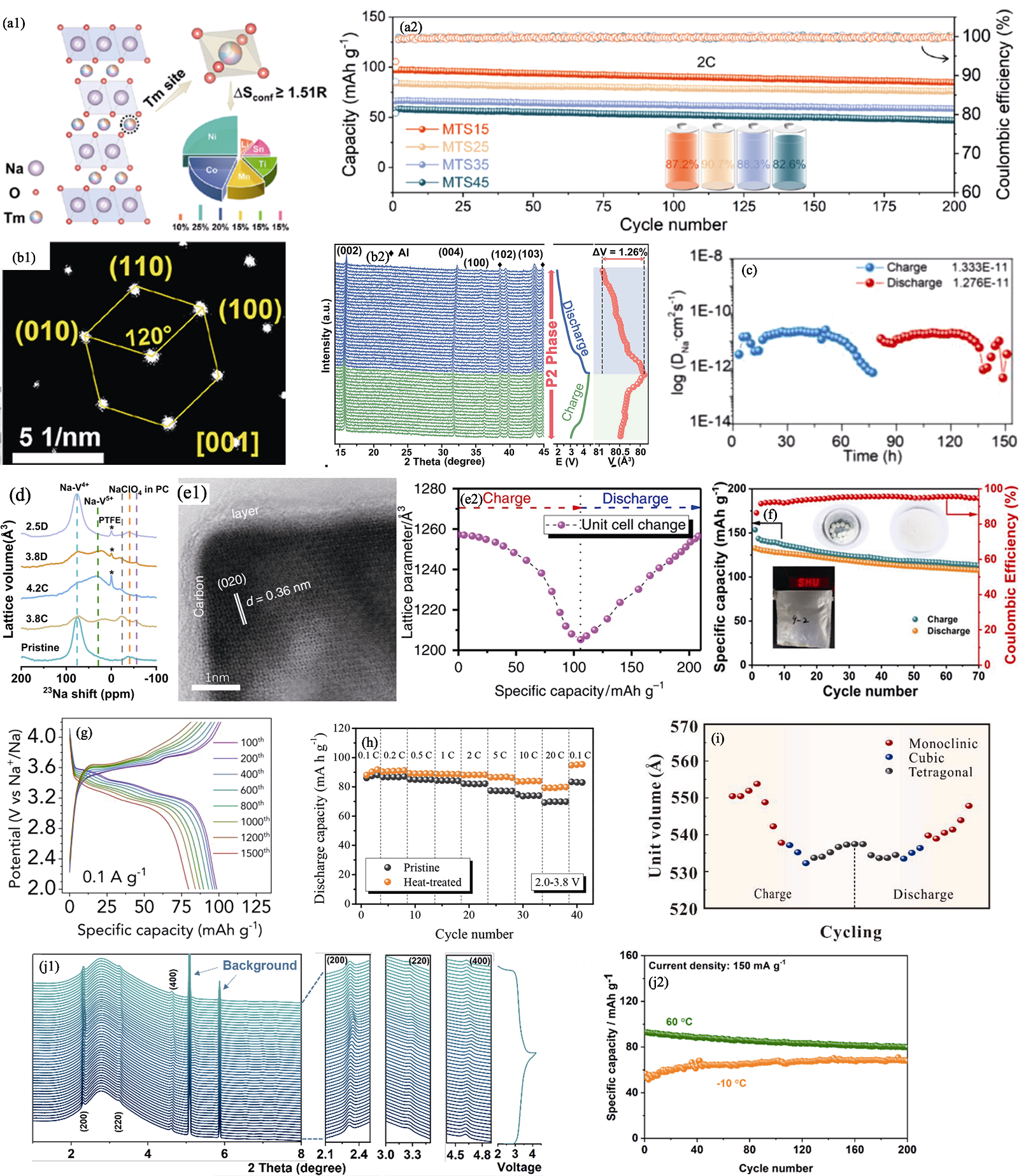
图3 结构优化策略[34⇓⇓⇓-38,40,42⇓⇓ -45]
Fig. 3 Strategies of structural optimization[34⇓⇓⇓-38,40,42⇓⇓ -45] (a1) Crystal structure schematic of MTS15 and (a2) cycling performance of MTS15, MTS25, MTS35, and MTS45[34]; (b1) Selected area electron diffraction pattern of P2-NCLMO[35]; (b2) In situ XRD patterns and corresponding volume variations of P2-NCLMO electrode in the first cycle at 0.2C[35]; (c) Calculated diffusion coefficient of Na+ in P2/O3-NaMnNiCuFeTiOF[36]; (d) High-resolution ex situ solid-state 23Na nuclear magnetic resonance spectra of Na3(VOPO4)2F/8% Ketjen black electrode under various states[37]; (e1) Bright field TEM image of nanosized Na4Fe3(PO4)2(P2O7) plates (NFPP-E) with carbon layers and (e2) volume change details during the charge/discharge process of NFPP-E[38]; (f) Cycling performance of full-cell based on MnHCF-S-170 cathode and soft carbon anode[40]; (g) Charge-discharge curves of KMF in different cycles at 0.1 A·g−1[42]; (h) Rate performances of pristine and HT samples under current densities from 0.1C to 20C[43]; (i) Unit volume of SC-HEPBA during charge and discharge process[44]; (j1) In-situ synchrotron-based powder XRD patterns with charge-discharge curves and (j2) cycling performance under 150 mA·g−1 and different temperatures[45]
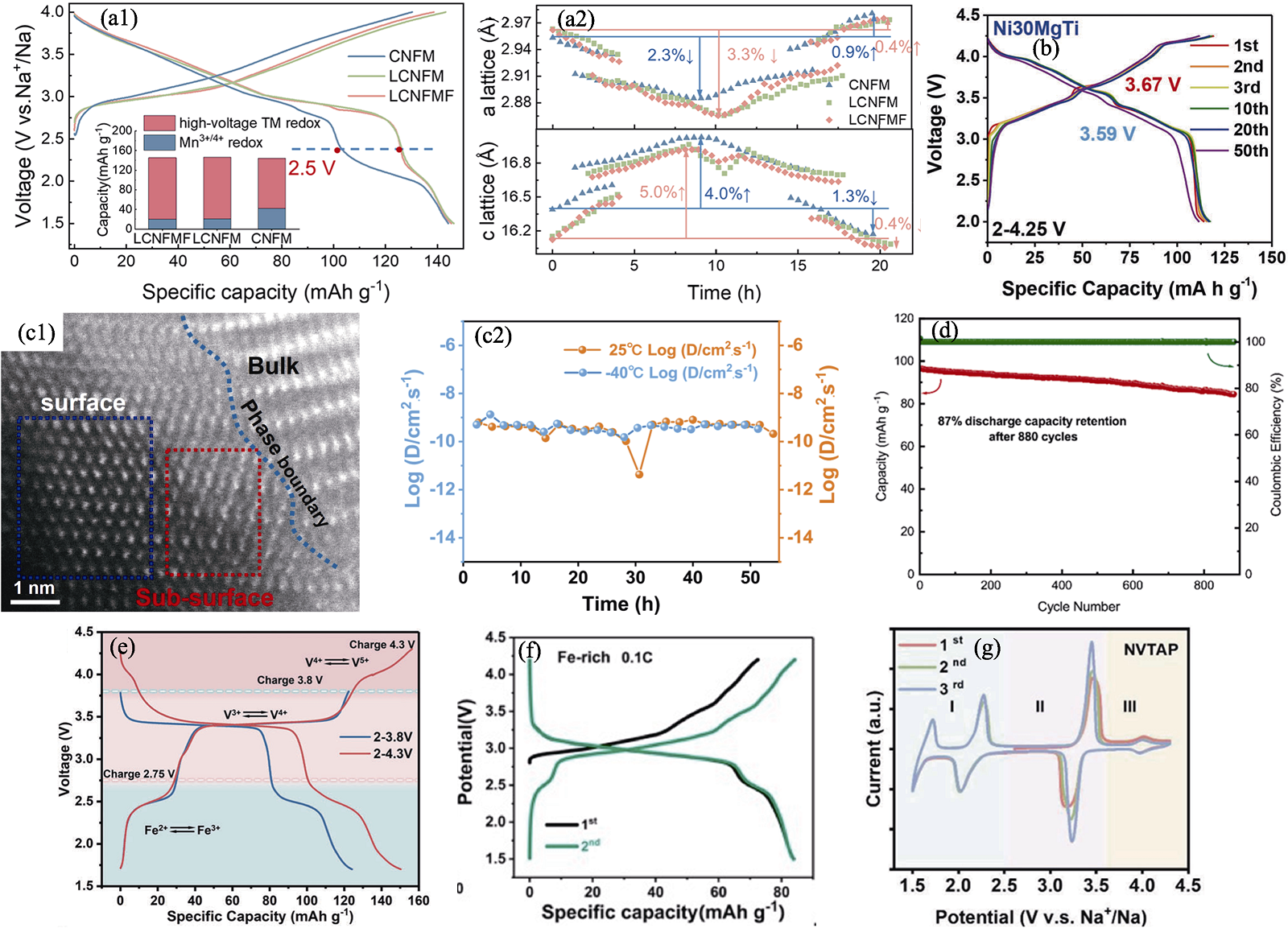
图4 化学元素掺杂策略[16,46⇓⇓⇓⇓ -51]
