无机材料学报 ›› 2013, Vol. 28 ›› Issue (1): 1-11.DOI: 10.3724/SP.J.1077.2012.12082 CSTR: 32189.14.SP.J.1077.2012.12082
• • 下一篇
施剑林, 陈 雨, 陈航榕
收稿日期:2012-02-11
修回日期:2012-04-10
出版日期:2013-01-10
网络出版日期:2012-12-20
基金资助:SHI Jian-Lin, CHEN Yu, CHEN Hang-Rong
Received:2012-02-11
Revised:2012-04-10
Published:2013-01-10
Online:2012-12-20
Supported by:摘要:
无机介孔纳米生物材料在药物靶向输送、组织工程、基因传输治疗、分子影像、无创手术增效治疗等医学领域具有广阔的应用前景, 对于诸如癌症等重大疾病的早期诊断与高效治疗具有重要的意义。本文以医学应用需求为背景, 以纳米合成化学为基础, 从多功能介孔纳米生物材料的设计入手, 结合本课题组的研究进展, 综述了介孔基纳米诊疗剂的研究现状和未来发展的趋势。通过对介孔SiO2纳米粒子进行功能化修饰, 赋予其特定的功能, 不仅可以作为临床分子影像(核磁共振成像、荧光成像以及各种成像模式的复合)的造影剂对疾病进行诊断, 并能同时高效地包覆和传输药物对疾病进行治疗(化疗、基因治疗、光动力学治疗或者无创手术治疗)。随着纳米生物技术的发展和纳米合成化学的进步, 设计和制备具有特定功能的满足临床需求的介孔氧化硅基纳米生物材料, 并系统地评价其细胞生物学效应和生物安全性, 将会真正实现其在临床上的应用, 从而造福人类。
中图分类号:
施剑林, 陈 雨, 陈航榕. 多功能介孔氧化硅基纳米诊疗剂的研究进展[J]. 无机材料学报, 2013, 28(1): 1-11.
SHI Jian-Lin, CHEN Yu, CHEN Hang-Rong. Progress on the Multifunctional Mesoporous Silica-based Nanotheranostics[J]. Journal of Inorganic Materials, 2013, 28(1): 1-11.
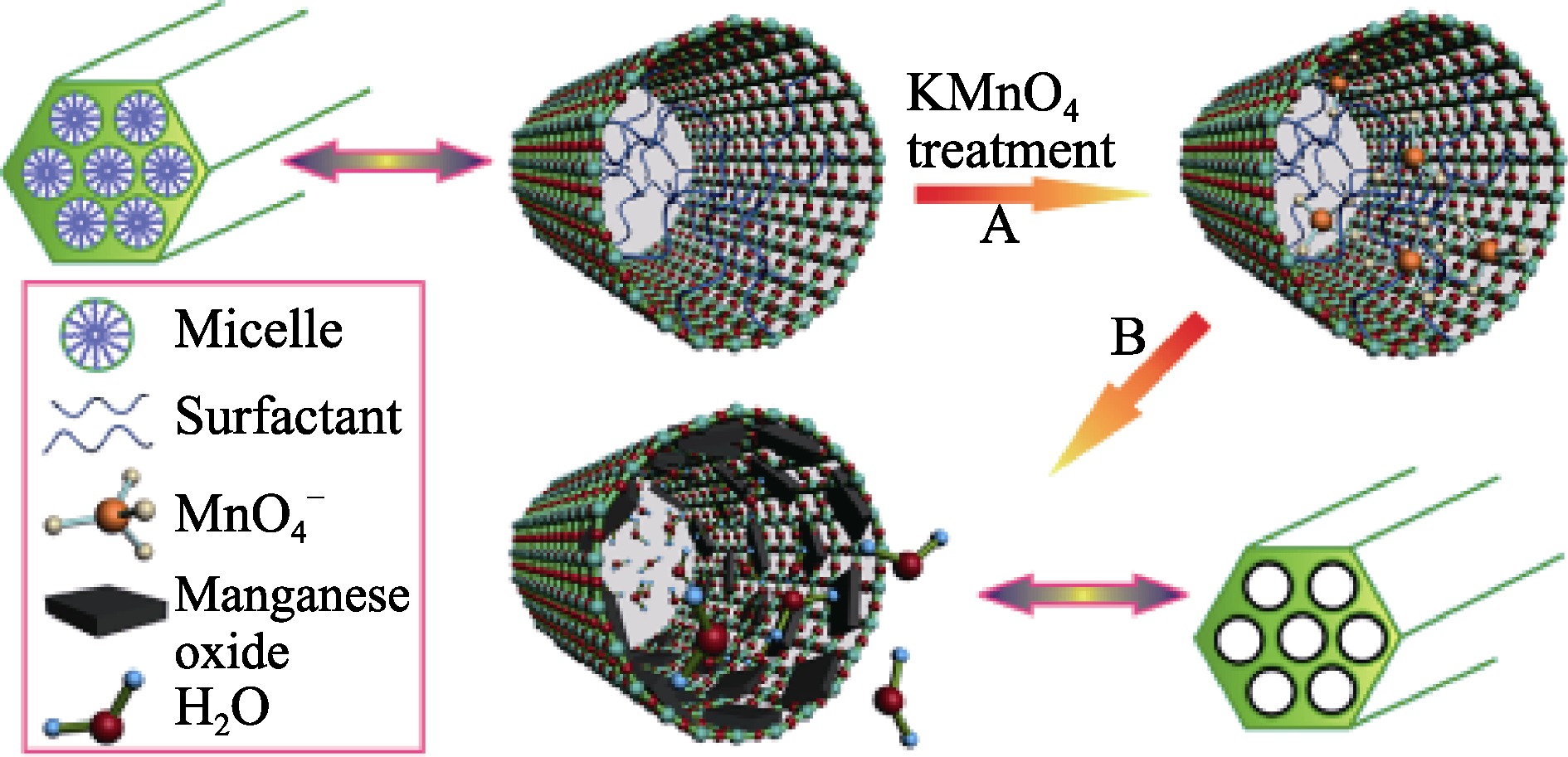
图1 通过氧化-还原法制备Mn顺磁中心均匀分散的介孔SiO2(Mn-MSNs)的示意图[38]
Fig. 1 Schematic representation for the preparation of manganese oxide-dispersed MSNs (Mn-MSNs) by the oxidation- reduction strategy[38]
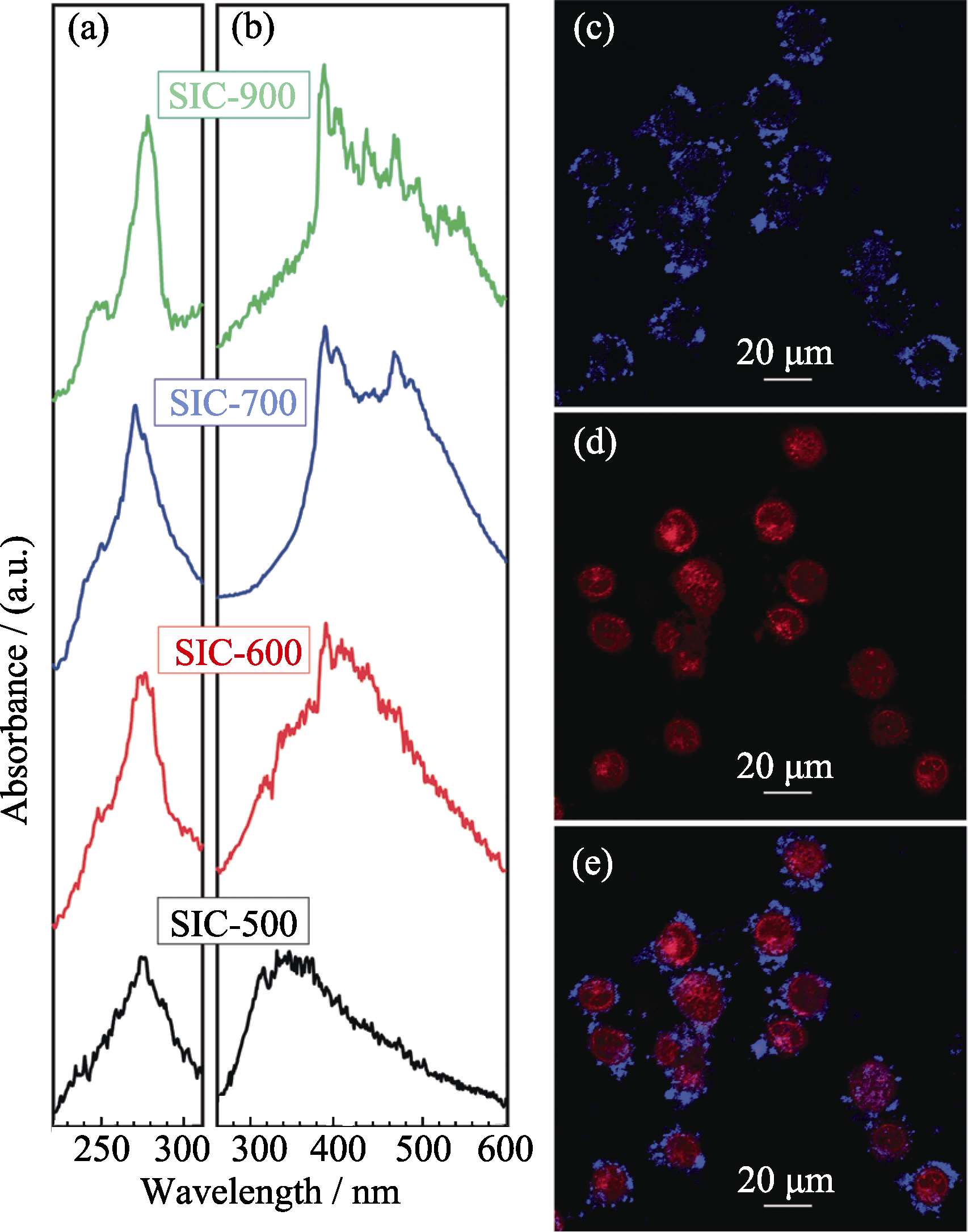
图3 氧缺失的荧光介孔SiO2的激发(a)和发射(b)光谱图; 荧光MSNs用于细胞标记(c)和阿霉素传输(d)的共聚焦图像(图像e是图像c和图像d的融合图像)[41]
Fig. 3 (a) Luminescent excitation and (b) emission spectra of oxygen-deficient fluorescent MSNs; (c) cell labeling and (b) doxorubicin delivery by obtained fluorescent MSNs (e is the merged figure of c and d)[41]

图4 (a)MFNEs的制备示意图; (b)MFNEs标记乳腺癌细胞MCF-7的共聚焦显微镜照片; (c1-c4)体内荷瘤大鼠注射MFNEs前(c1)和注射后(c2: 0.5 h、c3: 1 h、c4: 1.5 h)的MRI-T2图像[19]
Fig. 4 (a) Synthetic procedures of MFNEs; (b) Confocal fluorescent microscopic images of breast cancer MCF-7 cells labeled with MFNEs; (c1-c4) In vivo MRI of a tumor-bearing mouse before (c1) and after injection of MFNEs for different time intervals (c2: 0.5 h, c3: 1 h, c4: 1.5 h)[19]
