|
Boron Nitride Nanosheets Supported Cu2O Nanoparticles: Synthesis and Catalytic Reduction for 4-nitrophenol
Journal of Inorganic Materials
2019, 34 (8):
817-826.
DOI: 10.15541/jim20180487
Despite excellent catalytic capability, Cu2O nanomaterial exhibits weak stability which limits its application. In this study, a novel kind of Cu2O, Cu2O/BNNSs-OH, supported catalyst with highly catalytic efficiency and stability, was facilely fabricated via a controllable liquid phase reduction of ascorbic acid and combining with an annealing process. Cu2O/BNNSs-OH catalyst was synthesized by using boron nitride nanosheets (BNNSs), prepared by the “push-pull” effect of polyvinylpyrrolidone (PVP) and water phase change, as a supporter and spherical Cu2O nanoparticles (2-7 nm) prepared by forward titration (ascorbic acid→Cu 2+, solution with a pH 11) as active components. Morphology and structure of as-obtained samples were characterized by scanning electron microscopy (SEM), high resolution transmission electronic microscopy (HRTEM), atomic force microscopy (AFM), X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), and Raman spectroscopy. The results of the synthetic method showed that spherical Cu2O nanoparticles were uniformly dispersed on the carrier surface and BNNSs displayed some stabilization effect on Cu2O which could be prevented from being oxidized into CuO. Moreover, the catalytic activity was investigated by catalytic reduction reaction of 4-nitrophenol to 4-aminophenol. Cu2O/BNNSs-OH with high catalytic activity similar to the noble metal catalyst for the reduction of 4-nitrophenol is highly reusable for five successive cycles without significant degradation and activity loss. 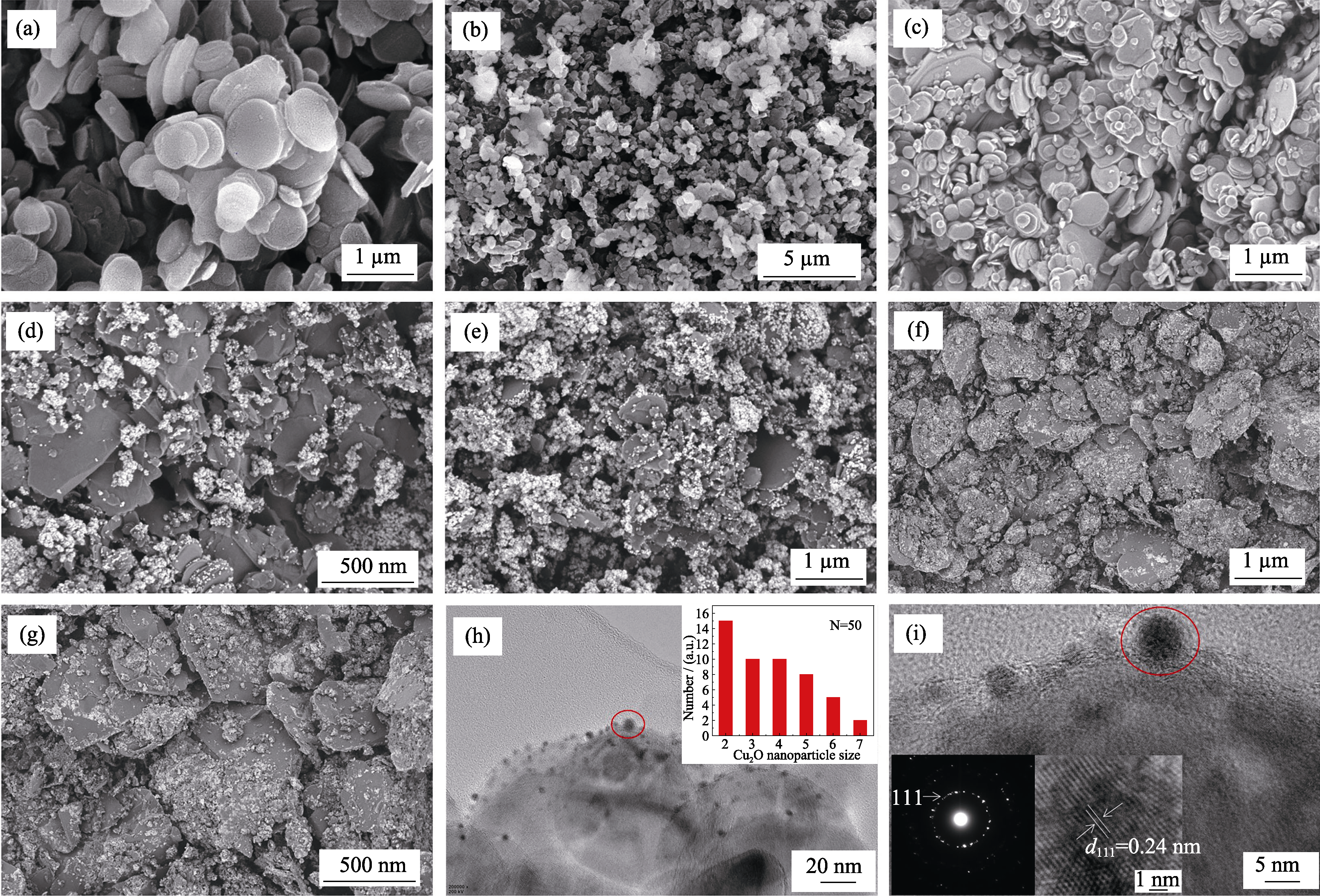
Fig. 7
SEM images of BNNSs-OH (a), CuO/BNNSs-OH (b), products prepared in ascorbic acid solution reduction system at different pH((c) pH 3, (d) pH 5, (e) pH 7, (f) pH 9, (g) pH 11), HRTEM images of Cu2O/BNNSs-OH (h-i) with inset in (h) showing the corresponding size distributions of Cu2O NPs with inset in (i) showing the corresponding selected SAED pattern and lattice fringe pattern at pH 11
Extracts from the Article
图7(a)为功能化氮化硼纳米片的SEM照片, 可以清晰地看出 BNNSs-OH 为平滑交错的片状结构。前驱体阶段(混合溶液pH=11)制备的CuO/ BNNSs-OH由大量细小的CuO颗粒构成, 其粒径相对较大, 且紧紧团聚在一起(图7(b))。抗坏血酸还原阶段, 不同pH下制备的铜基催化剂SEM照片如 图7(c~g)所示。当反应体系pH在3~7时, Cu颗粒粒径随着pH增加逐渐减小, 最终变为球形颗粒 (图7(c~e))。当反应体系pH升高至9时, 在亚微米级Cu颗粒的表面开始产生纳米级颗粒, 对应上述XRD结果可知, 新生的Cu2O NPs附着在原来的Cu颗粒上(图7(f))。高分辨TEM图说明反应体系pH为11时, 产物全部为2~7 nm左右的Cu2O球形纳米粒子, 高度分散于氮化硼纳米片的表面, 且Cu2O NPs具有清晰的晶格, 层间距为0.24 nm, 对应立方相Cu2O的(111)晶面[29](图7(h~i))。
Other Images/Table from this Article
|