Fig. 4 Strategies of chemical elements doping[16,46⇓⇓⇓⇓ -51] (a1) Initial charge-discharge curves of CNFM, Na0.89Li0.05Cu0.11Ni0.11Fe0.3Mn0.43O2 (LCNFM), and LCNFMF at 0.1C in a voltage range of 1.5-4.0 V with inset showing quantitative analysis results of reversible capacity contributions[46]; (a2) Changes of a/c-lattice parameters in three samples obtained by fitting in situ XRD data[46]; (b) Charge-discharge curves of Ni30MgTi[47]; (c1) STEM-HAADF image of interface between preconstructed layer and bulk phase and (c2) corresponding sodium ion diffusion coefficients of P2-NaMNNb calculated from GITT (galvanostatic intermittent titration technique) formula under 25 and −40 ℃[48]; (d) Cycling performance of NFVNP//HC at 5C in a voltage range of 1.5-3.8 V[49]; (e) Galvanostatic charge-discharge profiles at 0.5C in different voltage windows of 1.7-3.8 V and 1.7-4.3 V[16]; (f) Galvanostatic charge/discharge profiles of Fe-rich electrode at 0.1C from 1.5 V to 4.2 V[50]; (g) CV curves of NVTAP at 0.2 mV·s-1[51]
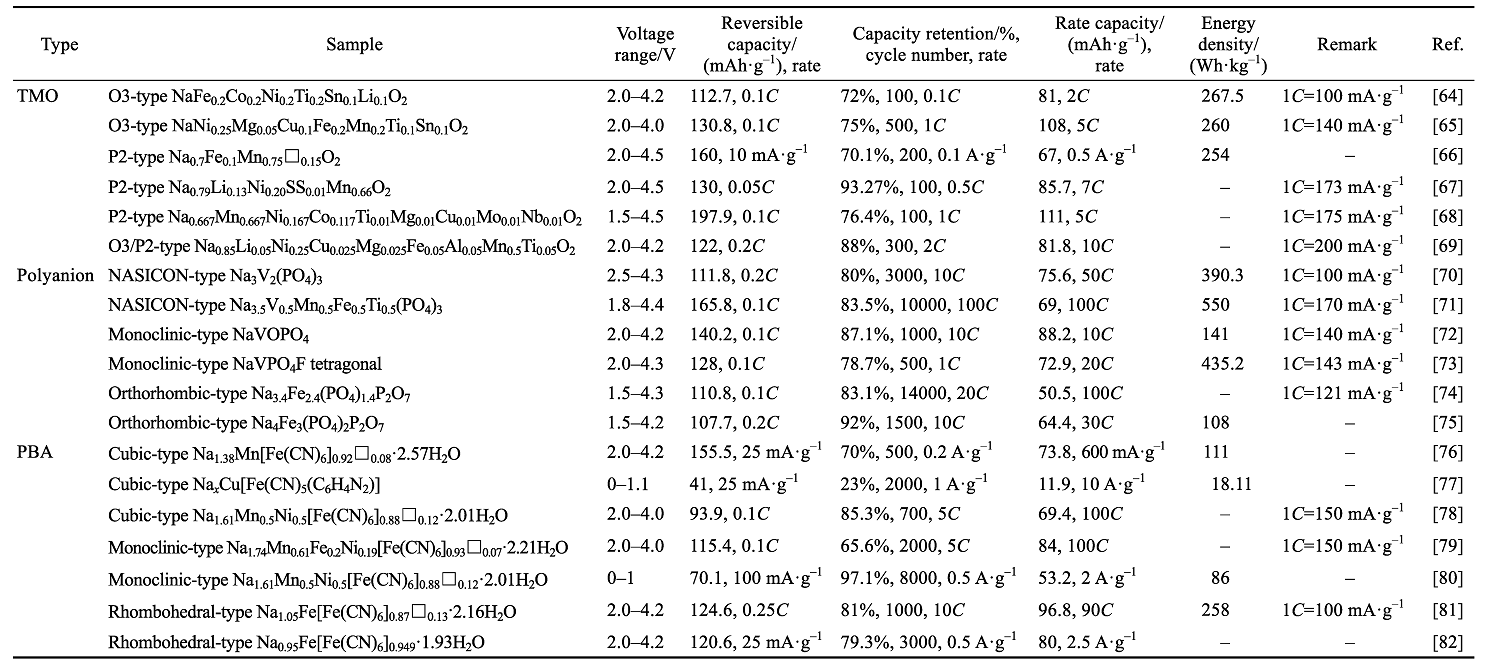 |
表2 不同种类钠离子电池正极材料性能汇总[64⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-82]
Table 2 Comprehensive overview of various cathode materials for sodium-ion batterio[64⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-82]
 |
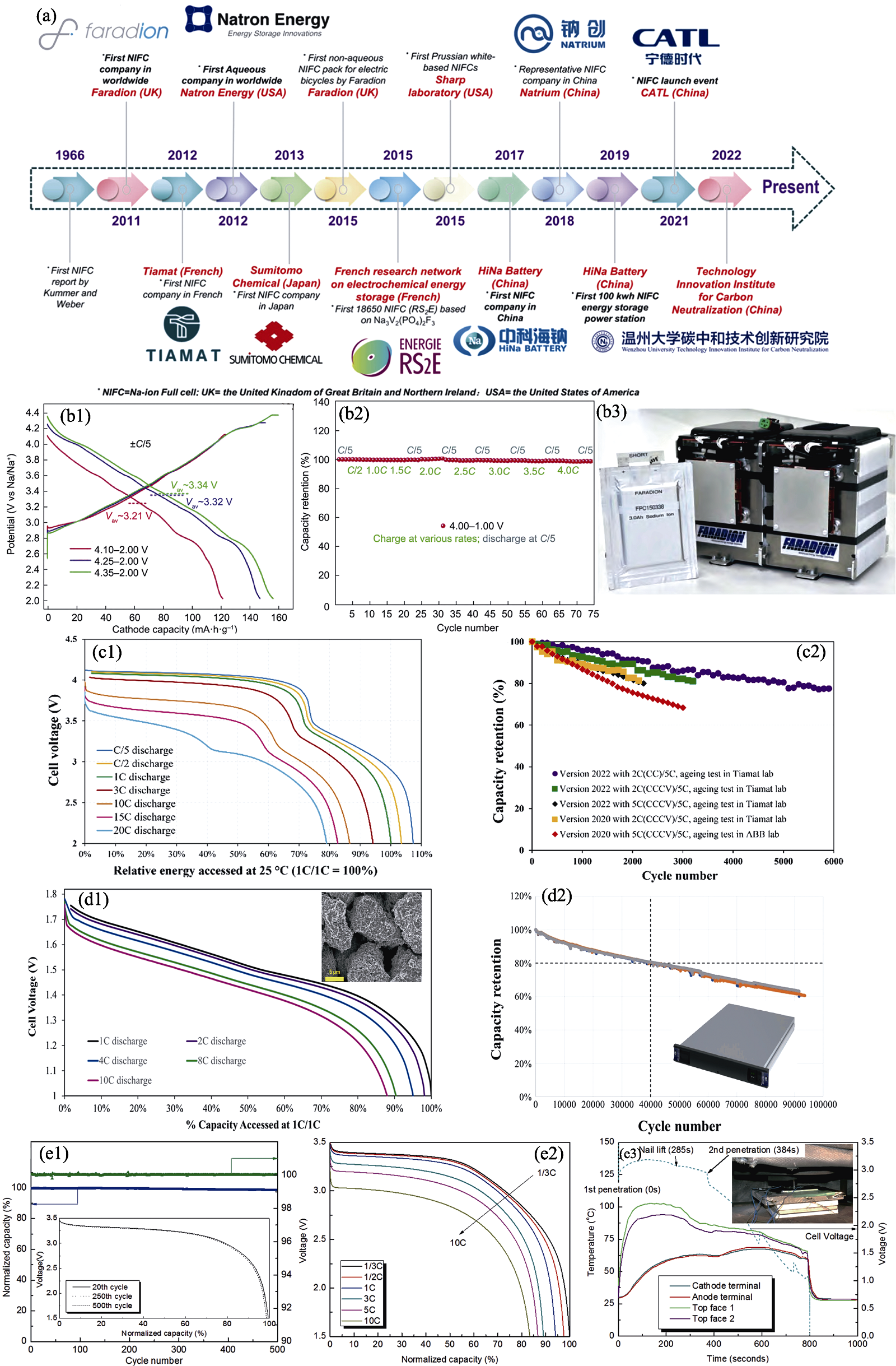
图5 钠离子电池国外产业化进展[83,85 -86,90 -91]
