|
Boron Nitride Nanosheets Supported Cu2O Nanoparticles: Synthesis and Catalytic Reduction for 4-nitrophenol
Journal of Inorganic Materials
2019, 34 (8):
817-826.
DOI: 10.15541/jim20180487
Despite excellent catalytic capability, Cu2O nanomaterial exhibits weak stability which limits its application. In this study, a novel kind of Cu2O, Cu2O/BNNSs-OH, supported catalyst with highly catalytic efficiency and stability, was facilely fabricated via a controllable liquid phase reduction of ascorbic acid and combining with an annealing process. Cu2O/BNNSs-OH catalyst was synthesized by using boron nitride nanosheets (BNNSs), prepared by the “push-pull” effect of polyvinylpyrrolidone (PVP) and water phase change, as a supporter and spherical Cu2O nanoparticles (2-7 nm) prepared by forward titration (ascorbic acid→Cu 2+, solution with a pH 11) as active components. Morphology and structure of as-obtained samples were characterized by scanning electron microscopy (SEM), high resolution transmission electronic microscopy (HRTEM), atomic force microscopy (AFM), X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), and Raman spectroscopy. The results of the synthetic method showed that spherical Cu2O nanoparticles were uniformly dispersed on the carrier surface and BNNSs displayed some stabilization effect on Cu2O which could be prevented from being oxidized into CuO. Moreover, the catalytic activity was investigated by catalytic reduction reaction of 4-nitrophenol to 4-aminophenol. Cu2O/BNNSs-OH with high catalytic activity similar to the noble metal catalyst for the reduction of 4-nitrophenol is highly reusable for five successive cycles without significant degradation and activity loss. 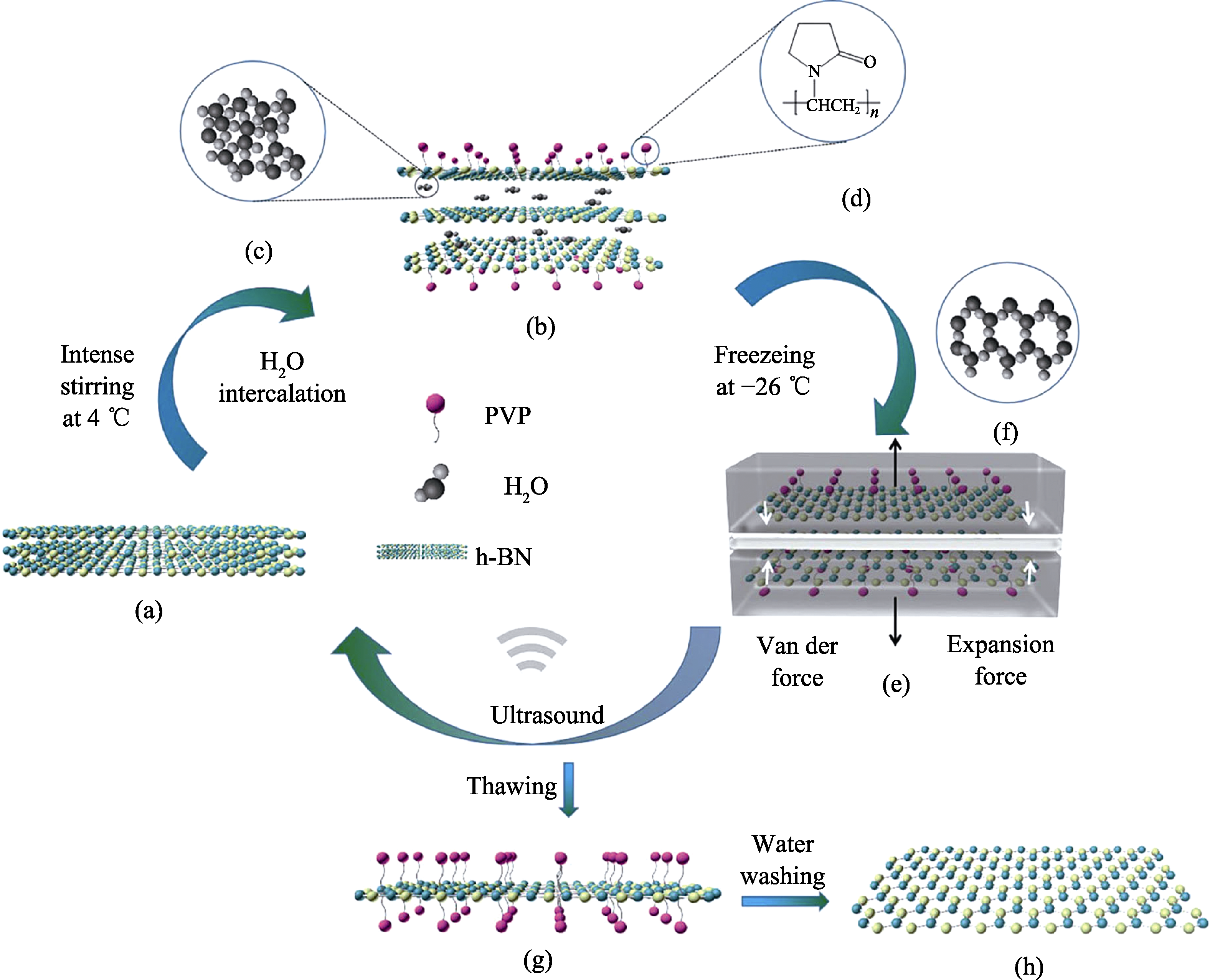
Fig. 1
Gentle water freezing-thawing exfoliation of h-BN triggered by freezing expansion force and against reaggregation by PVP coating
Extracts from the Article
冻融法剥离块状六方氮化硼机理如图1所示: PVP通过强烈的疏水作用吸附在六方氮化硼表面, 为剥离过程提供一种强烈的“拉”的驱动力。4 ℃时, 水的体积最小, 排列最为规整[19], 水分子巨大的比表面能及强烈的搅拌作用大大提高插层效率。当温度降至0 ℃以下时, 水分子逐渐聚集成蜂窝状结构, 水结冰产生的“体积膨胀力”(250 kPa)[20]能够克服层间的范德华力, 为剥离提供一种“推”的驱动力。经过反复冻融, 在PVP与水相变提供的“推-拉”协同作用下可以实现块状氮化硼的高效剥离。h-BN表面吸附的PVP可以有效防止BNNSs发生团聚。
Other Images/Table from this Article
|