无机材料学报 ›› 2020, Vol. 35 ›› Issue (12): 1295-1306.DOI: 10.15541/jim20200134 CSTR: 32189.14.10.15541/jim20200134
所属专题: 能源材料论文精选(一):锂离子电池(2020)
• 综述 • 下一篇
收稿日期:2020-03-16
修回日期:2020-04-22
出版日期:2020-12-20
网络出版日期:2020-05-10
作者简介:郑时有(1974–), 男, 教授. E-mail: syzheng@usst.edu.cn
基金资助:
ZHENG Shiyou( ),DONG Fei,PANG Yuepeng,HAN Pan,YANG Junhe
),DONG Fei,PANG Yuepeng,HAN Pan,YANG Junhe
Received:2020-03-16
Revised:2020-04-22
Published:2020-12-20
Online:2020-05-10
About author:ZHENG Shiyou(1974–), male, professor. E-mail: syzheng@usst.edu.cn
Supported by:摘要:
负极材料是锂离子电池的重要组成部分, 目前商用锂离子电池的负极材料石墨的理论比容量仅为372 mAh/g, 严重制约了锂离子电池的进一步发展。在众多的锂离子电池负极材料新体系中, 金属氧化物具有理论比容量高、价格低廉、环境相容性好等优点, 受到广泛关注, 但是其存在导电性差、充放电体积变化大等缺点。研究发现, 纳米化可以在保持金属氧化物优点的同时克服其缺点, 因此成为金属氧化物基负极材料的研究热点。本文对近期纳米金属氧化物基锂离子电池负极材料研究的主要成果进行综述, 着重关注几种具有代表性的金属氧化物及其复合物的纳米结构设计与性能优化, 并为后续相关研究提出建议。
中图分类号:
郑时有, 董飞, 庞越鹏, 韩盼, 杨俊和. 纳米金属氧化物基锂离子电池负极材料研究进展[J]. 无机材料学报, 2020, 35(12): 1295-1306.
ZHENG Shiyou, DONG Fei, PANG Yuepeng, HAN Pan, YANG Junhe. Research Progress on Nanostructured Metal Oxides as Anode Materials for Li-ion Battery[J]. Journal of Inorganic Materials, 2020, 35(12): 1295-1306.
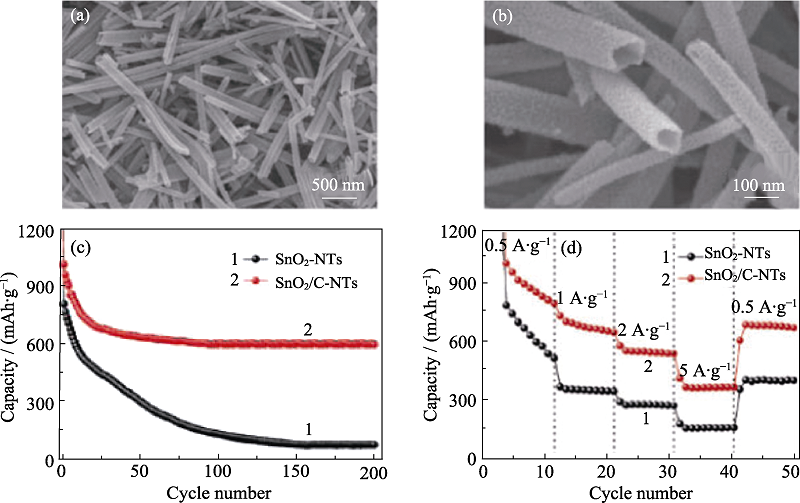
图1 (a, b)SnO2/C-NTs的SEM照片; (c)循环稳定性曲线以及(d)倍率性能曲线[23]
Fig. 1 (a, b) SEM images of SnO2/C-NTs; (c) Cycling performance at 500 mA/g, and (d) rate capabilities of SnO2-NTs and SnO2/C-NTs[23]
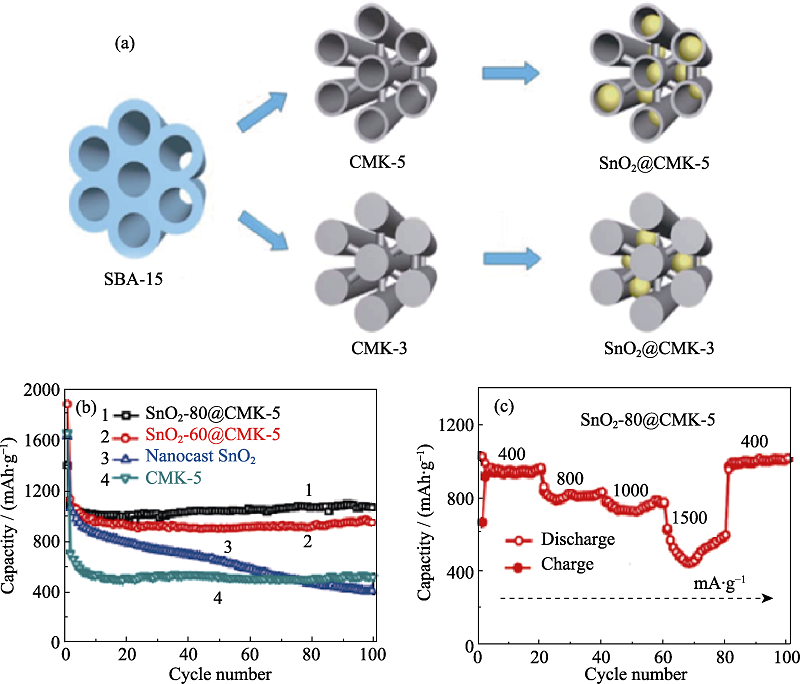
图2 (a)超细SnO2纳米颗粒固定在介孔碳的孔道中的合成原理示意图; SnO2/介孔碳复合电极材料的(b)循环稳定性曲线和(c)倍率性能曲线[31]
Fig. 2 (a) Illustration of the synthesis principles of ultrafine SnO2 NPs immobilized in the mesopore channels of mesoporous carbon; (b) Cycle performance at 200 mA/g between 0.005 and 3 V, and (c) rate performance of electrodes based on different SnO2 content[31]
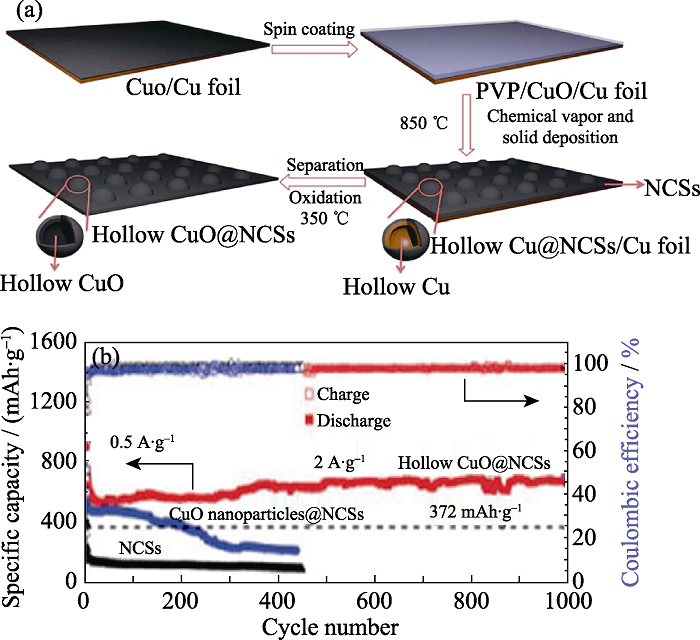
图4 (a)空心CuO@NCS复合材料的合成示意图, (b)在100 mA/g下的循环稳定性曲线[46]
Fig. 4 (a) Schematic illustration of the synthesis of hollow CuO@NCS composites; (b) Cycle performance of CuO@NCS at 100 mA/g[46]

图5 (a)颗粒与片上以及(b~d)片与片上的Fe2O3-石墨烯复合材料的TEM照片[57]
Fig. 5 TEM images of (a) Fe2O3-graphene particle-on-sheet composite, and (b-d) Fe2O3-graphene sheet-on-sheet composite[57]