Fig. 5 International Industrialization process of sodium-ion batteries[83,85 -86,90 -91] (a) Worldwide development history of sodium-ion batteries[85]; (b1) Charge/discharge profiles of Faradion’s second-generation cathode material at 0.2C within different voltage windows[86]; (b2) Fast-charge performance of Faradion’s second-generation cathode material||HC 0.1 Ah full cell, charging at 4C (15 min total charge) without obvious capacity drop[86]; (b3) Faradion 3.0 Ah Na-ion pouch cell with 400 Wh battery pack system[86]; (c1) Discharge voltage profiles at different discharge rates as a function of relative energy accessed under 25 ℃ (1C/1C=100%) and (c2) cycling life measured on different versions of Tiamat Na-ion cells at 25 ℃ and 100% DOD[90]; (d1) Discharge voltage profiles of Natron cell at various discharge C rates as a function of percentage of discharge capacity accessed at 1C/1C and (d2) cycling performance at 25 ℃, 10C/10C, and 100% DOD performed in Natron’s lab with inset showing photo of ABB-Natron pluggable battery module containing 32 Natron’s 4.6 Ah sodium-ion pouch cells[91]; (e1) Cycling performance, (e2) rate capability at room temperature and (e3) safety evaluation of Novasis pouch cells[83]
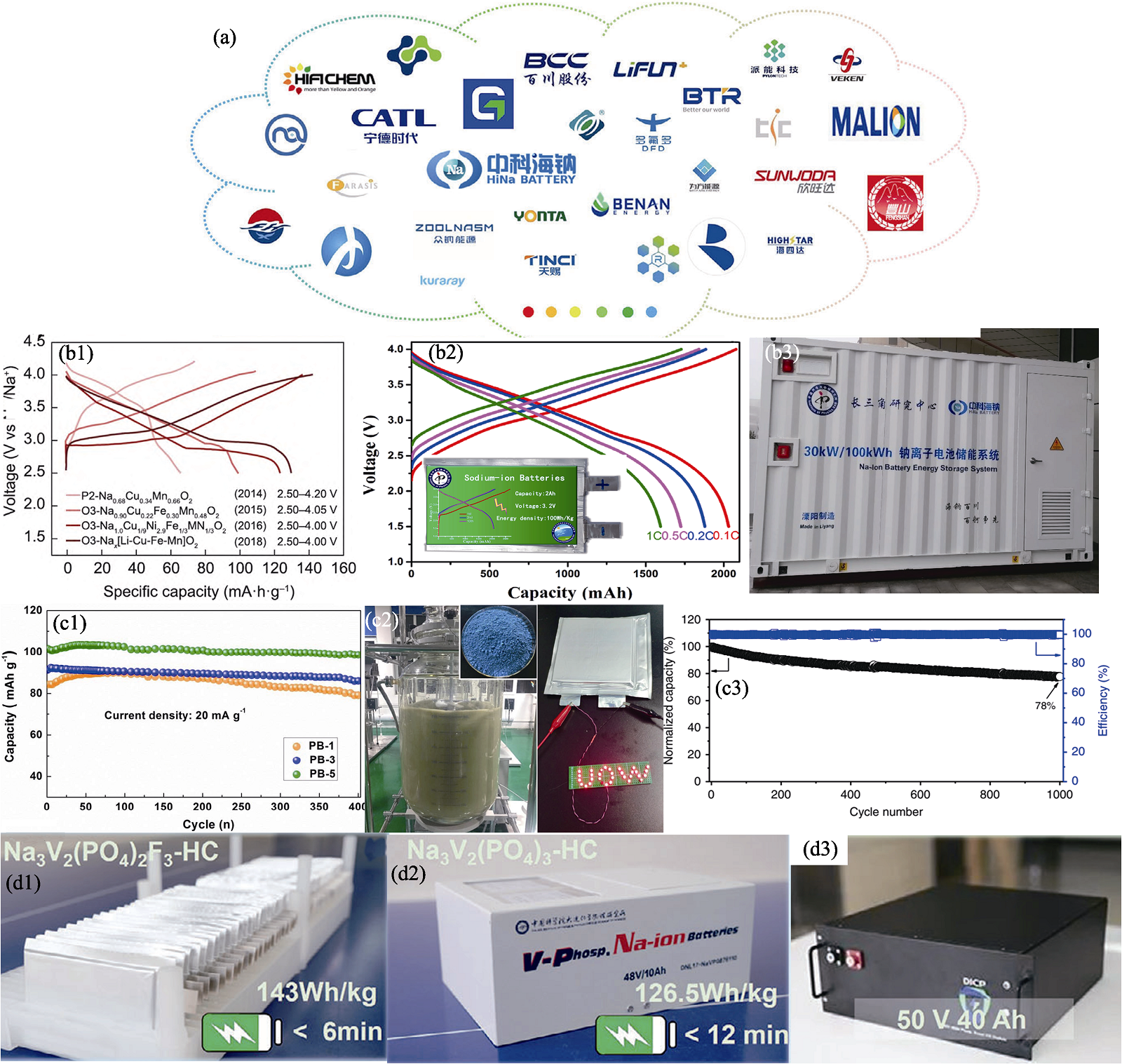
图6 钠离子电池国内产业化进展[97,99⇓ -101,104 -105,107]
Fig. 6 Domestic industrialization process of sodium-ion batteries[97,99⇓ -101,104 -105,107] (a) Companies dedicated to advancing sodium-ion batteries[97]; (b1) Typical first charge/discharge profiles of several Cu-based oxide cathode materials[99], (b2) rate capability at various constant rates from 0.1C to 1C[100] and (b3) first 30 kW/100 kWh Na-ion battery system for energy