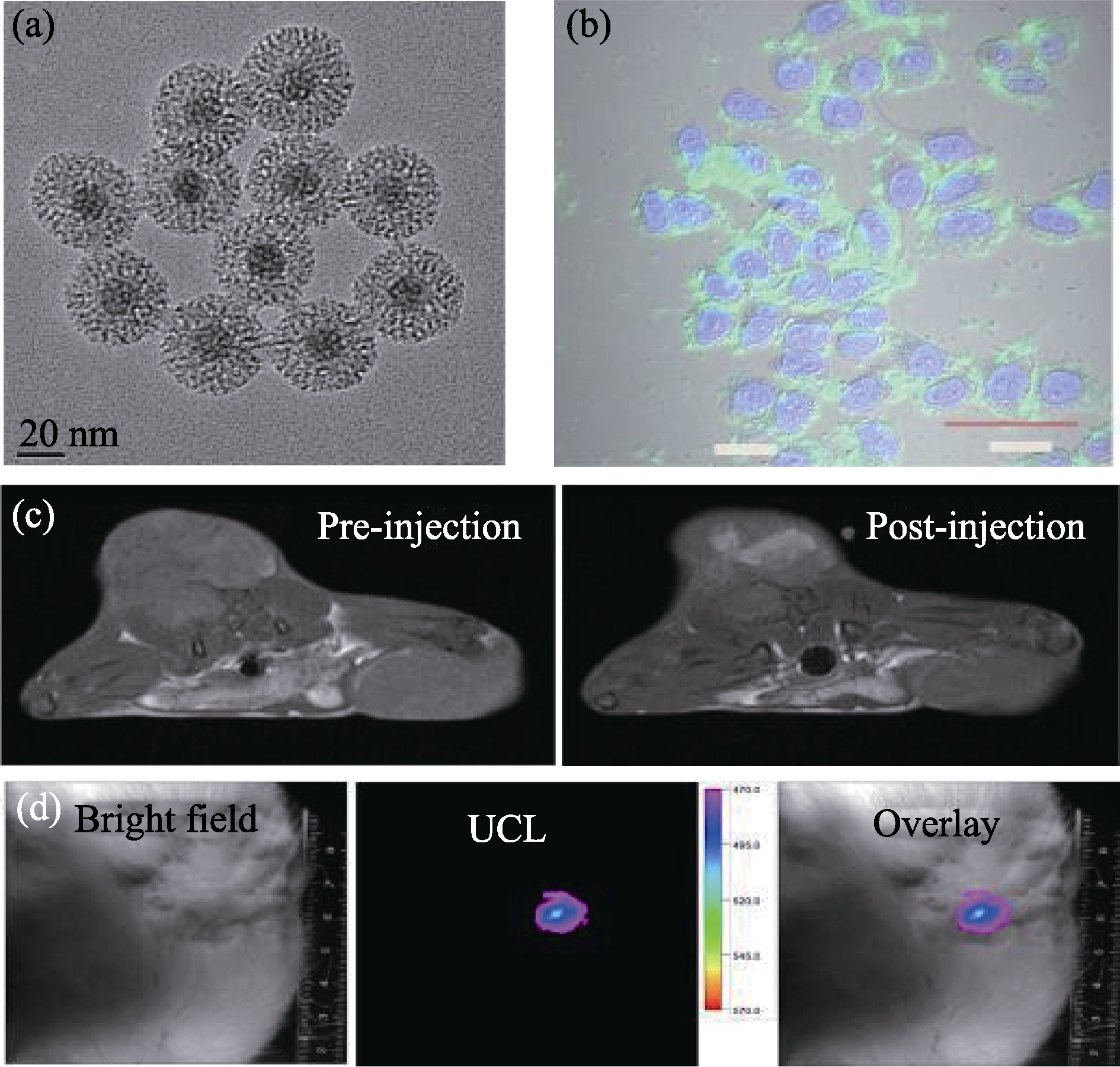
图5 (a)NaYF4:Tm/Yb/Gd@mSiO2的TEM照片; (b) NaYF4:Tm/Yb/Gd@mSiO2标记MCF-7细胞的共聚焦显微镜照片; (c)荷瘤大鼠在注射NaYF4:Tm/Yb/Gd@mSiO2前(左图)后(右图)的MRI-T1图像; (d)荷瘤大鼠在注射NaYF4:Tm/Yb/Gd@mSiO2后的上转换活体荧光图像, 从左到右分别为明场、荧光和融合后的图像[43]
Fig. 5 (a) TEM images of NaYF4:Tm/Yb/Gd@mSiO2 nanocomposites; (b) Confocol images of MCF-7 cells incubated with NaYF4:Tm/Yb/Gd@mSiO2; (c) In vivo MRI-T1 images of tumor (left: pre-injection, right: post-injection); (d) In vivo upconversion luminescence imaging of a tumor-bearing mouse after local injection at the tumor site, from left to right: bright field, upconversion luminescence and overlay images[43]
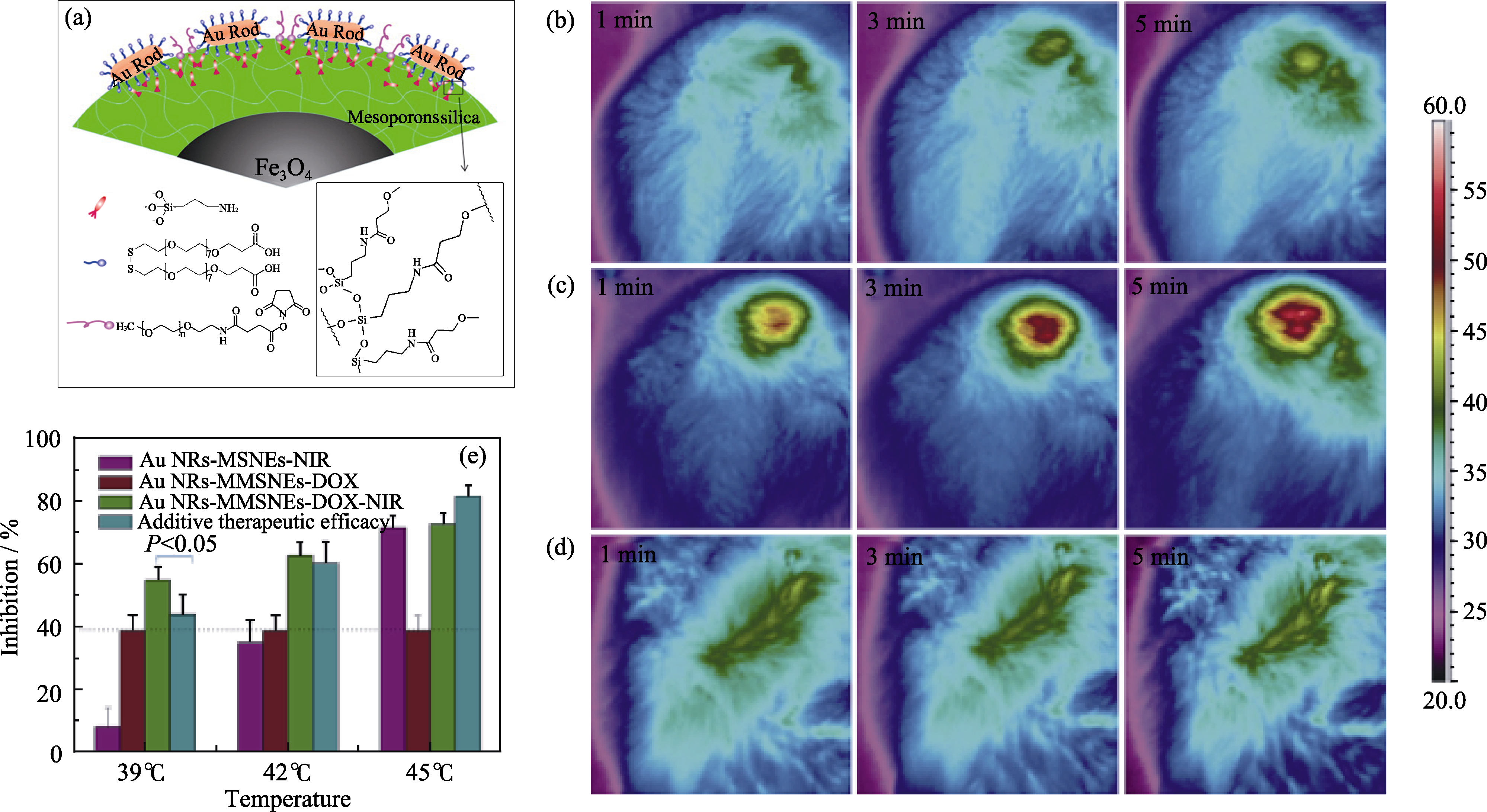
图6 (a)Au棒复合的磁性介孔纳米药物载体(GMMNs)的微观结构示意图; (b-d)注射GMMNs后肿瘤部位在1 W/cm2(b)和 2 W/cm2(c)的808 nm激光辐照下不同时间后的热成像监控图像, 其中(d)为注射PBS参照样在2 W/cm2的808 nm激光辐照下热成像监控图像; (e)不同处理条件下MCF-7细胞生长被抑制的速率, 其中紫色为GMMNs-NIR, 红色为GMMNx-DOX, 绿色为GMMNs-DOX-NIR[44]
Fig. 6 (a) Microscopic structure of GMMNs; (b-d) Thermographic surveillance of photothermal heating at different time points in GMMNs -injected tumor under 1 W/cm2 (b) and 2 W/cm2 (c) irradiations and PBS solution-injected tumor under 2 W/cm2 irradiation (d); (e) Comparison of inhibition rates for MCF cells treated by GMMNs-NIR (purple), GMMNs-DOX (red) and GMMNs-DOX-NIR (green)[44]
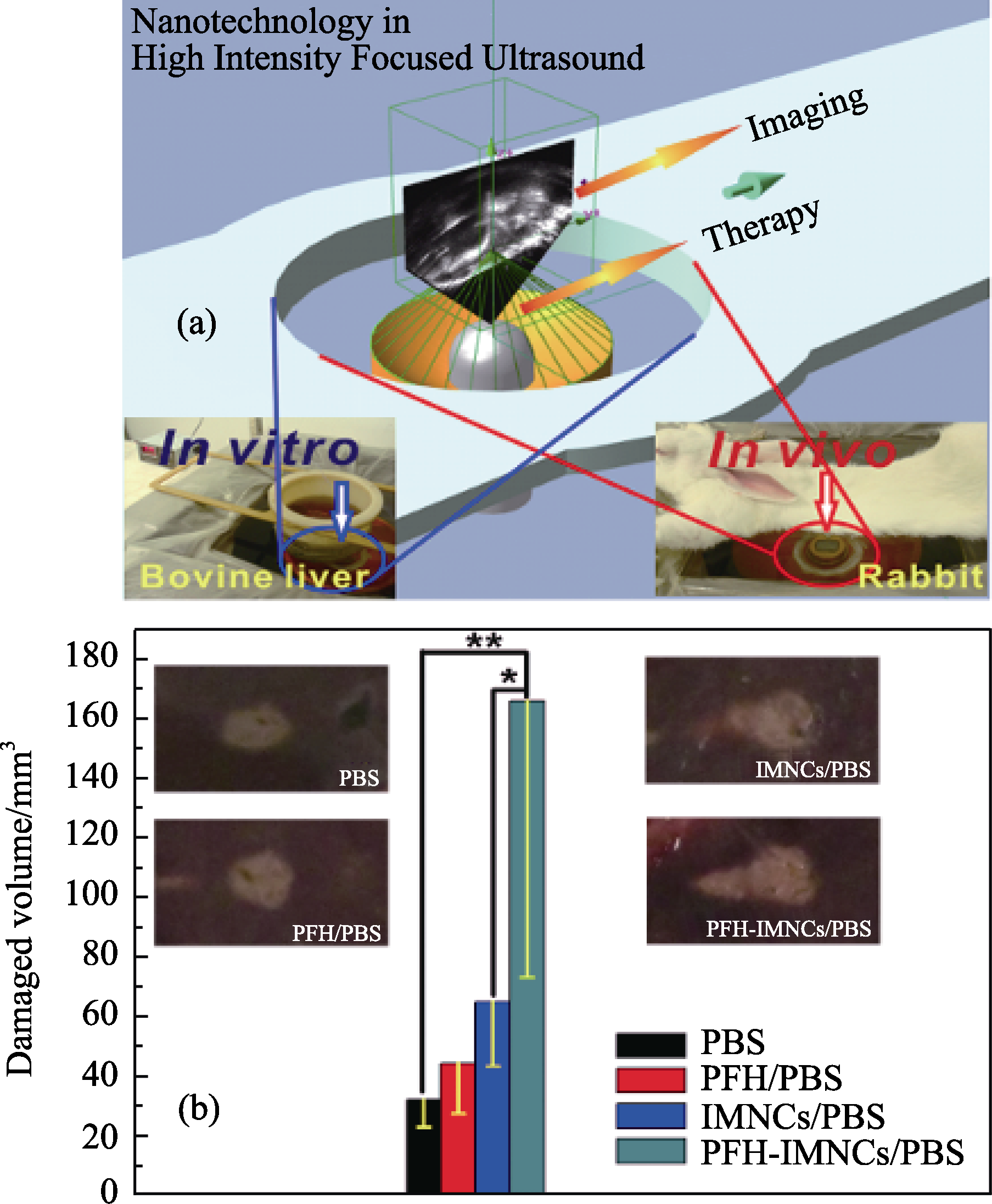
图7 (a)超声引导下HIFU治疗的示意图。通过超声成像, 为HIFU的辐照寻找治疗的靶点, 治疗过程又可以通过超声成像进行监控。体外实验以脱气牛肝为HIFU辐照评价基体, 体内实验以兔子为模型动物; (b)将PBS(200 μL)、PFH/ PBS (200 μL)、IMNCs/PBS(200 μL)和PFH-IMNCs/PBS(200 μL)注射到脱气牛肝后, 在相同的HIFU辐照条件下(150 W/cm2, 5 s; *P < 0.1, **P < 0.05)组织消融体积, 其中b中的插图为不同实验条件下消融后组织的数码照片图[45]