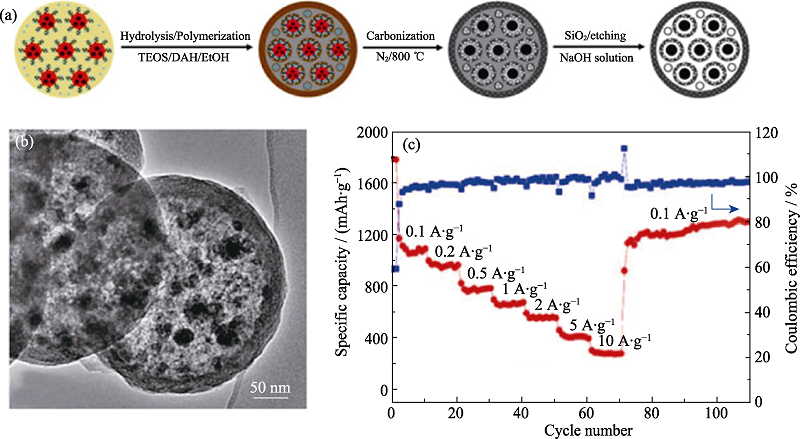
图6 Fe3O4@N-HPCNs的(a)合成过程示意图, (b)TEM照片, 以及(c)循环稳定性曲线[63]
Fig. 6 (a) Illustration for the synthetic procedure, (b) TEM image, and (c) rate capabilities of Fe3O4@N-HPCNs[63]
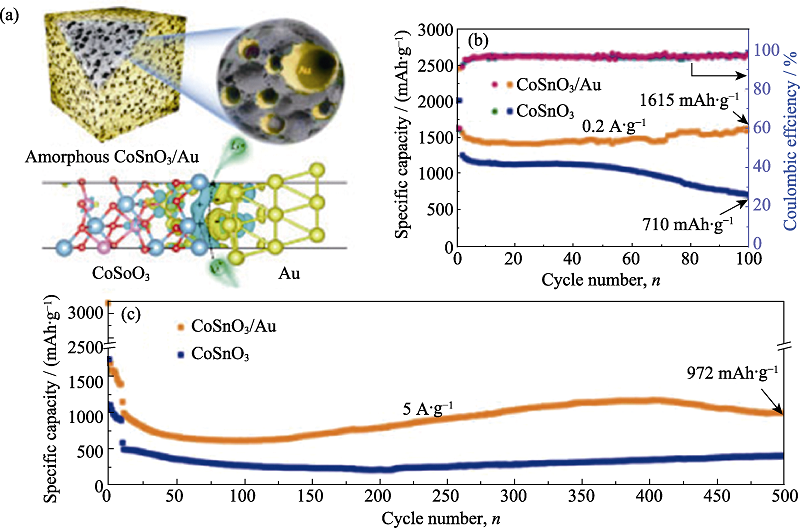
图7 (a)非晶多孔CoSnO3/Au复合纳米立方的结构示意图; 在(b) 0.2和(c) 5 A/g电流密度下的循环稳定性曲线[65]
Fig. 7 (a) Structure diagrams of the amorphous porous CoSnO3/Au composite nanocubes; Cycle performance of the amorphous porous CoSnO3/Au composite nanocubes at (b) 0.2 and (c) 5 A/g[65]
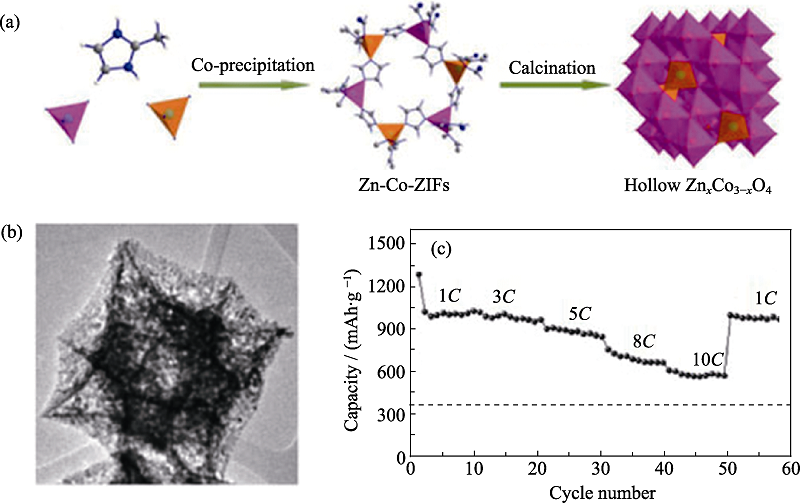
图8 尖晶石ZnxCo3-xO4空心多面体的(a)合成过程示意图, (b)TEM照片, 以及(c)倍率性能曲线[79]
Fig. 8 (a) Schematic illustration for the synthetic procedure, (b) TEM image, and (c) rate capabilities of spinel ZnxCo3-xO4 hollow polyhedron[79]
| Materials structure | First cyclic capacity/(mAh?g-1) (Current density/(A?g-1)) | Coulombic efficiency | Cycling performance/ (mAh?g-1) (Current density/ (A?g-1), cycle number) | Rate performance/(mAh?g-1) (Current density/(A?g-1)) | Ref. |
|---|---|---|---|---|---|
| SnO2 NPs (5~20 nm) | 1310 (0.1) | 69% | 800 (0.1, 100) | 850 (1); 800 (2) | [ |
| SnO2/C-NT (15 nm) | 483 (0.5) | 51% | 596 (0.5, 200) | 683 (1); 550 (2) | [ |
| SnO2 nanosheets (7.4 nm) | 1338 (0.1) | 55% | 763 (0.1, 300) | 460 (1); 280 (2) | [ |
| SnO2 HS (50 nm) | 736 (0.1) | 47% | 540 (0.1, 50) | 550 (1); 422 (2) | [ |
| SnO2/C (50~100 nm) | 998 (0.1) | 69% | 750 (0.1, 100) | 413 (1); 325 (2) | [ |
| C-SnO2/CNT (7 nm) | 1373 (1) | 52% | 950 (1, 100) | 1100 (1); 950 (2) | [ |
| SnO2@G-SWCNT (7 nm) | 1007 (0.1) | 53% | 785 (0.1, 100) | 510 (1); 426 (2) | [ |
| SnO2@CMK-5 (4~5 nm) | 694 (0.2) | 71% | 1039 (0.1, 100) | 770 (1) | [ |
| SnO2/C (2.8 nm) | 899 (1.4) | 44% | 560 (1.4, 100) | 700 (1.4); 538 (2.8) | [ |
| CuO spheres (400 nm) | 590 (0.45) | 66% | 400 (0.45, 50) | - | [ |
| CuO octahedra (5 nm) | 506 (0.5) | 70% | 785 (0.5, 50) | 488 (1); 370 (2) | [ |
| CuO labyrinths (20 nm) | 645 (0.1) | 66% | 330 (1, 100) | 340 (1.3); 255 (3.4) | [ |
| CuO NRAs (2~3 μm) | 751 (0.1) | 56% | 671 (0.1, 150) | 367 (1); 300 (2) | [ |