storage[101]; (c1) Cycling performance of Na1+xFe[Fe(CN)6] electrodes[104], (c2) digital images of synthesis of Prussian white Na2−xFeFe(CN)6 in 100 L reactor and powder of final product and (c3) cycling performance of pouch full cell[105]; (d1-d3) Practical application of sodium-ion batteries with polyanionic materials as cathodes[107]
| [1] | LI M, LU J, CHEN Z, et al. 30 years of lithium-ion batteries. Adv. Mater., 2018, 30(33):1800561. |
| [2] | HU M, HUANG L, LI H, et al. Research progress on hard carbon anode for Li/Na-ion batteries. J. Inorg. Mater., 2024, 39(1):32. |
| [3] | YANG B, QIAN Y, LI Q, et al. Critical summary and perspectives on state-of-health of lithium-ion battery. Renew. Sust. Energ. Rev., 2024, 190: 114077. |
| [4] | SHAO R, SUN Z, WANG L, et al. Resolving the origins of superior cycling performance of antimony anode in sodium-ion batteries: a comparison with lithium-ion batteries. Angew. Chem. Int. Ed., 2024, 136(11):e202320183. |
| [5] | VAALMA C, BUCHHOLZ D, WEIL M, et al. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater., 2018, 3(4):18013. |
| [6] | KUBOTA K, DAHBI M, HOSAKA T, et al. Towards K-ion and Na-ion batteries as “beyond Li-ion”. Chem. Rec., 2018, 18(4):459. |
| [7] | USISKIN R, LU Y, POPOVIC J, et al. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater., 2021, 6(11):1020. |
| [8] |
WANG J, ZHU Y F, SU Y, et al. Routes to high-performance layered oxide cathodes for sodium-ion batteries. Chem. Soc. Rev., 2024, 53(8):4230.