Fig. 7 (a) Schematic illustration of the high intensity focused ultrasound (HIFU) therapeutic principle. The HIFU radiates to the targeted site of the body and the process is monitored by the outside ultrasound imaging. The ex vivo experiment was conducted using bovine liver as a radiation substrate (left digital picture) while the in vivo experiment was carried out using rabbits as a model animal (the right digital picture); (b) Coagulated tissue volume of bovine liver by the intra-tissue injection of different agents such as PBS (200 μL), PFH/PBS (200 μL), IMNCs/PBS (200 μL) and PFH-IMNCs/PBS (200 μL) under the same irradiation power and duration (150 W/cm2, 5 s; *P < 0.1, **P < 0.05). Insets in b are the macroscopic appearances of bovine liver tissues exposed to HIFU with or without using the synergistic agents[45]
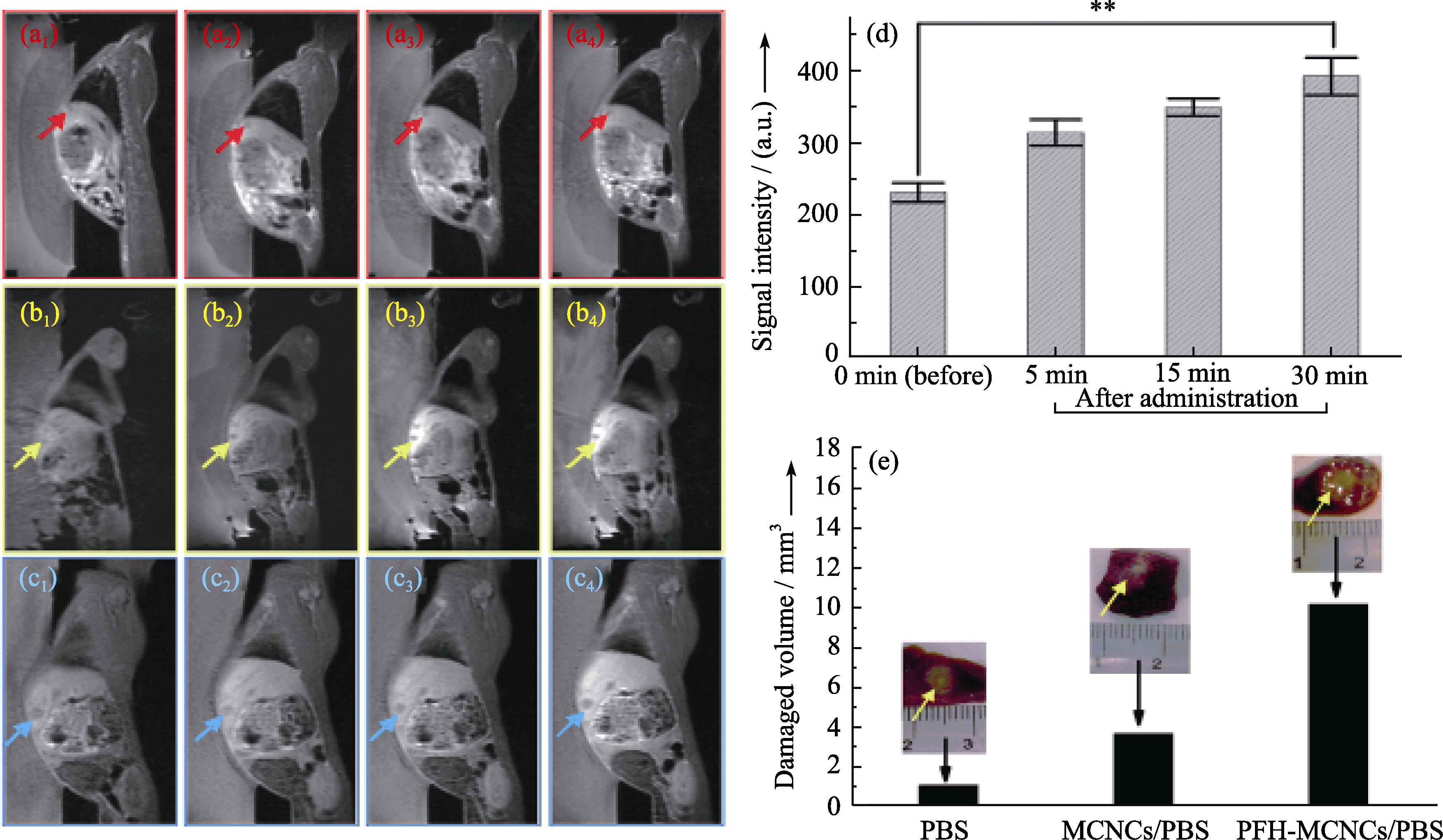
图8 耳静脉注射不同试剂前(a1, b1和c1)后(5 min: a2, b2和c2; 15 min: a3, b3和c3; 30 min: a4, b4和c4)兔VX2肿瘤模型的MRI-T1图像(PBS: a1-a4; MCNCs/PBS: b1-b4; PFH-MCNCs/PBS: c1-c4), 箭头指向肿瘤部位; (d)注射PFH-MCNCs/PBS前后肿瘤部位的MRI-T1信号值(**P < 0.005); (e)在辐照功率为150 W/cm2, 辐照时间为5 s的条件下体内兔VX2肿瘤部位消融坏死的体积, 其中插图为相应的肿瘤消融组织的数码照片[18]
Fig. 8 In vivo T1-weighted MR imaging of rabbits bearing VX2 liver tumor before (a1, b1 and c1) and after (5 min: a2, b2 and c2; 15 min: a3, b3 and c3; 30 min: a4, b4 and c4) administration of different agents (PBS: a1-a4; MCNCs/PBS: b1-b4; PFH-MCNCs/PBS: c1-c4) via ear vein. Arrows indicate the tumor. (d) T1-weighted MRI signal intensities of tumor tissue before and after intravenous administration of PFH-MCNCs/PBS (**P < 0.005); (e) In vivo coagulated necrotic tumor volume by MRI-guided HIFU exposure under the irradiation power of 150 W/cm2 and duration of 5 s in rabbit liver tumors after receiving different agents via ear vein (inset: digital pictures of tumor tissue after HIFU exposure)[18]
| [1] | Lammers T, Aime S, Hennink W E, et al. Theranostic nanomedicine. Accounts Chem. Res., 2011, 44(10): 1029-1038. |
| [2] | Kievit F M, Zhang M Q. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv. Mater., 2011, 23(36): H217-H247. |