| CuO spheres (10 nm) | 552 (0.67) | 55% | 750 (0.67, 350) | 650 (1.3); 600 (3.4) | [ |
| CuO/MWCNT (10 nm) | 462 (0.07) | 69% | 650 (0.07, 100) | 590 (1.3); 590 (2) | [ |
| Cu2O/CuO/rGO (500 nm) | 375 (0.3) | 75% | 570 (0.3, 100) | 350 (1.3); 250 (2.7) | [ |
| Cu@NCSs (45 nm) | 909 (0.5) | 62% | 602 (0.5, 200) | 760 (1); 570 (2) | [ |
| Graphene/Fe2O3 (40 nm) | 1074 (0.1) | 65% | 800 (0.1, 50) | - | [ |
| Fe2O3/CA (5~50 nm) | 836 (0.1) | 55% | 635 (0.1, 100) | 652 (0.4); 546 (0.8) | [ |
| RG-O/Fe2O3 (60 nm) | 1219 (0.1) | 72% | 1027 (0.1, 50) | 970 (0.4); 760 (0.8) | [ |
| Fe3O4@PCFs (10~60 nm) | 1014 (0.2) | 72% | 920 (0.2, 80) | 677 (1); 523 (2) | [ |
| Fe3O4/PPy (200 nm) | 493 (1) | 89% | 554 (1, 100) | 500 (1); 330 (2) | [ |
| Fe3O4-CNTs (50~100 nm) | 845 (0.05) | 77% | 702 (0.05, 50) | - | [ |
| Fe3O4@N-HPCNs (6 nm) | 521 (0.1) | 54% | 1240 (0.1, 100) | 700 (1); 600 (2) | [ |
| CoMoO4 NPs (2~10 nm) | 1051 (0.2) | 72% | 1185 (0.2, 100) | 900 (1); 850 (2) | [ |
| CoSnO3/Au cube (70 nm) | 1693 (0.2) | 68% | 1615 (0.2, 100) | 1425 (1); 1289 (2) | [ |
| NiFe2O4 NPs (20 nm) | 1177 (0.1) | 79% | 1390 (0.1, 20) | 823 (1); 725 (3) | [ |
| ZnxCo3-xO4 (10 nm) | 967 (0.1) | 76% | 990 (0.1, 50) | 1020 (0.9); 988 (2.7) | [ |
| NixCo3-xO4 (40 nm) | 1133 (0.1) | 70% | 1109 (0.1, 100) | 864 (1); 728 (2) | [ |
表1 不同金属氧化物负极材料的结构与综合电化学性能
Table 1 Structures and comprehensive electrochemical performances of different metal oxide anode materials
| Materials structure | First cyclic capacity/(mAh?g-1) (Current density/(A?g-1)) | Coulombic efficiency | Cycling performance/ (mAh?g-1) (Current density/ (A?g-1), cycle number) | Rate performance/(mAh?g-1) (Current density/(A?g-1)) | Ref. |
|---|---|---|---|---|---|
| SnO2 NPs (5~20 nm) | 1310 (0.1) | 69% | 800 (0.1, 100) | 850 (1); 800 (2) | [ |
| SnO2/C-NT (15 nm) | 483 (0.5) | 51% | 596 (0.5, 200) | 683 (1); 550 (2) | [ |
| SnO2 nanosheets (7.4 nm) | 1338 (0.1) | 55% | 763 (0.1, 300) | 460 (1); 280 (2) | [ |
| SnO2 HS (50 nm) | 736 (0.1) | 47% | 540 (0.1, 50) | 550 (1); 422 (2) | [ |
| SnO2/C (50~100 nm) | 998 (0.1) | 69% | 750 (0.1, 100) | 413 (1); 325 (2) | [ |
