无机材料学报 ›› 2023, Vol. 38 ›› Issue (7): 771-777.DOI: 10.15541/jim20220754 CSTR: 32189.14.10.15541/jim20220754
所属专题: 【信息功能】发光材料与器件(202506)
顾军毅1,2( ), 范武刚2, 张兆泉2, 姚琴2(
), 范武刚2, 张兆泉2, 姚琴2( ), 展红全1(
), 展红全1( )
)
收稿日期:2022-12-15
修回日期:2023-01-09
出版日期:2023-02-21
网络出版日期:2023-02-21
通讯作者:
展红全, 教授. E-mail: zhanhongquan@jci.edu.cn;作者简介:顾军毅(1997-), 男, 硕士研究生. E-mail: 2020028013@stu.jci.edu.cn
基金资助:
GU Junyi1,2( ), FAN Wugang2, ZHANG Zhaoquan2, YAO Qin2(
), FAN Wugang2, ZHANG Zhaoquan2, YAO Qin2( ), ZHAN Hongquan1(
), ZHAN Hongquan1( )
)
Received:2022-12-15
Revised:2023-01-09
Published:2023-02-21
Online:2023-02-21
Contact:
ZHAN Hongquan, professor. E-mail: zhanhongquan@jci.edu.cn;About author:GU Junyi (1997-), male, Master candidate. E-mail: 2020028013@stu.jci.edu.cn
Supported by:摘要:
镨的倍半氧化物(Pr2O3)是合成荧光粉和激光增益介质的重要原料, 由于其易发生镨的变价并在空气中吸水而受到的关注较少。本研究采用不同表征手段研究在空气和在氩氢混合气氛下Pr6O11还原为Pr2O3的过程机理以及两种粉体的物相、微观形貌、粒度及价态等, 并进一步分析以上两种氧化镨的发光特性与镨的价态关系。结果表明: 两种气氛下氧化镨的相变过程显著不同, 还原性的Ar/H2气氛可以加快Pr6O11的还原过程, 在800 ℃即可得到Pr2O3。含Pr3+的Pr2O3除了导带到价带跃迁导致的紫外吸收外, 还存在因f→f跃迁引发的可见光波段的吸收, 而Pr6O11对波长超过320 nm的紫外-可见光有较强吸收, 这与Pr4+和氧之间电荷转移有关。Pr2O3的荧光发射光谱中的宽谱带显示Pr3+的4f5d轨道的最低能级在1S0之下, 同时含Pr4+的Pr6O11在404 nm处的荧光强度降低63%, 这归因于Pr3+/Pr4+之间的能量耗散。这种荧光性能的差异可用于含谱的高氧离子迁移率陶瓷或晶体中Pr的价态分析。本工作研究的Pr6O11到Pr2O3在不同气氛下的转变过程及相关机理性能, 有望推动Pr2O3在不同领域的应用。
中图分类号:
顾军毅, 范武刚, 张兆泉, 姚琴, 展红全. 还原制备Pr2O3粉体及其结构和光学性能研究[J]. 无机材料学报, 2023, 38(7): 771-777.
GU Junyi, FAN Wugang, ZHANG Zhaoquan, YAO Qin, ZHAN Hongquan. Structure and Optical Property of Pr2O3 Powder Prepared by Reduction[J]. Journal of Inorganic Materials, 2023, 38(7): 771-777.