DOI PMID |
| [9] | ZHANG H, GAO Y, LIU X, et al. Long-cycle-life cathode materials for sodium-ion batteries toward large-scale energy storage systems. Adv. Energy Mater., 2023, 13(23):2300149. |
| [10] | LIU Q, HU Z, CHEN M, et al. Recent progress of layered transition metal oxide cathodes for sodium-ion batteries. Small, 2019, 15(32):1805381. |
| [11] |
GUPTA P, PUSHPAKANTH S, HAIDER M A, et al. Understanding the design of cathode materials for Na-ion batteries. ACS Omega, 2022, 7(7):5605.
DOI PMID |
| [12] | LI Y, CHEN M, LIU B, et al. Heteroatom doping: an effective way to boost sodium ion storage. Adv. Energy Mater., 2020, 10(27):2000927. |
| [13] | DENG J, LUO W B, CHOU S L, et al. Sodium-ion batteries: from academic research to practical commercialization. Adv. Energy Mater., 2018, 8(4):1701428. |
| [14] | HWANG J Y, MYUNG S T, SUN Y K. Sodium-ion batteries: present and future. Chem. Soc. Rev., 2017, 46: 3529. |
| [15] | HAO Z, SHI X, YANG Z, et al. The distance between phosphate- based polyanionic compounds and their practical application for sodium-ion batteries. Adv. Mater., 2024, 36(7):2305135. |
| [16] | ZHOU Y, XU G, LIN J, et al. Reversible multielectron redox chemistry in a NASICON-type cathode toward high-energy- density and long-life sodium-ion full batteries. Adv. Mater., 2023, 35(44):2304428. |
| [17] | LU Y, WANG L, CHENG J, et al. Prussian blue: a new framework of electrode materials for sodium batteries. Chem. Commun., 2012, 48(52):6544. |
| [18] | WANG J, DREYER S L, WANG K, et al. P2-type layered high- entropy oxides as sodium-ion cathode materials. Mater. Futures, 2022, 1(3):172. |
| [19] | ZHOU P, CHE Z, MA F, et al. Designing water air-stable P2-layered cathodes with delayed P2-O2 phase transition by composition and structure engineering for sodium-ion batteries at high voltage. Chem. Eng. J., 2020, 420: 127667. |
| [20] | SUN Y K. Direction for commercialization of O3-type layered cathodes for sodium-ion batteries. ACS Energy Lett., 2020, 5(4):1278. |
| [21] | ZHANG C, GAO R, ZHENG L, et al. New insights into the roles of Mg in improving the rate capability and cycling stability of O3-NaMn0.48Ni0.2Fe0.3Mg0.02O2 for sodium-ion batteries. ACS Appl. Mater. Interfaces, 2018, 10(13):10819. |
| [22] | GAO R M, ZHENG Z J, WANG P F, et al. Recent advances and prospects of layered transition metal oxide cathodes for sodium-ion batteries. Energy Storage Mater., 2020, 30: 9. |
| [23] | LIU J, KAN W H, LING C D. Insights into the high voltage layered oxide cathode materials in sodium-ion batteries: structural evolution and anion redox. J. Power Sources, 2021, 481: 229139. |
| [24] | XU C, ZHAO J, YANG C, et al. Polyanionic cathode materials for practical Na-ion batteries toward high energy density and long cycle life. ACS Cent. Sci., 2023, 9(9):1721. |
| [25] | LI Y, HE W X, ZHENG X Y, et al. Prussian blue cathode materials for aqueous sodium-ion batteries: preparation and electrochemical performance. J. Inorg. Mater., 2019, 34(4):365. |
| [26] |
SONG J, WANG L, LU Y, et al. Removal of interstitial H2O in hexacyanometallates for a superior cathode of a sodium-ion battery. J. Am. Chem. Soc., 2015, 137(7):2658.
