无机材料学报 ›› 2023, Vol. 38 ›› Issue (7): 830-838.DOI: 10.15541/jim20220662 CSTR: 32189.14.10.15541/jim20220662
所属专题: 【生物材料】骨骼与齿类组织修复(202506)
吴未1,2( ), BAKHET Shahd2, ASANTE Naomi Addai2, KAREEM Shefiu2, KOMBO Omar Ramadhan3, 李宾斌2, 戴红莲1,2(
), BAKHET Shahd2, ASANTE Naomi Addai2, KAREEM Shefiu2, KOMBO Omar Ramadhan3, 李宾斌2, 戴红莲1,2( )
)
收稿日期:2022-11-05
修回日期:2022-12-18
出版日期:2023-03-20
网络出版日期:2023-03-20
通讯作者:
戴红莲, 教授. E-mail: daihonglian@whut.edu.cn作者简介:吴 未(1998-), 女, 硕士. E-mail: 2625276216@qq.com
WU Wei1,2( ), BAKHET Shahd2, ASANTE Naomi Addai2, KAREEM Shefiu2, KOMBO Omar Ramadhan3, LI Binbin2, DAI Honglian1,2(
), BAKHET Shahd2, ASANTE Naomi Addai2, KAREEM Shefiu2, KOMBO Omar Ramadhan3, LI Binbin2, DAI Honglian1,2( )
)
Received:2022-11-05
Revised:2022-12-18
Published:2023-03-20
Online:2023-03-20
Contact:
DAI Honglian, professor. E-mail: daihonglian@whut.edu.cnAbout author:WU Wei (1998-), male, Master. E-mail: 2625276216@qq.com
Supported by:摘要:
磷酸三钙(β-TCP)陶瓷替代材料由于其与骨矿物成分相近及良好的生物相容性和骨传导性, 近年来被广泛关注, 常以纳米颗粒、支架和微球等形式用于骨修复。本研究制备了五种不同的磷酸三钙/磷酸三镁(TMP) (TCP、25% TMP、50% TMP、75% TMP和TMP)复合微球并作了相应表征。随着复合微球中TMP含量增加, 微球释放的Mg2+和Ca2+的累积浓度增加, 且TMP可以调节复合微球的降解速率。以小鼠胚胎成骨细胞前体细胞(MC3T3-E1)和人脐静脉内皮细胞(HUVECs)为模型, 评价了该复合微球的生物相容性、成血管和成骨作用。结果表明, 与TCP、TMP和75% TMP相比, 25% TMP和50% TMP复合微球具有更好的细胞相容性, 对HUVECs有一定的促增殖作用。因此, 含25% TMP和50% TMP的复合微球对血管生成和成骨具有更积极的作用。
中图分类号:
吴未, BAKHET Shahd, ASANTE Naomi Addai, KAREEM Shefiu, KOMBO Omar Ramadhan, 李宾斌, 戴红莲. 双相磷酸镁钙微球体外成血管和促成骨研究[J]. 无机材料学报, 2023, 38(7): 830-838.
WU Wei, BAKHET Shahd, ASANTE Naomi Addai, KAREEM Shefiu, KOMBO Omar Ramadhan, LI Binbin, DAI Honglian. In vitro Study of Biphasic Calcium Magnesium Phosphate Microspheres for Angiogenesis and Bone Formation[J]. Journal of Inorganic Materials, 2023, 38(7): 830-838.