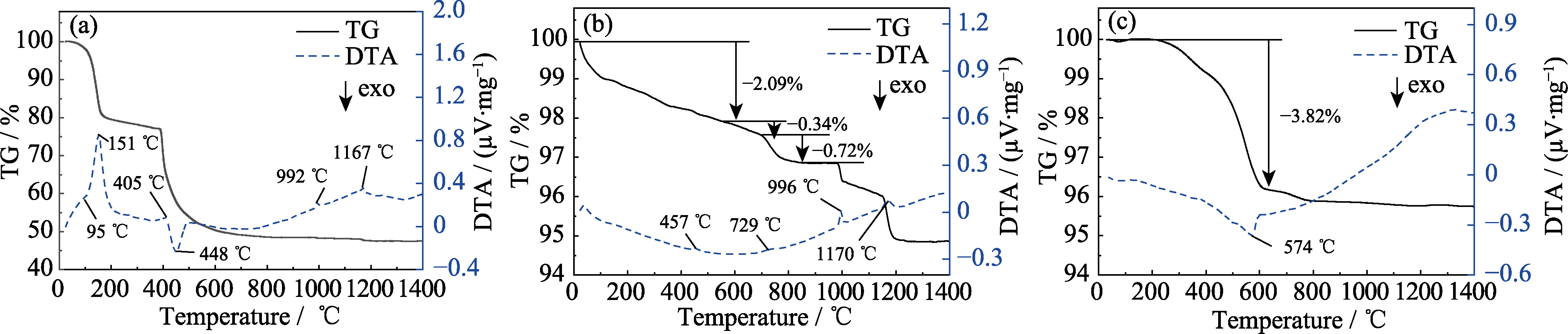
图1 不同气氛下草酸镨和Pr6O11的TG-DTA曲线
Fig. 1 TG-DTA curves of praseodymium oxalate and Pr6O11 in different atmospheres (a) Praseodymium oxalate tested in the air; (b, c) Pr6O11 tested in (b) air and (c) Ar/H2 atmosphere
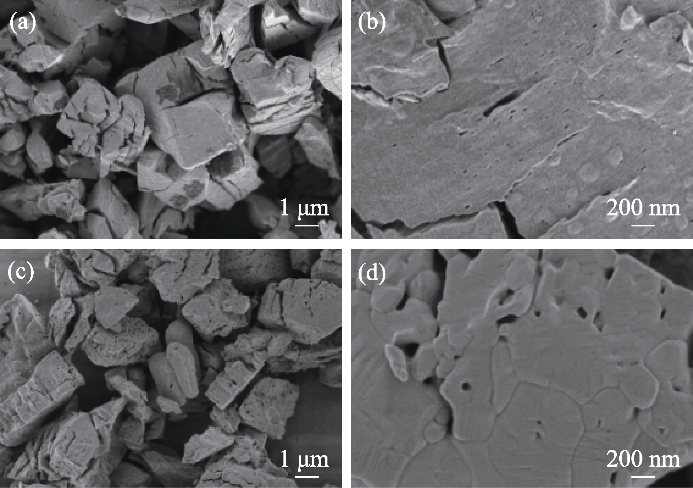
图3 Pr6O11和Pr2O3的SEM照片
Fig. 3 SEM images of Pr6O11 and Pr2O3 (a, b) SEM images of Pr6O11 at (a) low and (b) high magnification; (c, d) SEM images of Pr2O3 at (c) low and (d) high magnification
| Sample | SBET/(m2·g-1) | SLDPSA/(m2·g-1) |
|---|---|---|
| Pr6O11 | 5.8 | 2.0 |
| Pr2O3 | 1.9 | 1.5 |
表1 Pr6O11和Pr2O3比表面积
Table 1 Specific surface areas of Pr6O11 and Pr2O3
| Sample | SBET/(m2·g-1) | SLDPSA/(m2·g-1) |
|---|---|---|
| Pr6O11 | 5.8 | 2.0 |
| Pr2O3 | 1.9 | 1.5 |
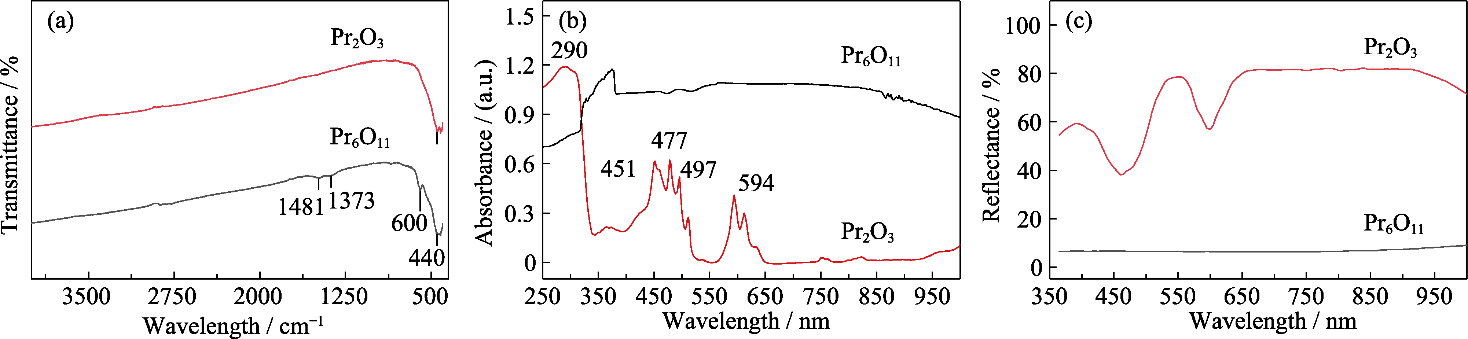
图6 Pr6O11和Pr2O3的红外和紫外-可见光光谱图
Fig. 6 Infrared and UV-Vis spectra of Pr6O11 and Pr2O3 (a) FT-IR spectra; (b) UV-Vis absorption spectra; (c) UV-Vis reflective spectra
| [1] | KOLAVEKAR S B, AYACHIT N H. Impact of Pr2O3 on the physical and optical properties of multi-component borate glasses. Materials Chemistry and Physics, 2021, 257: 123796. |
| [2] |
LAKSHMINARAYANA G, QIU J R, BRIK M G, et al. Photoluminescence of Pr3+-, Dy3+- and Tm3+-doped transparent nanocrystallized KNbGeO5 glasses. Journal of Physics D: Applied Physics, 2008, 41(17):175106.
DOI URL |
| [3] |
HAN C, WU J G, PU C H, et al. High piezoelectric coefficient of Pr2O3-doped Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramics. Ceramics International, 2012, 38(8):6359.
DOI URL |
| [4] |
SCHMEIßER D. The Pr2O3/Si(001) interface. Materials Science in Semiconductor Processing, 2003, 6(1/2/3):59.
DOI URL |
| [5] |
PU Z, QIN J W, AO B Y, et al. Intermediates of carbon monoxide oxidation on praseodymium monoxide molecules: insights from matrix-isolation IR spectroscopy and quantum-chemical calculations. Inorganic Chemistry, 2021, 60(11):7660.