| C-SnO2/CNT (7 nm) | 1373 (1) | 52% | 950 (1, 100) | 1100 (1); 950 (2) | [ |
| SnO2@G-SWCNT (7 nm) | 1007 (0.1) | 53% | 785 (0.1, 100) | 510 (1); 426 (2) | [ |
| SnO2@CMK-5 (4~5 nm) | 694 (0.2) | 71% | 1039 (0.1, 100) | 770 (1) | [ |
| SnO2/C (2.8 nm) | 899 (1.4) | 44% | 560 (1.4, 100) | 700 (1.4); 538 (2.8) | [ |
| CuO spheres (400 nm) | 590 (0.45) | 66% | 400 (0.45, 50) | - | [ |
| CuO octahedra (5 nm) | 506 (0.5) | 70% | 785 (0.5, 50) | 488 (1); 370 (2) | [ |
| CuO labyrinths (20 nm) | 645 (0.1) | 66% | 330 (1, 100) | 340 (1.3); 255 (3.4) | [ |
| CuO NRAs (2~3 μm) | 751 (0.1) | 56% | 671 (0.1, 150) | 367 (1); 300 (2) | [ |
| CuO spheres (10 nm) | 552 (0.67) | 55% | 750 (0.67, 350) | 650 (1.3); 600 (3.4) | [ |
| CuO/MWCNT (10 nm) | 462 (0.07) | 69% | 650 (0.07, 100) | 590 (1.3); 590 (2) | [ |
| Cu2O/CuO/rGO (500 nm) | 375 (0.3) | 75% | 570 (0.3, 100) | 350 (1.3); 250 (2.7) | [ |
| Cu@NCSs (45 nm) | 909 (0.5) | 62% | 602 (0.5, 200) | 760 (1); 570 (2) | [ |
| Graphene/Fe2O3 (40 nm) | 1074 (0.1) | 65% | 800 (0.1, 50) | - | [ |
| Fe2O3/CA (5~50 nm) | 836 (0.1) | 55% | 635 (0.1, 100) | 652 (0.4); 546 (0.8) | [ |
| RG-O/Fe2O3 (60 nm) | 1219 (0.1) | 72% | 1027 (0.1, 50) | 970 (0.4); 760 (0.8) | [ |
| Fe3O4@PCFs (10~60 nm) | 1014 (0.2) | 72% | 920 (0.2, 80) | 677 (1); 523 (2) | [ |
| Fe3O4/PPy (200 nm) | 493 (1) | 89% | 554 (1, 100) | 500 (1); 330 (2) | [ |
| Fe3O4-CNTs (50~100 nm) | 845 (0.05) | 77% | 702 (0.05, 50) | - | [ |
| Fe3O4@N-HPCNs (6 nm) | 521 (0.1) | 54% | 1240 (0.1, 100) | 700 (1); 600 (2) | [ |
| CoMoO4 NPs (2~10 nm) | 1051 (0.2) | 72% | 1185 (0.2, 100) | 900 (1); 850 (2) | [ |
| CoSnO3/Au cube (70 nm) | 1693 (0.2) | 68% | 1615 (0.2, 100) | 1425 (1); 1289 (2) | [ |
| NiFe2O4 NPs (20 nm) | 1177 (0.1) | 79% | 1390 (0.1, 20) | 823 (1); 725 (3) | [ |
| ZnxCo3-xO4 (10 nm) | 967 (0.1) | 76% | 990 (0.1, 50) | 1020 (0.9); 988 (2.7) | [ |
| NixCo3-xO4 (40 nm) | 1133 (0.1) | 70% | 1109 (0.1, 100) | 864 (1); 728 (2) | [ |
| [1] |
CHEN H, LING M, HENCZ L, et al. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy- storage devices. Chemical Reviews, 2018,118(18):8936-8982.
