|
Boron Nitride Nanosheets Supported Cu2O Nanoparticles: Synthesis and Catalytic Reduction for 4-nitrophenol
Journal of Inorganic Materials
2019, 34 (8):
817-826.
DOI: 10.15541/jim20180487
Despite excellent catalytic capability, Cu2O nanomaterial exhibits weak stability which limits its application. In this study, a novel kind of Cu2O, Cu2O/BNNSs-OH, supported catalyst with highly catalytic efficiency and stability, was facilely fabricated via a controllable liquid phase reduction of ascorbic acid and combining with an annealing process. Cu2O/BNNSs-OH catalyst was synthesized by using boron nitride nanosheets (BNNSs), prepared by the “push-pull” effect of polyvinylpyrrolidone (PVP) and water phase change, as a supporter and spherical Cu2O nanoparticles (2-7 nm) prepared by forward titration (ascorbic acid→Cu 2+, solution with a pH 11) as active components. Morphology and structure of as-obtained samples were characterized by scanning electron microscopy (SEM), high resolution transmission electronic microscopy (HRTEM), atomic force microscopy (AFM), X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), and Raman spectroscopy. The results of the synthetic method showed that spherical Cu2O nanoparticles were uniformly dispersed on the carrier surface and BNNSs displayed some stabilization effect on Cu2O which could be prevented from being oxidized into CuO. Moreover, the catalytic