DOI URL |
| [6] |
BAKHT M K, SADEGHI M, AHMADI S J, et al. Preparation of radioactive praseodymium oxide as a multifunctional agent in nuclear medicine: expanding the horizons of cancer therapy using nanosized neodymium oxide. Nuclear Medicine Communications, 2013, 34(1):5.
DOI URL |
| [7] |
SHLYAKHTINA A V, ABRANTES J C C, GOMES E, et al. Effect of Pr3+/Pr4+ ratio on the oxygen ion transport and thermomechanical properties of the pyrochlore and fluorite phases in the ZrO2-Pr2O3 system. International Journal of Hydrogen Energy, 2016, 41(23):9982.
DOI URL |
| [8] |
PAWLAK D A, LUKASIEWICZ T, CARPENTER M, et al. Czochralski crystal growth, microstructure and spectroscopic properties of PrAlO3 perovskite. Journal of Crystal Growth, 2005, 282(1/2):260.
DOI URL |
| [9] |
GUZIK A, TALIK E, PAJACZKOWSKA A, et al. Magnetic properties of manganese doped PrAlO3 monocrystalline fibres. Materials Science-Poland, 2014, 32(4):633.
DOI URL |
| [10] | LIU Z G, ZHENG Z R, HUANG X Q, et al. The Pr4+ ions in Mg doped PrGaO3 perovskites. Journal of Alloys and Compounds, 2004, 363(1/2):60. |
| [11] |
NIGRO R L, TORO R G, MALANDRINO G, et al. A simple route to the synthesis of Pr2O3 high-k thin films. Advanced Materials, 2003, 15(13):1071.
DOI URL |
| [12] | GAZULLA M F, VENTURA M J, ANDREU C, et al. Praseodymium oxides. complete characterization by determining oxygen content. Microchemical Journal, 2019, 148: 291. |
| [13] | FU X M, SUN H, YANG Z Z. Study on preparation of nano- granular Pr6O11 by thermal decomposition and its optical absorption properties. Rare Metals and Cemented Carbides, 2019, 47(5):33. |
| [14] |
MASO N, BELTRAN H, MUNOZ R, et al. Optimization of praseodymium-doped cerium pigment synthesis temperature. Journal of the American Ceramic Society, 2003, 86(3):425.