| [3] | Lammers T, Kiessling F, Hennink W E, et al. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol. Pharm., 2010, 7(6): 1899-1912. |
| [4] | Slowing II, Vivero-Escoto J L, Wu C W, et al. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev., 2008, 60(11): 1278-1288. |
| [5] | Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed., 2007, 46(40): 7548-7558. |
| [6] | Liong M, Angelos S, Choi E, et al. Mesostructured multifunctional nanoparticles for imaging and drug delivery. J. Mater. Chem., 2009, 19(35): 6251-6257. |
| [7] | Wang S B. Ordered mesoporous materials for drug delivery. Micro. Meso. Mater., 2009, 117(1/2): 1-9. |
| [8] | Kresge C T, Leonowicz M E, Roth W J, et al. Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism. Nature, 1992, 359(6397): 710-712. |
| [9] | Piao Y, Burns A, Kim J, et al. Designed fabrication of silica-based nanostructured particle systems for nanomedicine applications. Adv. Funct. Mater., 2008, 18(23): 3745-3758. |
| [10] | He Q J, Shi J L. Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem., 2011, 21(16): 5845-5855. |
| [11] | Vallet-Regi M, Ramila A, del Real R P, et al. A new property of MCM-41: drug delivery system. Chem. Mater., 2001, 13(2): 308-311. |
| [12] | Coti K K, Belowich M E, Liong M, et al. Mechanised nanoparticles for drug delivery. Nanoscale, 2009, 1(1): 16-39. |
| [13] | Lu J, Liong M, Li Z X, et al. Biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small, 2010, 6(16): 1794-1805. |
| [14] | Li L L, Tang F Q, Liu H Y, et al. In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy. ACS Nano, 2010, 4(11): 6874-6882. |
| [15] | He Q J, Zhang J M, Shi J L, et al. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials, 2010, 31(6): 1085-1092. |
| [16] | Huang X L, Li L L, Liu T L, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano, 2011, 5(7): 5390-5399. |