DOI PMID |
| [27] | WANG Y, FENG Z, CUI P, et al. Pillar-beam structures prevent layered cathode materials from destructive phase transitions. Nat. Commun., 2021, 12: 13. |
| [28] | BERSUKER I B. Jahn-Teller and pseudo-Jahn-Teller effects: from particular features to general tools in exploring molecular and solid state properties. Chem. Rev., 2020, 121(3):1463. |
| [29] | ESHETU G G, ELIA G A, ARMAND M, et al. Electrolytes and interphases in sodium-based rechargeable batteries: recent advances and perspectives. Adv. Energy Mater., 2020, 10(20):2000093. |
| [30] | WANG Q, MARIYAPPAN S, ROUSSE G, et al. Unlocking anionic redox activity in O3-type sodium 3d layered oxides via Li substitution. Nat. Mater., 2021, 20: 353. |
| [31] | REN M, ZHAO S, GAO S, et al. Homeostatic solid solution in layered transition-metal oxide cathodes of sodium-ion batteries. J. Am. Chem. Soc., 2022, 145(1):224. |
| [32] | KIM Y, PARK H, SHIN K, et al. Rational design of coating ions via advantageous surface reconstruction in high-nickel layered oxide cathodes for lithium-ion batteries. Adv. Energy Mater., 2021, 11(38):2101112. |
| [33] | ZHANG R, YANG S, LI H, et al. Air sensitivity of electrode materials in Li/Na ion batteries: issues and strategies. InfoMat, 2022, 4(6):e12305. |
| [34] | WANG H, GAO X, ZHANG S, et al. High-entropy Na-deficient layered oxides for sodium-ion batteries. ACS Nano, 2023, 17(13):12530. |
| [35] | WANG Y, ZHAO X, JIN J, et al. Boosting the reversibility and kinetics of anionic redox chemistry in sodium-ion oxide cathodes via reductive coupling mechanism. J. Am. Chem. Soc., 2023, 145(41):22708. |
| [36] | ZHOU P, CHE Z, LIU J, et al. High-entropy P2/O3 biphasic cathode materials for wide-temperature rechargeable sodium-ion batteries. Energy Storage Mater., 2023, 57: 618. |
| [37] | SHEN X, ZHOU Q, HAN M, et al. Rapid mechanochemical synthesis of polyanionic cathode with improved electrochemical performance for Na-ion batteries. Nat. Commun., 2021, 12: 2848. |
| [38] | CHEN M, HUA W, XIAO J, et al. NASICON-type air-stable and all-climate cathode for sodium-ion batteries with low cost and high-power density. Nat. Commun., 2019, 10: 1480. |
| [39] | WANG K, LIU Z, LIN C, et al. Development of quasi-solid-state Na-ion battery based on water-minimal Prussian blue cathode. J. Inorg. Mater., 2024, 39(9):1005. |
| [40] | PENG J, GAO Y, ZHANG H, et al. Ball milling solid-state synthesis of highly crystalline Prussian blue analogue Na2-xMnFe(CN)6 cathodes for all-climate sodium-ion batteries. Angew. Chem. Int. Ed., 2022, 61(32):e202205867. |
| [41] | SHANG Y, LI X, SONG J, et al. Unconventional Mn vacancies in Mn-Fe Prussian blue analogs: suppressing Jahn-Teller distortion for ultrastable sodium storage. Chem, 2020, 6(7): 1804. |
| [42] |
LI X, SHANG Y, YAN D, et al. Topotactic epitaxy self-assembly of potassium manganese hexacyanoferrate superstructures for highly reversible sodium-ion batteries. ACS Nano, 2022, 16(1):453.
DOI PMID |
| [43] | WANG W, GANG Y, PENG J, et al. Effect of eliminating water in Prussian blue cathode for sodium-ion batteries. Adv. Funct. Mater., 2022, 32(25):2111727. |
| [44] | HUANG Y, ZHANG X, JI L, et al. Boosting the sodium storage performance of Prussian blue analogs by single-crystal and high- entropy approach. Energy Storage Mater., 2023, 58: 1. |
| [45] | PENG J, ZHANG B, HUA W, et al. A disordered Rubik's cube-inspired framework for sodium-ion batteries with ultralong cycle lifespan. Angew. Chem. Int. Ed., 2023, 62(6):e202215865. |
| [46] |
DING F, WANG H, ZHANG Q, et al. Tailoring electronic structure to achieve maximum utilization of transition metal redox for high-entropy Na layered oxide cathodes. J. Am. Chem. Soc., 2023, 145(25):13592.
DOI PMID |
| [47] | LIU Z, WU J, ZENG J, et al. Co-free layered oxide cathode material with stable anionic redox reaction for sodium-ion batteries. Adv. Energy Mater., 2023, 13(29):2301471. |
| [48] | SHI Q, QI R, FENG X, et al. Niobium-doped layered cathode material for high-power and low-temperature sodium-ion batteries. Nat. Commun., 2022, 13: 3205. |
| [49] | ZHAO Q Y, LI J Y, CHEN M J, et al. Bimetal substitution enabled energetic polyanion cathode for sodium-ion batteries. Nano Lett., 2022, 22(23):9685. |
| [50] | WANG J, ZENG W, ZHU J, et al. Fe-rich pyrophosphate with prolonged high-voltage-plateaus and suppressed voltage decay as sodium-ion battery cathode. Nano Energy, 2023, 116: 108822. |
| [51] | LI Z, SUN C, LI M, et al. Na2.5VTi0.5Al0.5(PO4)3 as long lifespan cathode for fast charging sodium-ion batteries. Adv. Funct. Mater., 2024, 34(23):2315114. |
| [52] | BI Z, HUANG W, MU S, et al. Dual-interface reinforced flexible solid garnet batteries enabled by in-situ solidified gel polymer electrolytes. Nano Energy, 2021, 90: 106498. |
| [53] | SHI R, LIU K, ZUO M, et al. Interface-reinforced solid-state electrochromic Li-ion batteries enabled by in-situ liquid-solid transitional plastic glues. J. Energy Chem., 2024, 98: 96. |
| [54] | BI Z, SUN Q, JIA M, et al. Molten salt driven conversion reaction enabling lithiophilic and air-stable garnet surface for solid-state lithium batteries. Adv. Funct. Mater., 2022, 32(52):2208751. |
| [55] | BI Z, SHI R, LIU X, et al. In situ conversion reaction triggered alloy@antiperovskite hybrid layers for lithiophilic and robust lithium/garnet interfaces. Adv. Funct. Mater., 2023, 33(43):2307701. |
| [56] | HUANG G, KONG Q, YAO W, et al. High proportion of active nitrogen-doped hard carbon based on mannich reaction as anode material for high-performance sodium-ion batteries. ChemSusChem, 2023, 16(7):e202202070. |
| [57] | HOU Z, ZHANG X, CHEN J, et al. Towards high-performance aqueous sodium ion batteries: constructing hollow NaTi2(PO4)3@C nanocube anode with Zn metal-induced pre-sodiation and deep eutectic electrolyte. Adv. Energy Mater., 2022, 12(14):2104053. |
| [58] | CHE C, WU F, LI Y, et al. Challenges and breakthroughs in enhancing temperature tolerance of sodium-ion batteries. Adv. Mater., 2024, 36(28):2402291. |
| [59] | ZHANG Y, XU J, LI Z, et al. All-climate aqueous Na-ion batteries using “water-in-salt” electrolyte. Sci. Bull., 2022, 67(2):161. |
| [60] | LIU X, ZHENG X, QIN X, et al. Temperature-responsive solid- electrolyte-interphase enabling stable sodium metal batteries in a wide temperature range. Nano Energy, 2022, 103: 107746. |
| [61] | LIU M, YANG Z, SHEN Y, et al. Chemically presodiated Sb with a fluoride-rich interphase as a cycle-stable anode for high-energy sodium ion batteries. J. Mater. Chem. A, 2021, 9(9):5639. |