DOI URL PMID |
| [2] | XIONG Q Q, JI Z G. Controllable growth of MoS2/C flower-like microspheres with enhanced electrochemical performance for lithium ion batteries. Journal of Alloys and Compounds, 2016,673:215-219. |
| [3] | ZHANG Y, XIA X, LIU B, et al. Multiscale graphene-based materials for applications in sodium ion batteries. Advanced Energy Materials, 2019,9(8):1803342. |
| [4] | LIN Y, GAO M X, ZHU D, et al. Effects of carbon coating and iron phosphides on the electrochemical properties of LiFePO4/C. Journal of Power Sources, 2008,184(2):444-448. |
| [5] | ZHANG S, GU H, PAN H, et al. A novel strategy to suppress capacity and voltage fading of Li- and Mn-rich layered oxide cathode material for lithium-ion batteries. Advanced Energy Materials, 2017,7(6):1601066. |
| [6] | GENG K M, WU J J, GENG H B, et al. N-doped carbon- encapsulated cobalt nanoparticles on N-doped graphene nanosheets as a high-capacity anode material for lithium-ion storage. Chinese Journal of Inorganic Chemistry, 2016,32(9):1495-1502. |
| [7] |
ZHANG X F, ZHAO Y, PATEL Y, et al. Potentiometric measurement of entropy change for lithium batteries. Physical Chemistry Chemical Physics, 2017,19(15):9833-9842.
DOI URL PMID |
| [8] | DONG H, KOENIG G M. A review on synthesis and engineering of crystal precursors produced via coprecipitation for multicomponent lithium-ion battery cathode materials. CrystEngComm, 2020,22(9):1514-1530. |
| [9] | SHEN S, ZHANG S, DENG S, et al. Bioinspired large-scale production of multidimensional high-rate anodes for both liquid & solid-state lithium ion batteries. Journal of Materials Chemistry A, 2019,7(40):22958-22966. |
| [10] |
AN W, GAO B, MEI S, et al. Scalable synthesis of ant-nest-like bulk porous silicon for high-performance lithium-ion battery anodes. Nature Communications, 2019,10(1):1447.
DOI URL PMID |
| [11] | WANG J, CHENG Z N, GUO Y Z, et al. Preparation and electrochemical performance of ordered mesoporous Si/C composite for anode material. Journal of Inorganic Materials, 2018,33(3):313-319. |
| [12] | GUO S L, KANG S, LU W Q. Ge nanoparticles in MXene sheets: one-step synthesis and highly improved electrochemical property in lithium-ion batteries. Journal of Inorganic Materials, 2020,35(1):105-111. |
| [13] |
YE X, LIN Z, LIANG S, et al. Upcycling of electroplating sludge into ultrafine Sn@C nanorods with highly stable lithium storage performance. Nano Letters, 2019,19(3):1860-1866.
DOI URL PMID |
| [14] | SCHULZE M C, BELSON R M, KRAYNAK L A, et al. Electrodeposition of Sb/CNT composite films as anodes for Li- and Na-ion batteries. Energy Storage Materials, 2020,25:572-584. |
| [15] | MCNULTY D, GEANEY H, BUCKLEY D, et al. High capacity binder-free nanocrystalline GeO2 inverse opal anodes for Li-ion batteries with long cycle life and stable cell voltage. Nano Energy, 2018,43:11-21. |
| [16] | LI W, WANG K, CHENG S, et al. A two-dimensional hybrid of SbOx nanoplates encapsulated by carbon flakes as a high performance sodium storage anode. Journal of Materials Chemistry A, 2017,5(3):1160-1167. |
| [17] | WANG D, GAO M, PAN H, et al. High performance amorphous- Si@SiOx/C composite anode materials for Li-ion batteries derived from ball-milling and in situ carbonization. Journal of Power Sources, 2014,256:190-199. |
| [18] |
XIA X, ZHANG Y, CHAO D, et al. Solution synthesis of metal oxides for electrochemical energy storage applications. Nanoscale, 2014,6(10):5008-5048.