| [17] | Chen Y, Chen H R, Zeng D P, et al. Core/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano, 2010, 4(10): 6001-6013. |
| [18] | Chen Y, Chen H R, Sun Y, et al. Multifunctional mesoporous composite nanocapsules for highly efficient MRI-guided high-intensity focused ultrasound cancer surgery. Angew. Chem. Int. Ed., 2011, 50(52): 12505-12509. |
| [19] | Chen Y, Chen H R, Zhang S J, et al. Multifunctional mesoporous nanoellipsoids for biological bimodal imaging and magnetically targeted delivery of anticancer drugs. Adv. Funct. Mater., 2011, 21(2): 270-278. |
| [20] | Lee C H, Cheng S H, Wang Y J, et al. Near-infrared mesoporous silica nanoparticles for optical imaging: characterization and in vivo biodistribution. Adv. Funct. Mater., 2009, 19(2): 215-222. |
| [21] | Zhao W R, Gu J L, Zhang L X, et al. Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J. Am. Chem. Soc., 2005, 127(25): 8916-8917. |
| [22] | Deng Y, Qi D, Deng C, et al. Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J. Am. Chem. Soc., 2008, 130(1): 28-29. |
| [23] | Kim J, Lee J E, Lee J, et al. Magnetic fluorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals. J. Am. Chem. Soc., 2006, 128(3): 688-689. |
| [24] | Kim J, Kim H S, Lee N, et al. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew. Chem. Int. Ed., 2008, 47(44): 8438-8441. |
| [25] | Lee J E, Lee N, Kim H, et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J. Am. Chem. Soc., 2010, 132(2): 552-557. |
| [26] | Gan Q, Lu X Y, Yuan Y A, et al. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles- capped mesoporous silica. Biomaterials, 2011, 32(7): 1932-1942. |
| [27] | Viswanathan S, Kovacs Z, Green K N, et al. Alternatives to gadolinium-based metal chelates for magnetic resonance imaging. Chem. Rev., 2010, 110(5): 2960-3018. |