| Gene | Primer sequence |
|---|---|
| VEGF | AGGAGTACCCCGACGAGATAGA CACATCTGCTGTGCTGTAGGAA |
| FGF | ACAGGAGCGACCAGCACATT TTGGTGTCTGCGAGCCGTAT |
| COL I | CACTGCAAGAACAGCGTAGC AAGTTCCGGTGTGACTCGTG |
| OPN | ACACTTTCACTCCAATCGTCCCTAC GGACTCCTTAGACTCACCGCTCTT |
Table 1 Primer sequences used in RT-qPCR
| Gene | Primer sequence |
|---|---|
| VEGF | AGGAGTACCCCGACGAGATAGA CACATCTGCTGTGCTGTAGGAA |
| FGF | ACAGGAGCGACCAGCACATT TTGGTGTCTGCGAGCCGTAT |
| COL I | CACTGCAAGAACAGCGTAGC AAGTTCCGGTGTGACTCGTG |
| OPN | ACACTTTCACTCCAATCGTCCCTAC GGACTCCTTAGACTCACCGCTCTT |
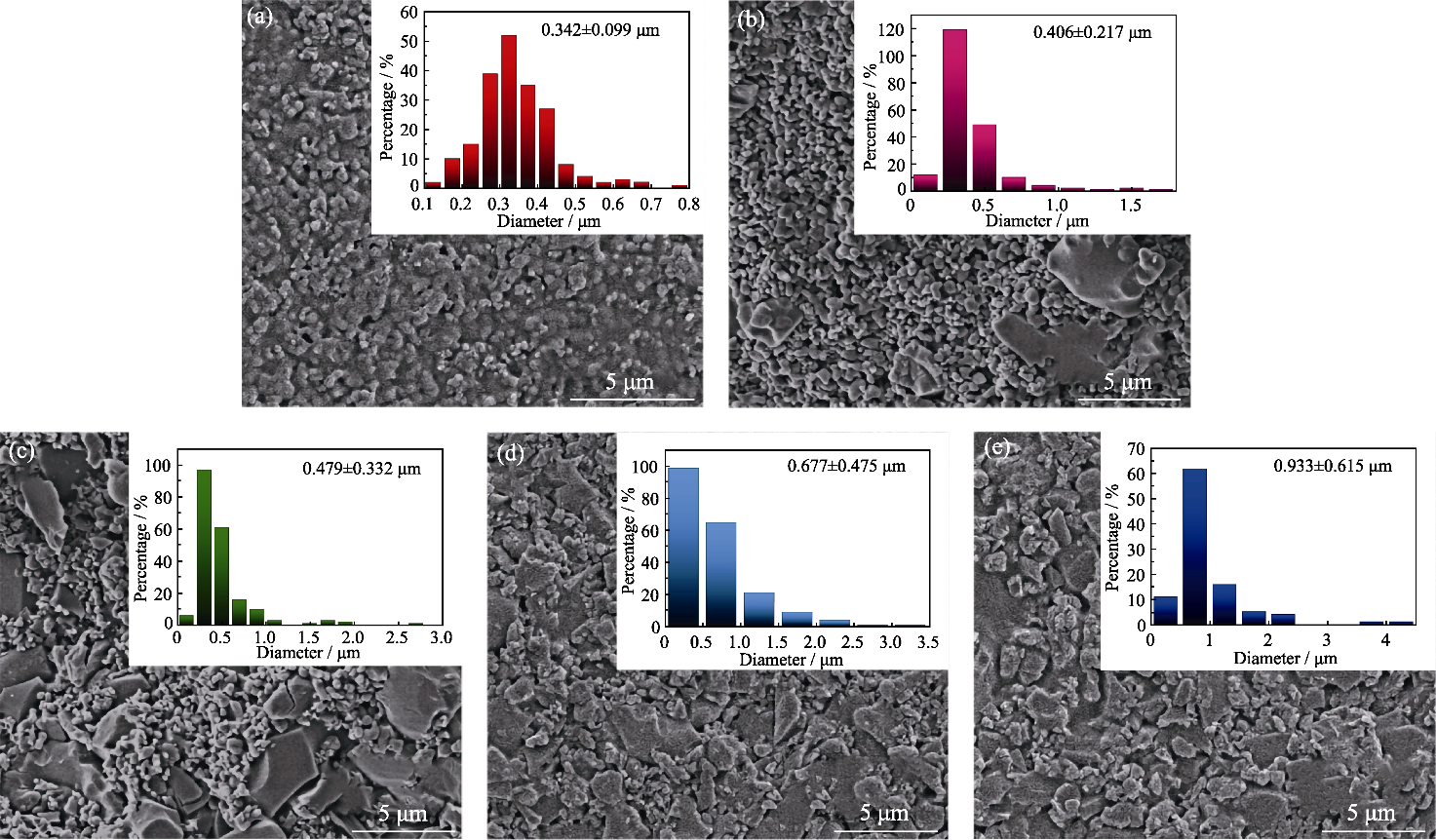
Fig. 2 SEM images of different microsphere composites with insets showing their corresponding particle size distributions (a)TCP; (b) 25% TMP; (c) 50% TMP; (d) 75% TMP; (e) TMP
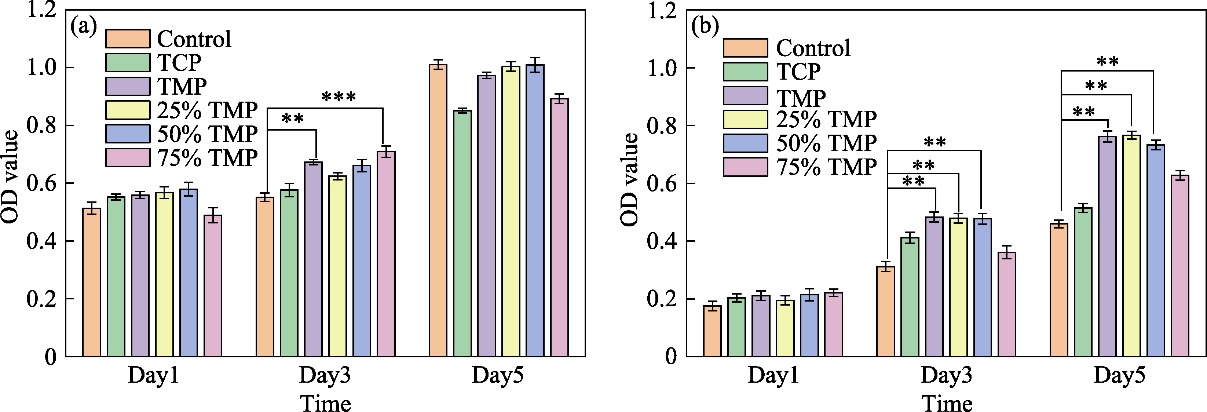