| [62] | MU J J, LIU Z M, LAI Q S, et al. An industrial pathway to emerging presodiation strategies for increasing the reversible ions in sodium-ion batteries and capacitors. Energy Mater., 2022, 2(6):200043. |
| [63] | LIU T, XIANG P, LI Y, et al. In situ forming Na-Sn alloy/Na2S interface layer for ultrastable solid state sodium batteries. Adv. Funct. Mater., 2024, 34(32):2316528. |
| [64] | TIAN K, HE H, LI X, et al. Boosting electrochemical reaction and suppressing phase transition with a high-entropy O3-type layered oxide for sodium-ion batteries. J. Mater. Chem. A, 2022, 10(28):14943. |
| [65] | DING F, ZHAO C, XIAO D, et al. Using high-entropy configuration strategy to design Na-ion layered oxide cathodes with superior electrochemical performance and thermal stability. J. Am. Chem. Soc., 2022, 144(18):8286. |
| [66] | TANG Y, ZHANG Q, ZUO W, et al. Sustainable layered cathode with suppressed phase transition for long-life sodium-ion batteries. Nat. Sustain., 2024, 7(3):348. |
| [67] | FENG J, LIU Y, FANG D, et al. Reusing the steel slag to design a gradient-doped high-entropy oxide for high-performance sodium ion batteries. Nano Energy, 2023, 118: 109030. |
| [68] | MA S, ZOU P, XIN H L. Extending phase-variation voltage zones in P2-type sodium cathodes through high-entropy doping for enhanced cycling stability and rate capability. Mater. Today Energy, 2023, 38: 101446. |
| [69] | MU J, CAI T, DONG W, et al. Biphasic high-entropy layered oxide as a stable and high-rate cathode for sodium-ion batteries. Chem. Eng. J., 2023, 471: 144403. |
| [70] | ZHOU Y, XU G, LIN J, et al. A multicationic-substituted configurational entropy-enabled NASICON cathode for high- power sodium-ion batteries. Nano Energy, 2024, 128: 109812. |
| [71] | LI M, SUN C, YUAN X, et al. A configuration entropy enabled high-performance polyanionic cathode for sodium-ion batteries. Adv. Funct. Mater., 2024, 34(21):2314019. |
| [72] | SHEN X, HAN M, LI X, et al. Regulated synthesis of α-NaVOPO4 with an enhanced conductive network as a high-performance cathode for aqueous Na-ion batteries. ACS Appl. Mater. Interfaces, 2022, 14(5):6841. |
| [73] | LING M, JIANG Q, LI T, et al. The mystery from tetragonal NaVPO4F to monoclinic NaVPO4F: crystal presentation, phase conversion, and Na-storage kinetics. Adv. Energy Mater., 2021, 11(21):2100627. |
| [74] | FAN Z, SONG W, YANG N, et al. Insights into the phase purity and storage mechanism of nonstoichiometric Na3.4Fe2.4(PO4)1.4P2O7 cathode for high-mass-loading and high-power-density sodium-ion batteries. Angew. Chem. Int. Ed., 2024, 63(8):e202316957. |
| [75] | ZHANG L M, HE X D, WANG S, et al. Hollow-sphere-structured Na4Fe3(PO4)2(P2O7)/C as a cathode material for sodium-ion batteries. ACS Appl. Mater. Interfaces, 2021, 13(22):25972. |
| [76] | TANG Y, LI W, FENG P, et al. High-performance manganese hexacyanoferrate with cubic structure as superior cathode material for sodium-ion batteries. Adv. Funct. Mater., 2020, 30(10):1908754. |
| [77] | PAN T Y, WU C Y, NI C S, et al. Improvement in cycling stability of Prussian blue analog-based aqueous sodium-ion batteries by ligand substitution and electrolyte optimization. Electrochim. Acta, 2022, 427: 140778. |
| [78] | XU Z, SUN Y, XIE J, et al. Scalable preparation of Mn/Ni binary Prussian blue as sustainable cathode for harsh-condition-tolerant sodium- ion batteries. ACS Sustainable Chem. Eng., 2022, 10(40):13277. |
| [79] | XU Z, SUN Y, XIE J, et al. High-performance Ni/Fe-codoped manganese hexacyanoferrate by scale-up synthesis for practical Na-ion batteries. Mater. Today Sustain., 2022, 18: 100113. |
| [80] | SHEN L, JIANG Y, LIU Y, et al. High-stability monoclinic nickel hexacyanoferrate cathode materials for ultrafast aqueous sodium ion battery. Chem. Eng. J., 2020, 388: 124228. |
| [81] | TANG Y, WANG L, HU J, et al. Epitaxial nucleation of NaxFeFe(CN)6@rGO with improved lattice regularity as ultrahigh-rate cathode for sodium-ion batteries. Adv. Energy Mater., 2024, 14(7):2303015. |
| [82] | ANG C, LU W, ZHANG Y, et al. Toward ultrahigh rate and cycling performance of cathode materials of sodium ion battery by introducing a bicontinuous porous structure. Adv. Mater., 2024, 36(26):2402005. |
| [83] | BAUER A, SONG J, VAIL S, et al. The scale-up and commercialization of nonaqueous Na-ion battery technologies. Adv. Energy Mater., 2018, 8(17):1702869. |
| [84] | GOIKOLEA E, PALOMARES V, WANG S, et al. Na-ion batteries— approaching old and new challenges. Adv. Energy Mater., 2020, 10(44):2002055. |
| [85] | GAO Y, ZHANG H, PENG J, et al. A 30-year overview of sodium-ion batteries. Carbon Energy, 2024, 6(6):e464. |
| [86] | ZHAO L, ZHANG T, LI W, et al. Engineering of sodium-ion batteries: opportunities and challenges. Engineering, 2022, 24: 172. |
| [87] | RUDOLA A, RENNIE A J, HEAP R, et al. Commercialisation of high energy density sodium-ion batteries: Faradion's journey and outlook. J. Mater. Chem. A, 2021, 9(13):8279. |
| [88] | SAYERS R, BARKER J, HEAP R. Compositions containing doped nickelate compounds: WO2015177544A1. 2015-05-20. |
| [89] | EDELSTEIN S. Faradion electric bike: prototype powered by sodium-ion batteries. (2015-05-24)[2024-08-06]. http://www.greencarreports.com/news/1098434_faradion-electric-bike-prototype-powered-bysodium-ion-batteries. |
| [90] | HE M, MEJDOUBI A E, CHARTOUNI D, et al. High power NVPF/HC-based sodium-ion batteries. J. Power Sources, 2023, 588: 233741. |
| [91] | HE M, DAVIS R, CHARTOUNI D, et al. Assessment of the first commercial Prussian blue based sodium-ion battery. J. Power Sources, 2022, 548: 232036. |
| [92] | LIU Q, HU Z, CHEN M, et al. The cathode choice for commercialization of sodium-ion batteries: layered transition metal oxides versus Prussian blue analogs. Adv. Funct. Mater., 2020, 30(14):1909530. |
| [93] | KUZE S, KAGEURA J I, MATSUMOTO S, et al. Development of a sodium ion secondary battery. Sumitomo Kagaku, 2013, 2013: 1. |
| [94] | RS2E network. French researchers develop sodium-ion battery in 18650 format;performance comparable to Li-ion. (2015-11-27) [2024-08-06]. https://www.greencarcongress.com/2015/11/20151127-rs2e.html. |
| [95] | HALL N, BOULINEAU S, CROGUENNEC L, et al. Method for preparing a Na3V2(PO4)2F3 particulate material: WO2017/064189A1. 2017-04-20. |
| [96] |
WANG L, SONG J, QIAO R, et al. Rhombohedral Prussian white as cathode for rechargeable sodium-ion batteries. J. Am. Chem. Soc., 2015, 137(7):2548.