| [28] | Terreno E, Castelli D D, Viale A, et al. Challenges for molecular magnetic resonance imaging. Chem. Rev., 2010, 110(5): 3019-3042. |
| [29] | Taylor K M L, Kim J S, Rieter W J, et al. Mesoporous silica nanospheres as highly efficient MRI contrast agents. J. Am. Chem. Soc., 2008, 130(7): 2154-2155. |
| [30] | Hsiao J K, Tsai C P, Chung T H, et al., Mesoporous silica nanoparticles as a delivery system of gadolinium for effective human stem cell tracking. Small, 2008, 4(9): 1445-1452. |
| [31] | Penfield J G, Reilly R F. What nephrologists need to know about gadolinium. Nat. Clin. Pract. Nephrol., 2007, 3(12): 654-668. |
| [32] | Tromsdorf U I, Bruns O T, Salmen S C, et al. A highly effective, nontoxic T-1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett., 2009, 9(12): 4434-4440. |
| [33] | Perez-Rodriguez J, Lai S, Ehst B D, et al. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment-report of 33 cases. Radiology, 2009, 250(2): 371-377. |
| [34] | Na H B, Lee J H, An K J, et al. Development of a T-1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Ed., 2007, 46(28): 5397-5401. |
| [35] | Peng Y K, Lai C W, Liu C L, et al. A new and facile method to prepare uniform hollow MnO/functionalized mSiO2 core/shell nanocomposites. ACS Nano, 2011, 5(5): 4177-4187. |
| [36] | Schladt T D, Shukoor M I, Schneider K, et al. Au@MnO nanoflowers: hybrid nanocomposites for selective dual functionalization and imaging. Angew. Chem. Int. Ed., 2010, 49(23): 3976-3980. |
| [37] | Kim T, Momin E, Choi J, et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T(1) contrast agents for labeling and MRI tracking of adipose-derived mesenchyrnal stem cells. J. Am. Chem. Soc., 2011, 133(9): 2955-2961. |
| [38] | Chen Y, Chen H, Zhang S, et al. Structure-property relationships in manganese oxide - mesoporous silica nanoparticles used for T1-weighted MRI and simultaneous anti-cancer drug delivery. Biomaterials, 2012, 33(7): 2388-2398. |