DOI URL |
| [15] |
FUCHI S, ISHIKAWA W, NISHIMURA S, et al. Luminescence properties of Pr6O11-doped and PrF3-doped germanate glasses for wideband NIR phosphor. Journal of Materials Science: Materials in Electronics, 2016, 28(10):7042.
DOI |
| [16] |
WILKENS H, GEVERS S, RÖHE S, et al. Structural changes of ultrathin cub-PrO2(111)/Si(111) films due to thermally induced oxygen desorption. The Journal of Physical Chemistry C, 2014, 118(6):3056.
DOI URL |
| [17] |
LV P, ZHANG L J, KOPPALA S, et al. Decomposition study of praseodymium oxalate as a precursor for praseodymium oxide in the microwave field. ACS Omega, 2020, 5(34):21338.
DOI PMID |
| [18] |
ANENBURG M, BURNHAM A D, HAMILTON J L. Quadrivalent praseodymium in planetary materials. American Mineralogist, 2020, 105(12): 1802.
DOI URL |
| [19] |
TREU B L, FAHRENHOLTZ W G, O'KEEFE M J. Thermal decomposition behavior of praseodymium oxides, hydroxides, and carbonates. Inorganic Materials, 2011, 47(9):974.
DOI URL |
| [20] |
THANGADURAI V, HUGGINS R A, WEPPNER W. Mixed ionic- electronic conductivity in phases in the praseodymium oxide system. Journal of Solid State Electrochemistry, 2001, 5(7/8):531.
DOI URL |
| [21] |
NETZ A, CHU W F, THANGADURAI V, et al. Investigations of praseodymium oxide electrodes in lithium concentration cells. Ionics, 1999, 5(5/6):426.
DOI URL |
| [22] |
GONG Y Y, CHU R Q, XU Z J, et al. Varistor, dielectric, and luminescent properties of Pr6O11-doped TiO2 multifunctional ceramics. Journal of the American Ceramic Society, 2016, 99(9):2995.
DOI URL |
| [23] | POMIRO F J, GAVIRÍA J P, FOUGA G G, et al. Chlorination of Pr2O3 and Pr6O11. crystal structure, magnetic and spectroscopic properties of praseodymium oxychloride. Journal of Alloys and Compounds, 2019, 776: 919. |
| [24] | SU L W, ZHANG Y F, ZHAN X Y, et al. Pr6O11: temperature- dependent oxygen vacancy regulation and catalytic performance for lithium-oxygen batteries. ACS Applied Materials & Interfaces, 2022, 14(36):40975. |
| [25] | WANG Y, DENG G F, CAI C L, et al. Mechanism and kinetics of thermal decomposition of dysprosium oxalate with six water. Nonferrous Metals Science and Engineering, 2017, 8(6):98. |
| [26] |
DEVANGAD P, TAMBOLI M, SHAMEEM K M M, et al. Spectroscopic identification of rare earth elements in phosphate glass. Laser Physics, 2018, 28(1):015703.