Fig. 5 Cell viabilities of (a) MC3T3-E1 and (b) HUVECs assessed by CCK-8 assay (a) MC3T3-E1 and (b) HUVECs assayed on day 1, 3, 5 cultured with different microspheres concentration extracts *: p < 0.01; **: p < 0.005; ***: p < 0.0002; Colorful figures are available on website
| [1] |
NARITA K, KOBAYASHI E, SATO TJMT. Sintering behavior and mechanical properties of magnesium/β-tricalcium phosphate composites sintered by spark plasma sintering. Materials Transactions, 2016, 57(9):1620.
DOI URL |
| [2] | CHUTHATHIP M, AHMAD-FAUZI M N, YANNY-MARLIANA B I, et al. Effect of magnesium oxide on physical and biological properties in β-tricalcium phosphate ceramic. Journal of Physics Conference Series, 2018, 1082(1):012026. |
| [3] |
BASU S, BASU B. Doped biphasic calcium phosphate: synthesis and structure. Journal of Asian Ceramic Societies, 2019, 7(3):265.
DOI |
| [4] |
GHIȚULICĂ C D, CUCURUZ A, VOICU G, et al. Ceramics based on calcium phosphates substituted with magnesium ions for bone regeneration. International Journal of Applied Ceramic Technology, 2020, 17(1):342.