| [39] | Pan J, Wan D, Gong J L. PEGylated liposome coated QDs/mesoporous silica core-shell nanoparticles for molecular imaging. Chem. Commun., 2011, 47(12): 3442-3444. |
| [40] | Qian H S, Guo H C, Ho P C L, et al. Mesoporous-silica-coated up-conversion fluorescent nanoparticles for photodynamic therapy. Small, 2009, 5(20): 2285-2290. |
| [41] | He Q J, Shi J L, Cui X Z, et al. Synthesis of oxygen-deficient luminescent mesoporous silica nanoparticles for synchronous drug delivery and imaging. Chem. Commun., 2011, 47(28): 7947-7949. |
| [42] | Feng J, Song S Y, Deng R P, et al. Novel multifunctional nanocomposites: magnetic mesoporous silica nanospheres covalently bonded with near-infrared luminescent lanthanide complexes. Langmuir, 2010, 26(5): 3596-3600. |
| [43] | Liu J, Bu W, Zhang S, et al. Controlled synthesis of uniform and monodisperse upconversion core/mesoporous silica shell nanocomposites for bimodal imaging. Chem. Eur. J., 2012, 18(8): 2335-2341. |
| [44] | Ma M, Chen H, Chen Y, et al. Au capped magnetic core/mesoporous silica shell nanoparticles for combined photothermo-/chemo-therapy and multimodal imaging. Biomaterials, 2012, 33(3): 989-998. |
| [45] | Chen Y, Gao Y, Chen H, et al. Engineering inorganic nanoemulsions/nanoliposomes by fluoride-silica chemistry for efficient delivery/Co-delivery of hydrophobic agents. Adv. Funct. Mater., 2012, 22(8): 1586-1597. |
| [46] | Wang X, Chen H, Chen Y, et al. Perfluorohexane-encapsulated mesoporous silica nanocapsules as enhancement agents for highly efficient high intensity focused ultrasound (HIFU). Adv. Mater., 2012, 24(6): 785-791. |
| [47] | Takegami K, Kaneko Y, Watanabe T, et al. Heating and coagulation volume obtained with high-intensity focused ultrasound therapy: comparison of perflutren protein-type A microspheres and MRX-133 in rabbits. Radiology, 2005, 237(1): 132-136. |