DOI URL PMID |
| [19] |
LIANG C, GAO M, PAN H, et al. Lithium alloys and metal oxides as high-capacity anode materials for lithium-ion batteries. Journal of Alloys and Compounds, 2013,575:246-256.
DOI URL |
| [20] | IDOTA Y, KUBOTA T, MATSUFUJI A, et al. Tin-based amorphous oxide: a high-capacity lithium-ion-storage material. Science, 1997,276(5317):1395. |
| [21] |
WANG R, XU C, SUN J, et al. Solvothermal-induced 3D macroscopic SnO2/nitrogen-doped graphene aerogels for high capacity and long-life lithium storage. ACS Applied Materials & Interfaces, 2014,6(5):3427-3436.
DOI URL PMID |
| [22] | WANG L P, LECONTE Y, FENG Z, et al. Novel preparation of N-doped SnO2 nanoparticles via laser-assisted pyrolysis: demonstration of exceptional lithium storage properties. Advanced Materials, 2017,29(6):1603286. |
| [23] |
ZHOU X, YU L, LOU X W. Nanowire-templated formation of SnO2/carbon nanotubes with enhanced lithium storage properties. Nanoscale, 2016,8(15):8384-8389.
DOI URL PMID |
| [24] | ZHANG X, JIANG B, GUO J, et al. Large and stable reversible lithium-ion storages from mesoporous SnO2 nanosheets with ultralong lifespan over 1000 cycles. Journal of Power Sources, 2014,268:365-371. |
| [25] | LIU S, WANG R, LIU M, et al. Fe2O3@SnO2 nanoparticle decorated graphene flexible films as high-performance anode materials for lithium-ion batteries. Journal of Materials Chemistry A, 2014,2(13):4598-4604. |
| [26] |
BHASKAR A, DEEPA M, RAO T N. Size-controlled SnO2 hollow spheres via a template free approach as anodes for lithium ion batteries. Nanoscale, 2014,6(18):10762-10771.
DOI URL PMID |
| [27] |
PANG Y, WANG J, YANG J, et al. Fully reversible lithium storage of tin oxide enabled by self-doping and partial amorphization. Nanoscale, 2019,11(27):12915-12923.
DOI URL PMID |
| [28] |
ZHOU D, SONG W L, FAN L Z. Hollow core-shell SnO2/C fibers as highly stable anodes for lithium-ion batteries. ACS Applied Materials & Interfaces, 2015,7(38):21472-21478.
DOI URL PMID |
| [29] |
MA C, ZHANG W, HE Y S, et al. Carbon coated SnO2 nanoparticles anchored on CNT as a superior anode material for lithium-ion batteries. Nanoscale, 2016,8(7):4121-4126.
DOI URL PMID |
| [30] |
WANG J, FANG F, YUAN T, et al. Three-dimensional graphene/ single-walled carbon nanotube aerogel anchored with SnO2 nanoparticles for high performance lithium storage. ACS Applied Materials & Interfaces, 2017,9(4):3544-3553.
DOI URL PMID |
| [31] | HAN F, LI W C, LI M R, et al. Fabrication of superior performance SnO2@C composites for lithium-ion anodes using tubular mesoporous carbon with thin carbon walls and high pore volume. Journal of Materials Chemistry, 2012,22(19):9645-9651. |
| [32] | JAHEL A, GHIMBEU C M, MONCONDUIT L, et al. Confined ultrasmall SnO2 particles in micro/mesoporous carbon as an extremely long cycle-life anode material for Li-ion batteries. Advanced Energy Materials, 2014,4(11):1400025. |
| [33] | NAGPURE S C, BHUSHAN B, BABU S S. Multi-scale characterization studies of aged Li-ion large format cells for improved performance: an overview. Journal of The Electrochemical Society, 2013,160(11):A2111-A2154. |
| [34] | HE S, LI J, WANG J, et al. Facile synthesis and lithium storage performance of hollow CuO microspheres. Materials Letters, 2014,129:5-7. |
| [35] | XIANG J Y, TU J P, ZHANG L, et al. Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries. Journal of Power Sources, 2010,195(1):313-319. |
| [36] | WAN M, JIN D, FENG R, et al. Pillow-shaped porous CuO as anode material for lithium-ion batteries. Inorganic Chemistry Communications, 2011,14(1):38-41. |
| [37] | HU Y, HUANG X, WANG K, et al. Kirkendall-effect-based growth of dendrite-shaped CuO hollow micro/nanostructures for lithium-ion battery anodes. Journal of Solid State Chemistry, 2010,183(3):662-667. |
| [38] | CHEN K, XUE D. Room-temperature chemical transformation route to CuO nanowires toward high-performance electrode materials. The Journal of Physical Chemistry C, 2013,117(44):22576-22583. |
| [39] | JUNG H R, CHO S J, KIM K N, et al. Electrochemical properties of electrospun CuxO (x=1, 2)-embedded carbon nanofiber with EXAFS analysis. Electrochimica Acta, 2011,56(19):6722-6731. |
| [40] | MA Z, RUI K, ZHANG Q, et al. Self-templated formation of uniform F-CuO hollow octahedra for lithium ion batteries. Small, 2017,13(10):1603500. |
| [41] | JIA S, WANG Y, LIU X, et al. Hierarchically porous CuO nano- labyrinths as binder-free anodes for long-life and high-rate lithium ion batteries. Nano Energy, 2019,59:229-236. |
| [42] | YIN D, HUANG G, NA Z, et al. CuO nanorod arrays formed directly on Cu foil from MOFs as superior binder-free anode material for lithium-ion batteries. ACS Energy Letters, 2017,2(7):1564-1570. |
| [43] | XU Y, JIAN G, ZACHARIAH M R, et al. Nano-structured carbon- coated CuO hollow spheres as stable and high rate anodes for lithium- ion batteries. Journal of Materials Chemistry A, 2013,1(48):15486-15490. |
| [44] |
KO S, LEE J I, YANG H S, et al. Mesoporous CuO particles threaded with CNTs for high-performance lithium-ion battery anodes. Advanced Materials, 2012,24(32):4451-4456.