activity was investigated by catalytic reduction reaction of 4-nitrophenol to 4-aminophenol. Cu2O/BNNSs-OH with high catalytic activity similar to the noble metal catalyst for the reduction of 4-nitrophenol is highly reusable for five successive cycles without significant degradation and activity loss. 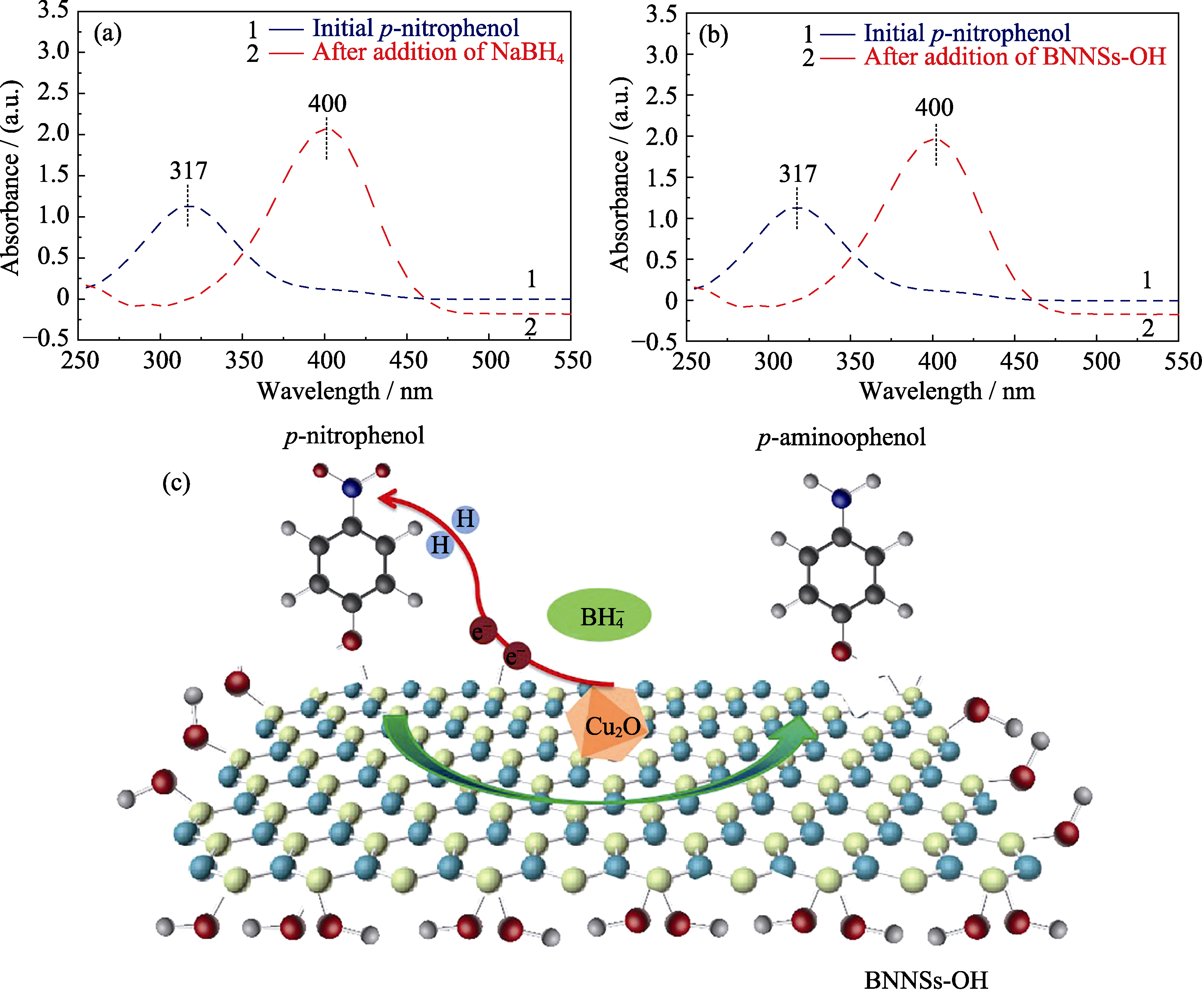
Fig. 10
UV-Vis absorption spectra of 4-NP solution before and after NaBH4 (a) and BNNSs-OH (b) additions, and schematic of the reduction of 4-NP to 4-AP over the Cu2O/ BNNSs-OH (c)
Extracts from the Article
为了更深入地了解4-NP催化还原反应, 本研究分别对反应过程中的NaBH4, BNNSs-OH及Cu2O NPs催化剂对4-NP的光谱吸收峰的影响进行考查。如图10(a)所示, 4-NP在317 nm处有特征吸收峰, 当加入NaBH4后, 4-NP中的羟基被去质子化, 在碱性条件下转化成4-NP阴离子, 吸收峰从317 nm红移至400 nm, 此时溶液由淡黄色变为黄色。虽然NaBH4是强还原剂, 但在无催化剂作用的情况下也不能直接还原4-NP离子, 这是因为4-NP阴离子与BH4-之间强烈的相互排斥作用以及两者氧化还原电位之间的巨大差异(EH3BO3(aq.)/BH4-(aq.)= -1.33 V和E4-NP(aq.)/4-AP(aq.)=0.76 V), 故通过NaBH4将4-NP还原为4-AP在动力学上是不可行的。用BNNSs-OH代替NaBH4加入到上述体系后, 也会发生光谱吸收峰移动(图10(b)), 这是由于BNNSs-OH表面和边缘含有大量的-OH, 可以当作吸附和4-NP离子化的活性位点。在催化剂的作用下, 可以克服BH4-到4-NP阴离子电子转移的动力学限制, 促进反应快速进行。
基于以上研究, Cu2O/BNNSs-OH催化还原对硝基苯酚的作用机理如图10(c)所示。BNNSs-OH和Cu2O NPs在催化还原4-NP反应过程中具有协同作用。4-NP被BNNSs-OH吸附并离子化, NaBH4仅被当作电子供体和氢源(式(1))[31], Cu2O NPs作为电子和氢原子的中转位点, 传递表面氢原子和电子至对硝基苯酚阴离子, 最终使-NO2加入质子移除氧转化为-NH2(式(2))[31]。而Cu单质及CuO颗粒尺寸较大且电子迁移能力相对较弱, Cu2O-Cu基催化剂因为Cu2O附着在亚微米级的Cu上, 也在一定程度上减缓反应进行, 故与Cu2O NPs相比催化活性相对较差。
Other Images/Table from this Article
|