| [48] | Kennedy J E, Haar G R, Cranston D. High intensity focused ultrasound: surgery of the future?Br. J. Radiol., 2003, 76(909): 590-599. |
| [49] | Bailey M R, Khokhlova V A, Sapozhnikov O A, et al. Physical mechanisms of the therapeutic effect of ultrasound - a review. Acoust. Phys., 2003, 49(4): 369-388. |
| [50] | Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics, 2010, 50(2): 221-229. |
| [1] | 魏相霞, 张晓飞, 徐凯龙, 陈张伟. 增材制造柔性压电材料的现状与展望[J]. 无机材料学报, 2024, 39(9): 965-978. |
| [2] | 杨鑫, 韩春秋, 曹玥晗, 贺桢, 周莹. 金属氧化物电催化硝酸盐还原合成氨研究进展[J]. 无机材料学报, 2024, 39(9): 979-991. |
| [3] | 刘鹏东, 王桢, 刘永锋, 温广武. 硅泥在锂离子电池中的应用研究进展[J]. 无机材料学报, 2024, 39(9): 992-1004. |
| [4] | 李世奇, 鲍群群, 胡萍, 施剑林. 基于乙二胺四乙酸插层锌铝双金属氢氧化物的晚期肿瘤抗转移免疫治疗研究[J]. 无机材料学报, 2024, 39(9): 1044-1052. |
| [5] | 黄洁, 汪刘应, 王滨, 刘顾, 王伟超, 葛超群. 基于微纳结构设计的电磁性能调控研究进展[J]. 无机材料学报, 2024, 39(8): 853-870. |
| [6] | 陈乾, 苏海军, 姜浩, 申仲琳, 余明辉, 张卓. 超高温氧化物陶瓷激光增材制造及组织性能调控研究进展[J]. 无机材料学报, 2024, 39(7): 741-753. |
| [7] | 王伟明, 王为得, 粟毅, 马青松, 姚冬旭, 曾宇平. 以非氧化物为烧结助剂制备高导热氮化硅陶瓷的研究进展[J]. 无机材料学报, 2024, 39(6): 634-646. |
| [8] | 蔡飞燕, 倪德伟, 董绍明. 高熵碳化物超高温陶瓷的研究进展[J]. 无机材料学报, 2024, 39(6): 591-608. |
| [9] | 吴晓晨, 郑瑞晓, 李露, 马浩林, 赵培航, 马朝利. SiCf/SiC陶瓷基复合材料高温环境损伤原位监测研究进展[J]. 无机材料学报, 2024, 39(6): 609-622. |
| [10] | 赵日达, 汤素芳. 多孔碳陶瓷化改进反应熔渗法制备陶瓷基复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 623-633. |
| [11] | 方光武, 谢浩元, 张华军, 高希光, 宋迎东. CMC-EBC损伤耦合机理及一体化设计研究进展[J]. 无机材料学报, 2024, 39(6): 647-661. |
| [12] | 张幸红, 王义铭, 程源, 董顺, 胡平. 超高温陶瓷复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 571-590. |
| [13] | 张慧, 许志鹏, 朱从潭, 郭学益, 杨英. 大面积有机-无机杂化钙钛矿薄膜及其光伏应用研究进展[J]. 无机材料学报, 2024, 39(5): 457-466. |
| [14] | 李宗晓, 胡令祥, 王敬蕊, 诸葛飞. 氧化物神经元器件及其神经网络应用[J]. 无机材料学报, 2024, 39(4): 345-358. |
| [15] | 于嫚, 高荣耀, 秦玉军, 艾希成. 上转换发光纳米材料对钙钛矿太阳能电池迟滞效应和离子迁移动力学的影响[J]. 无机材料学报, 2024, 39(4): 359-366. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||