DOI URL PMID |
| [45] | WU S, FU G, LYU W, et al. A single-step hydrothermal route to 3D hierarchical Cu2O/CuO/rGo nanosheets as high-performance anode of lithium-ion batteries. Small, 2018,14(5):1702667. |
| [46] | TAN Y, JIA Z, SUN J, et al. Controllable synthesis of hollow copper oxide encapsulated into N-doped carbon nanosheets as high-stability anodes for lithium-ion batteries. Journal of Materials Chemistry A, 2017,5(46):24139-24144. |
| [47] | LU X, WANG R, BAI Y, et al. Facile preparation of a three- dimensional Fe3O4/macroporous graphene composite for high- performance Li storage. Journal of Materials Chemistry A, 2015,3(22):12031-12037. |
| [48] | WANG R, XU C, SUN J, et al. Three-dimensional Fe2O3 nanocubes/nitrogen-doped graphene aerogels: nucleation mechanism and lithium storage properties. Scientific Reports, 2014,4(1):7171. |
| [49] |
CAI J X, LI Z P, LI W, et al. Synthesis and electrochemical performance of Fe2O3 nanofibers as anode materials for LIBs. Journal of Inorganic Materials, 2018,33(3):301-306.
DOI URL |
| [50] | WANG P, GAO M, PAN H, et al. A facile synthesis of Fe3O4/C composite with high cycle stability as anode material for lithium- ion batteries. Journal of Power Sources, 2013,239:466-474. |
| [51] |
QIN X, ZHANG H, WU J, et al. Fe3O4 nanoparticles encapsulated in electrospun porous carbon fibers with a compact shell as high-performance anode for lithium ion batteries. Carbon, 2015,87:347-356.
DOI URL |
| [52] |
ZHONG Y, FAN H, CHANG L, et al. Novel iron oxide nanotube arrays as high-performance anodes for lithium ion batteries. Journal of Power Sources, 2015,296:255-260.
DOI URL |
| [53] |
ZHAO B, ZHENG Y, YE F, et al. Multifunctional iron oxide nanoflake/ graphene composites derived from mechanochemical synthesis for enhanced lithium storage and electrocatalysis. ACS Applied Materials & Interfaces, 2015,7(26):14446-14455.
DOI URL PMID |
| [54] | PARK Y, OH M, PARK J S, et al. Electrochemically deposited Fe2O3 nanorods on carbon nanofibers for free-standing anodes of lithium-ion batteries. Carbon, 2015,94:9-17. |
| [55] |
QU J, YIN Y X, WANG Y Q, et al. Layer structured α-Fe2O3 nanodisk/reduced graphene oxide composites as high-performance anode materials for lithium-ion batteries. ACS Applied Materials & Interfaces, 2013,5(9):3932-3936.
DOI URL PMID |
| [56] | ZOU Y, KAN J, WANG Y. Fe2O3-graphene rice-on-sheet nanocomposite for high and fast lithium ion storage. The Journal of Physical Chemistry C, 2011,115(42):20747-20753. |
| [57] | KAN J, WANG Y. Large and fast reversible Li-ion storages in Fe2O3-graphene sheet-on-sheet sandwich-like nanocomposites. Scientific Reports, 2013,3(1):3502. |
| [58] | LIU N, SHEN J, LIU D. A Fe2O3 nanoparticle/carbon aerogel composite for use as an anode material for lithium ion batteries. Electrochimica Acta, 2013,97:271-277. |
| [59] |
ZHU X, ZHU Y, MURALI S, et al. Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano, 2011,5(4):3333-3338.
DOI URL PMID |
| [60] | PANG Y, WANG J, ZHOU Z, et al. Core-shell Fe3O4@Fe ultrafine nanoparticles as advanced anodes for Li-ion batteries. Journal of Alloys and Compounds, 2018,735:833-839. |
| [61] | ZHAO J, ZHANG S, LIU W, et al. Fe3O4/ppy composite nanospheres as anode for lithium-ion batteries with superior cycling performance. Electrochimica Acta, 2014,121:428-433. |
| [62] | GUO Q, GUO P, LI J, et al. Fe3O4-CNTs nanocomposites: inorganic dispersant assisted hydrothermal synthesis and application in lithium ion batteries. Journal of Solid State Chemistry, 2014,213:104-109. |
| [63] | MAO J, NIU D, ZHENG N, et al. Fe3O4-embedded and N-doped hierarchically porous carbon nanospheres as high-performance lithium ion battery anodes. ACS Sustainable Chemistry & Engineering, 2019,7(3):3424-3433. |
| [64] | MUSA N, WOO H J, TEO L P, et al. Optimization of Li2SnO3 synthesis for anode material application in Li-ion batteries. Materials Today: Proceedings, 2017,4(4, Part C):5169-5177. |
| [65] |
HE T, FENG J, RU J, et al. Constructing heterointerface of metal atomic layer and amorphous anode material for high-capacity and fast lithium storage. ACS Nano, 2019,13(1):830-838.