DOI URL |
| [5] | MAJI K, DASGUPTA S. Effect of β-tricalcium phosphate nanoparticles additions on the properties of gelatin-chitosan scaffolds. Bioceramics Development & Applications, 2017, 7(2):1000103. |
| [6] |
MURAKAMI S, MIYAJI H, NISHIDA E, et al. Dose effects of beta-tricalcium phosphate nanoparticles on biocompatibility and bone conductive ability of three-dimensional collagen scaffolds. Dental Materials Journal, 2017, 36(5):573.
DOI PMID |
| [7] | FANG Z Z. Sintering of advanced materials. Cambridge: Elsevier, 2010: 33- 85. |
| [8] | KAUR I, ELLIS L J, ROMER I, et al. Dispersion of nanomaterials in aqueous media: towards protocol optimization. Journal of Visualized Experiments, 2017, 130: e56074. |
| [9] |
XUE W, DAHLQUIST K, BANERJEE A, et al. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. Journal of Materials Science: Materials in Medicine, 2008, 19(7):2669.
DOI URL |
| [10] |
GUO X, LONG Y, LI W, et al. Osteogenic effects of magnesium substitution in nano-structured β-tricalcium phosphate produced by microwave synthesis. Journal of Materials Science, 2019, 54(16):11197.
DOI |
| [11] |
ELIAZ N, METOKI N J M. Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials, 2017, 10(4):334.
DOI URL |
| [12] |
RAO R R, ROOPA H N, KANNAN T S. Solid state synthesis and thermal stability of HAP and HAP-β-TCP composite ceramic powders. Journal of Materials Science: Materials in Medicine, 1997, 8(8):511.
DOI URL |
| [13] |
RUIZ-AGUILAR C, OLIVARES-PINTO U, AGUILAR-REYES E A, et al. Characterization of β-tricalcium phosphate powders synthesized by Sol-Gel and mechanosynthesis. Boletín de la Sociedad Española de Cerámica y Vidrio, 2018, 57(5):213.
DOI URL |
| [14] |
ANDO J. Tricalcium phosphate and its variation. Bulletin of the Chemical Society of Japan, 1958, 31(2):196.
DOI URL |
| [15] | OLSSON M. Chemical stability of grain boundariesinβ-tricalcium phosphate ceramics: β-TCP as bone substitute material. Department of Chemistry-Ångström, 2012, 42586904. |
| [16] | SGLAVO VM, FRASNELLI M. Effect of Mg2+ doping on beta- alpha phase transition in tricalcium phosphate (TCP) bioceramics. Acta Biomaterialia, 2016, 33: 283. |
| [17] |
MA Y, DAI H, HUANG X, et al. 3D printing of bioglass-reinforced β-TCP porous bioceramic scaffolds. Journal of Materials Science, 2019, 54(14):10437.
DOI |
| [18] | GALLO M, SANTONI B L G, DOUILLARD T, et al. Effect of grain orientation and magnesium doping on β-tricalcium phosphate resorption behavior. Acta Biomaterialia, 2019, 89: 391. |
| [19] |
TAVARES D D S, CASTRO L D O, SOARES G D D A, et al. Synthesis and cytotoxicity evaluation of granular magnesium substituted β-tricalcium phosphate. Journal of Applied Oral Science, 2013, 21(1):37.
DOI URL |
| [20] |
LEE D, SFEIR C, KUMTA P N J M S, et al. Novel in-situ synthesis and characterization of nanostructured magnesium substituted β-tricalcium phosphate (β-TCMP). Materials Science, 2009, 29(1):69.
DOI URL |
| [21] |
MARCHI J, DANTAS A, GREIL P, et al. Influence of Mg-substitution on the physicochemical properties of calcium phosphate powders. Materials Research Bulletin, 2007, 42(6):1040.
DOI URL |
| [22] |
RYU H-S, HONG KS, LEE J-K, et al. Magnesia-doped HA/β-TCP ceramics and evaluation of their biocompatibility. Biomaterials, 2004, 25(3):393.
DOI URL |
| [23] |
ZHANG X, JIANG F, GROTH T, et al. Preparation, characterization and mechanical performance of dense β-TCP ceramics with/ without magnesium substitution. Journal of Materials Science: Materials in Medicine, 2008, 19(9):3063.