DOI URL |
| [27] | JIN T T, ZHANG Z J, ZHANG H, et al. Crystal structure, phase transition and optical properties of ν-PrBO3. Journal of Inorganic Materials, 2013, 28(10):1153. |
| [28] | ZHANG X W, ZHANG S, LI X D, et al. Temperature response properties of Pr3+ in Ca2LuScGa2Ge2O12 with garnet structure. Journal of the Chinese Society of Rare Earths, 2022, 40(5):752. |
| [29] | KUNIMI S, FUJIHARA S. Synthesis and luminescent properties of CeO2:Pr4+,Eu3+ red pigments. ECS Journal of Solid State Science and Technology, 2012, 1(1):32. |
| [30] | KRISHNA CHANDAR N, JAYAVEL R. Structural, morphological and optical properties of solvothermally synthesized Pr(OH)3 nanoparticles and calcined Pr6O11 nanorods. Materials Research Bulletin, 2014, 50: 417. |
| [1] | 叶君豪, 周真真, 胡辰, 王雁斌, 荆延秋, 李廷松, 程梓秋, 吴俊林, IVANOV Maxim, HRENIAK Dariusz, 李江. 共沉淀纳米粉体制备Yb:Sc2O3透明陶瓷的微结构与光学性能[J]. 无机材料学报, 2025, 40(2): 215-224. |
| [2] | 吕朝阳, 徐勇, 杨久延, 涂广升, 涂兵田, 王皓. MgF2助剂对MgAl1.9Ga0.1O4透明陶瓷的制备与光学性能的影响[J]. 无机材料学报, 2024, 39(5): 531-538. |
| [3] | 李悦, 张旭良, 景芳丽, 胡章贵, 吴以成. 铈掺杂硼酸钙镧晶体的生长与性能研究[J]. 无机材料学报, 2023, 38(5): 583-588. |
| [4] | 王海东, 王燕, 朱昭捷, 李坚富, LAKSHMINARAYANA Gandham, 涂朝阳. Dy3+掺杂SrGdGa3O7晶体的晶体生长, 结构、光学和可见光荧光特性[J]. 无机材料学报, 2023, 38(12): 1475-1482. |
| [5] | 李文俊, 王皓, 涂兵田, 谌强国, 郑凯平, 王为民, 傅正义. 宽光谱透过Mg0.9Al2.08O3.97N0.03透明陶瓷的制备与性能研究[J]. 无机材料学报, 2022, 37(9): 969-975. |
| [6] | 刘强, 王倩, 陈鹏辉, 李晓英, 章立轩, 谢腾飞, 李江. 两步烧结法制备红色Ce:8YSZ透明陶瓷及其性能研究[J]. 无机材料学报, 2022, 37(8): 911-917. |
| [7] | 刘子玉, TOCI Guido, PIRRI Angela, PATRIZI Barbara, 冯亚刚, 陈肖朴, 胡殿君, 田丰, 吴乐翔, VANNINIMatteo, 李江. 固体激光用Nd:Lu2O3透明陶瓷的制备和光学性能研究[J]. 无机材料学报, 2021, 36(2): 210-216. |
| [8] | 黄新友, 刘玉敏, 刘洋, 李晓英, 冯亚刚, 陈肖朴, 陈鹏辉, 刘欣, 谢腾飞, 李江. 醇水共沉淀法制备Yb:YAG透明陶瓷及其性能研究[J]. 无机材料学报, 2021, 36(2): 217-224. |
| [9] | 韦家蓓, TOCIGuido, PIRRIAngela, PATRIZIBarbara, 冯亚刚, VANNINIMatteo, 李江. 共沉淀纳米粉体制备Yb:CaF2激光陶瓷及其性能研究[J]. 无机材料学报, 2019, 34(12): 1341-1348. |
| [10] | 刘小元, 刘宝丹, 姜亚南, 王柯, 周洋, 杨兵, 张兴来, 姜辛. 形貌可控及光学吸收性能可调的钙钛矿型SrTiO3纳米结构的原位生长[J]. 无机材料学报, 2019, 34(1): 65-71. |
| [11] | 杨锁龙, 王晓方, 蒋春丽, 赵雅文, 曾荣光, 王怀胜, 赖新春. InP量子点的掺杂及其光学性能[J]. 无机材料学报, 2016, 31(10): 1051-1057. |
| [12] | 周 鼎, 施 鹰, 范灵聪, 林德宝, 孙泽清, 徐家跃. Ce, Pr离子双掺LuAG透明陶瓷制备及光学性能[J]. 无机材料学报, 2016, 31(10): 1099-1102. |
| [13] | 杨雨佳, 王 晶, 何慧芬. 铽离子掺杂锆酸钡粉体制备及其光学性能研究[J]. 无机材料学报, 2016, 31(1): 27-33. |
| [14] | 刘 婧, 刘 军, 李 江, 林 丽, 潘裕柏, 程晓农, 郭景坤. 球磨转速对Nd:YAG透明陶瓷的显微结构及光学性能的影响[J]. 无机材料学报, 2015, 30(6): 581-587. |
| [15] | 杨 睿, 介万奇, 孙晓燕, 杨 敏, 呼 唤, 蔺 云. 温度梯度溶液法生长的Cr掺杂的ZnTe晶体的表征[J]. 无机材料学报, 2015, 30(4): 401-407. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||