DOI URL PMID |
| [66] | TAN H, CHO H W, WU J J. Binder-free ZnO@ZnSnO3 quantum dots core-shell nanorod array anodes for lithium-ion batteries. Journal of Power Sources, 2018,388:11-18. |
| [67] |
GE X, LI Z, WANG C, et al. Metal-organic frameworks derived porous core/shell structured ZnO/ZnCo2O4/C hybrids as anodes for high-performance lithium-ion battery. ACS Applied Materials & Interfaces, 2015,7(48):26633-26642.
DOI URL PMID |
| [68] | LI G, LI W, XU K, et al. Sponge-like NiCo2O4/MnO2 ultrathin nanoflakes for supercapacitor with high-rate performance and ultra- long cycle life. Journal of Materials Chemistry A, 2014,2(21):7738-7741. |
| [69] | RAI A K, GIM J, THI T V, et al. High rate capability and long cycle stability of Co3O4/CoFe2O4 nanocomposite as an anode material for high-performance secondary lithium ion batteries. The Journal of Physical Chemistry C, 2014,118(21):11234-11243. |
| [70] |
ISLAM M, ALI G, JEONG M G, et al. Study on the electrochemical reaction mechanism of NiFe2O4 as a high-performance anode for Li-ion batteries. ACS Applied Materials & Interfaces, 2017,9(17):14833-14843.
URL PMID |
| [71] |
LIAO L X, WANG M, FANG T, et al. Synthesis and characterization of ZnFe2O4 anode for lithium ion battery. Journal of Inorganic Materials, 2016,31(1):34-38.
DOI URL |
| [72] |
WANG L, BOCK D C, LI J, et al. Synthesis and characterization of CuFe2O4 nano/submicron wire-carbon nanotube composites as binder-free anodes for Li-ion batteries. ACS Applied Materials & Interfaces, 2018,10(10):8770-8785.
DOI URL PMID |
| [73] |
DENG S, ZHU H, WANG G, et al. Boosting fast energy storage by synergistic engineering of carbon and deficiency. Nature Communications, 2020,11(1):132.
DOI URL PMID |
| [74] | SHEN S, GUO W, XIE D, et al. A synergistic vertical graphene skeleton and S-C shell to construct high-performance TiNb2O7- based core/shell arrays. Journal of Materials Chemistry A, 2018,6(41):20195-20204. |
| [75] | YAO Z, XIA X, ZHANG Y, et al. Superior high-rate lithium-ion storage on Ti2Nb10O29 arrays via synergistic TiC/C skeleton and N-doped carbon shell. Nano Energy, 2018,54:304-312. |
| [76] |
WU J, PAN G, ZHONG W, et al. Rational synthesis of Cr0.5Nb24.5O62 microspheres as high-rate electrodes for lithium ion batteries. Journal of Colloid and Interface Science, 2020,562:511-517.
DOI URL PMID |
| [77] |
ETTE P M, CHITHAMBARARAJ A, PRAKASH A S, et al. MoS2 nanoflower-derived interconnected CoMoO4 nanoarchitectures as a stable and high rate performing anode for lithium-ion battery applications. ACS Applied Materials & Interfaces, 2020,12(10):11511-11521.
DOI URL PMID |
| [78] | YU Z, BAI Y, ZHANG S, et al. Metal-organic framework-derived Zn0.975Co0.025S/CoS2 embedded in N, S-codoped carbon nanotube/ nanopolyhedra as an efficient electrocatalyst for overall water splitting. Journal of Materials Chemistry A, 2018,6(22):10441-10446. |
| [79] | WU R, QIAN X, ZHOU K, et al. Porous spinel ZnxCo3-xO4 hollow polyhedra templated for high-rate lithium-ion batteries. ACS Nano, 2014,8(6):6297-6303. |
| [80] | WU L L, WANG Z, LONG Y, et al. Multishelled NixCo3-xO4 hollow microspheres derived from bimetal-organic frameworks as anode materials for high-performance lithium-ion batteries. Small, 2017,13(17):1604270. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 谭博文, 耿双龙, 张锴, 郑百林. 硅电极组分梯度设计抑制力-化学耦合劣化[J]. 无机材料学报, 2025, 40(7): 772-780. |
| [3] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [4] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [5] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [6] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [7] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [8] | 杨茗凯, 黄泽皑, 周芸霄, 刘彤, 张魁魁, 谭浩, 刘梦颖, 詹俊杰, 陈国星, 周莹. 基于Cu与金属氧化物-KCl熔融介质的甲烷热解制备少层石墨烯与氢气联产研究[J]. 无机材料学报, 2025, 40(5): 473-480. |
| [9] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [10] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [11] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [12] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [13] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [14] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [15] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||