DOI URL |
| [24] |
ONUMA K, IIJIMA M J C. Nanoparticles in β-tricalcium phosphate substrate enhance modulation of structure and composition of an octacalcium phosphate grown layer. CrystEngComm, 2017, 19(44):6660.
DOI URL |
| [25] |
SADER M S, LEGEROS R Z, SOARES G A. Human osteoblasts adhesion and proliferation on magnesium-substituted tricalcium phosphate dense tablets. Journal of Materials Science: Materials in Medicine, 2009, 20(2):521.
DOI URL |
| [26] |
LIN L C, CHANG S J, KUO S M, et al. Preparation and evaluation of β-TCP/polylactide microspheres as osteogenesis materials. Journal of Applied Polymer Science, 2008, 108(5):3210.
DOI URL |
| [27] | YUAN Z, WEI P, HUANG Y, et al. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomaterialia. 2019, 85: 294. |
| [28] | WANG J, XU J, HOPKINS C, et al. Biodegradable magnesium ased implants in orthopedics: a general review and perspectives. Advanced Science, 2020, 7(8):201902443. |
| [29] |
LIN S, YANG G, JIANG F, et al. Bone regeneration: a magnesiumnriched 3D culture system that mimics the bone development microenvironment for vascularized bone regeneration. Advanced Science, 2019, 6(12):1900209.
DOI URL |
| [30] | PAN C, SUN X, XU G, et al. The effects of β-TCP on mechanical properties, corrosion behavior and biocompatibility of beta- TCP/Zn-Mg composites. Materials Science & Engineering C, 2020, 108: 110397. |
| [31] |
ZHANG H, SHEN Y, XIONG Y, et al. Microstructural, mechanical properties and strengthening mechanism of DLP produced β-tricalcium phosphate scaffolds by incorporation of MgO/ZnO/58S bioglass. Ceramics International, 2021, 47(18):25863.
DOI URL |
| [32] |
ZHANG J. TANG L, QI H, et al. Dual function of magnesium in bone biomineralization, Advanced Healthcare Materials, 2019, 8(21):1901030.
DOI URL |
| [33] | LIN X, GE J, WEI D, et al. Surface degradation-enabled osseointegrative, angiogenic and antiinfective properties of magnesium- modified acrylic bone cement. Journal of Orthopaedic Translation. 2019, 17: 121. |
| [34] |
HE F, TIAN Y, FANG X, et al. Porous calcium phosphate composite bioceramic beads. Ceramics International, 2018, 44(11):13430.
DOI URL |
| [35] | HO V H, TRIPATHI G, GWON J, et al. Novel TOCNF reinforced injectable alginate/β-tricalcium phosphate microspheres for bone regeneration. Materials & Design, 2020, 194: 108892. |
| [36] |
MURAKAMI M, NGUYEN L T, HATANAKA K, et al. FGF-dependent regulation of VEGF receptor 2 expression in mice. The Journal of Clinical Investigation, 2011, 121(7):2668.
DOI URL |
| [37] |
OLIVARES-NAVARRETE R, HYZY S L, GITTENS R A, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. The Spine Journal, 2013, 13(11):1563.
DOI URL |
| [38] |
MATKAR P N, ARIYAGUNARAJAH R, LEONG-POI H, et al. Friends turned foes: angiogenic growth factors beyond angiogenesis. Biomolecules, 2017, 7(4):74.
DOI URL |
| [39] |
CHIM S M, TICKNER J, CHOW S T, et al. Angiogenic factors in bone local environment. Cytokine Growth Factor Reviews, 2013, 24(3):297.
DOI URL |
| [40] |
TAN A W, LIAU L L, CHUA K H, et al. Enhanced in vitro angiogenic behaviour of human umbilical vein endothelial cells on thermally oxidized TiO2 nanofibrous surfaces. Scientific Reports, 2016, 6(1):21828
DOI |
| [41] |
PRZYBYLSKI M. A review of the current research on the role of bFGF and VEGF in angiogenesis. Journal of Wound Care, 2009, 18(12):516.