DOI PMID |
| [97] | LU X, LI S, LI Y, et al. From lab to application: challenges and opportunities in achieving fast charging with polyanionic cathodes for sodium-ion batteries. Adv. Mater., 2024, 36(36):2407359. |
| [98] | XU S Y, WU X Y, LI Y M, et al. Novel copper redox-based cathode materials for room-temperature sodium-ion batteries. Chin. Phys. B, 2014, 23(11):118202. |
| [99] | MU L, XU S, LI Y, et al. Prototype sodium-ion batteries using an air-stable and Co/Ni-free O3-layered metal oxide cathode. Adv. Mater., 2015, 27(43):6928. |
| [100] | LI Y, HU Y S, QI X, et al. Advanced sodium-ion batteries using superior low cost pyrolyzed anthracite anode: towards practical applications. Energy Storage Mater., 2016, 5: 191. |
| [101] | HU Y S, KOMABA S, FORSYTH M, et al. A new emerging technology: Na-ion batteries. Small Methods, 2019, 3(4):1900184. |
| [102] |
容晓晖, 陆雅翔, 戚兴国, 等. 钠离子电池: 从基础研究到工程化探索. 储能科学与技术, 2020, 9(2):515.
DOI |
| [103] | 电池网. Natrium energy signed a contract of 80,000 mt of sodium-ion battery cathode material project, further accelerating the industrialisation. (2022-06-14)[2024-08-06]. https://news.metal.com/newscontent/101860656/natrium-energy-signed-a-contract-of-80000-mt-of-sodiumion-battery-cathode-material-project-further-accelerating-the-industrialisation/. |
| [104] | LI W J, CHOU S L, WANG J Z, et al. Facile method to synthesize Na-enriched Na1+xFeFe(CN)6 frameworks as cathode with superior electrochemical performance for sodium-ion batteries. Chem. Mater., 2015, 27(6): 1997. |
| [105] |
WANG W, GANG Y, HU Z, et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat. Commun., 2020, 11(1):980.
DOI PMID |
| [106] | Contemporary Amperex Technology Co., Ltd. The first-generation sodium-ion battery launch event. (2021-07-29)[2024-08-06]. https://www.catl.com/en/news/685.html. |
| [107] | 邱艳玲, 韩建鑫, 王雨霄. 我部研制出48V/10Ah磷酸盐基钠离子电池储能系统并成功开展应用示范. (2021-11-24)[2024-08-06]. http://energystorage.dicp.ac.cn/info/1061/5239.htm. |
| [1] | 余升阳, 苏海军, 姜浩, 余明辉, 姚佳彤, 杨培鑫. 激光增材制造超高温氧化物陶瓷孔隙缺陷形成及抑制研究进展[J]. 无机材料学报, 2025, 40(9): 944-956. |
| [2] | 闫共芹, 王晨, 蓝春波, 洪雨昕, 叶维超, 付向辉. Al掺杂P2型Na0.8Ni0.33Mn0.67-xAlxO2钠离子电池正极材料的制备与电化学性能[J]. 无机材料学报, 2025, 40(9): 1005-1012. |
| [3] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [4] | 肖晓琳, 王玉祥, 谷佩洋, 朱圳荣, 孙勇. 二维无机材料调控病损皮肤组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 860-870. |
| [5] | 马景阁, 吴成铁. 无机生物材料用于毛囊和毛发再生的研究[J]. 无机材料学报, 2025, 40(8): 901-910. |
| [6] | 张洪健, 赵梓壹, 吴成铁. 无机生物材料调控神经细胞功能及神经化组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 849-859. |
| [7] | 艾敏慧, 雷波. 微纳米生物活性玻璃: 功能化设计与血管化皮肤再生[J]. 无机材料学报, 2025, 40(8): 921-932. |
| [8] | 王宇彤, 常江, 徐合, 吴成铁. 硅酸盐生物陶瓷/玻璃促创面修复的研究进展:作用、机制和应用方式[J]. 无机材料学报, 2025, 40(8): 911-920. |
| [9] | 马文平, 韩雅卉, 吴成铁, 吕宏旭. 无机活性材料在类器官研究领域的应用[J]. 无机材料学报, 2025, 40(8): 888-900. |
| [10] | 罗晓民, 乔志龙, 刘颍, 杨晨, 常江. 无机生物活性材料调控心肌再生的研究进展[J]. 无机材料学报, 2025, 40(8): 871-887. |
| [11] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [12] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [13] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [14] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [15] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||