PMID |
| [42] | CHEN Y, OU Y, DONG J, et al. Osteopontin promotes collagen I synthesis in hepatic stellate cells by miRNA-129-5p inhibition. Experimental Cell Research, 2017, 363(1):343. |
| [43] | BHASKAR B, OWEN R, BAHMAEE H, et al. Composite porous scaffold of PEG/PLA support improved bone matrix deposition in vitro compared to PLA-only scaffolds, Journal of Biomedical Research Part A, 2018, 106(5):1334. |
| [1] | 余艺平, 肖鹏, 赵长浩, 徐梦迪, 姚立冬, 李伟, 王松. 耐高温层状Ta/Ta0.5Hf0.5C金属陶瓷的高频等离子体风洞烧蚀行为研究[J]. 无机材料学报, 2025, 40(7): 790-798. |
| [2] | 余乐洋阳, 赵芳霞, 张舒心, 徐以祥, 牛亚然, 张振忠, 郑学斌. 感应等离子球化技术制备喷涂用高熵硼化物粉体[J]. 无机材料学报, 2025, 40(7): 808-816. |
| [3] | 魏志帆, 陈国清, 祖宇飞, 刘渊, 李明浩, 付雪松, 周文龙. ZrB2-HfSi2复相陶瓷显微组织及其核-周结构形成机制[J]. 无机材料学报, 2025, 40(7): 817-825. |
| [4] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [5] | 何国强, 张恺恒, 王震涛, 包健, 席兆琛, 方振, 王昌昊, 王威, 王鑫, 姜佳沛, 李祥坤, 周迪. Ba(Nd1/2Nb1/2)O3: 一种被低估的K40微波介质陶瓷[J]. 无机材料学报, 2025, 40(6): 639-646. |
| [6] | 张家维, 陈宁, 程原, 王博, 朱建国, 金城. Bi4Ti3O12铋层状压电陶瓷的A/B位掺杂及其电学性能[J]. 无机材料学报, 2025, 40(6): 690-696. |
| [7] | 唐莹, 李洁, 相怀成, 方维双, 林慧兴, 杨俊峰, 方亮. Rattling效应: 一种影响微波介质陶瓷谐振频率温度系数的新机制[J]. 无机材料学报, 2025, 40(6): 656-666. |
| [8] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [9] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [10] | 周阳阳, 张艳艳, 于子怡, 傅正钱, 许钫钫, 梁瑞虹, 周志勇. 通过Bi3+自掺杂增强CaBi4Ti4O15基陶瓷压电性能[J]. 无机材料学报, 2025, 40(6): 719-728. |
| [11] | 杨燕, 张发强, 马名生, 王墉哲, 欧阳琪, 刘志甫. 基于CuO-TiO2-Nb2O5复合氧化物烧结助剂的ZnAl2O4陶瓷低温烧结研究[J]. 无机材料学报, 2025, 40(6): 711-718. |
| [12] | 黄子鹏, 贾文晓, 李玲霞. (Ti0.5W0.5)5+掺杂MgNb2O6陶瓷的晶体结构与太赫兹介电性能[J]. 无机材料学报, 2025, 40(6): 647-655. |
| [13] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [14] | 尹长志, 成名飞, 雷微程, 蔡弋炀, 宋小强, 付明, 吕文中, 雷文. Ga3+掺杂对SrAl2Si2O8陶瓷晶体结构及微波介电性能的影响[J]. 无机材料学报, 2025, 40(6): 704-710. |
| [15] | 吴鲁康, 傅正钱, 于子怡, 杨俊, 周斌, 陈学锋, 许钫钫. 电子能量损失谱在BaTiO3基多层陶瓷电容器中的应用研究[J]. 无机材料学报, 2025, 40(6): 683-689. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||