|
Boron Nitride Nanosheets Supported Cu2O Nanoparticles: Synthesis and Catalytic Reduction for 4-nitrophenol
Journal of Inorganic Materials
2019, 34 (8):
817-826.
DOI: 10.15541/jim20180487
Despite excellent catalytic capability, Cu2O nanomaterial exhibits weak stability which limits its application. In this study, a novel kind of Cu2O, Cu2O/BNNSs-OH, supported catalyst with highly catalytic efficiency and stability, was facilely fabricated via a controllable liquid phase reduction of ascorbic acid and combining with an annealing process. Cu2O/BNNSs-OH catalyst was synthesized by using boron nitride nanosheets (BNNSs), prepared by the “push-pull” effect of polyvinylpyrrolidone (PVP) and water phase change, as a supporter and spherical Cu2O nanoparticles (2-7 nm) prepared by forward titration (ascorbic acid→Cu 2+, solution with a pH 11) as active components. Morphology and structure of as-obtained samples were characterized by scanning electron microscopy (SEM), high resolution transmission electronic microscopy (HRTEM), atomic force microscopy (AFM), X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), and Raman spectroscopy. The results of the synthetic method showed that spherical Cu2O nanoparticles were uniformly dispersed on the carrier surface and BNNSs displayed some stabilization effect on Cu2O which could be prevented from being oxidized into CuO. Moreover, the catalytic activity was investigated by catalytic reduction reaction of 4-nitrophenol to 4-aminophenol. Cu2O/BNNSs-OH with high catalytic activity similar to the noble metal catalyst for the reduction of 4-nitrophenol is highly reusable for five successive cycles without significant degradation and activity loss. 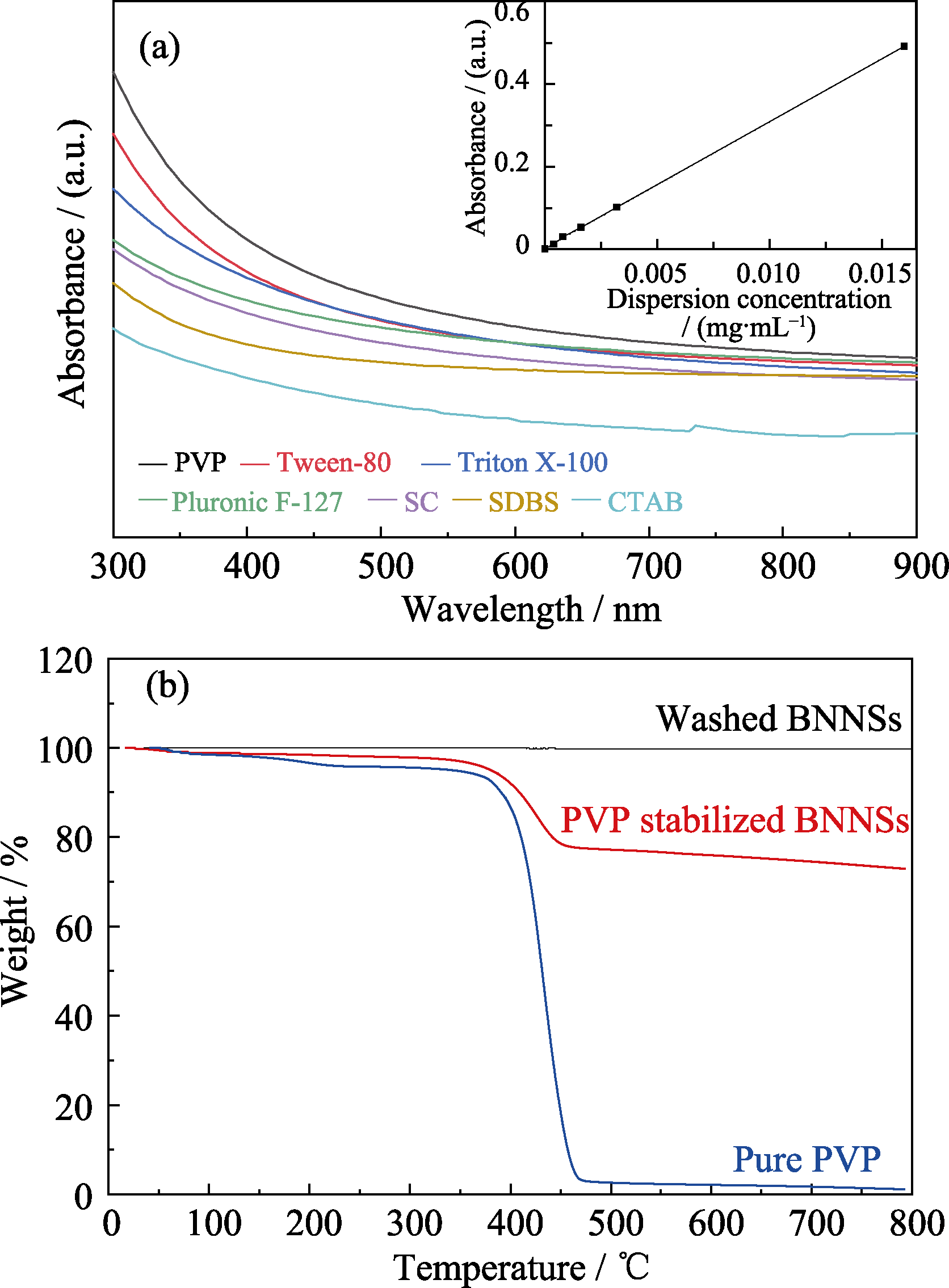
Fig. 2
Absorption spectra for h-BN dispersions stabilized with various surfactants (a), TGA curves of washed BNNSs, PVP stabilized BNNSs, and pure PVP (b)
Extracts from the Article
图2(a)为利用不同表面活性剂剥离得到氮化硼纳米片的紫外-可见(UV-Vis)吸收光谱, 包括离子型表面活性剂SC、SDBS及CTAB和非离子型表面活性剂PVP、Pluronic、F-127、Triton X-100及Tween 80。BNNSs在300 nm后无明显吸收峰, 故采用300 nm处的吸光度值计算氮化硼纳米片浓度。根据朗伯比尔定律, A=αlc (A为吸光度, α为摩尔吸收系数, l为吸收层厚度, c为分散液浓度), 通过绘制标准曲线得α=3177 L?g-1?m-1, 可计算BNNSs浓度[21]。结果表明, PVP辅助剥离得到的分散液中BNNSs浓度最高。这是因为离子型表面活性剂与水的相互作用较强, 而非离子型表面活性剂却通过强烈的疏水作用吸附在h-BN表面, PVP则因分子链中的吡咯烷酮基团与h-BN之间强烈的π-π作用[22], 为剥离提供强烈的“拉”的驱动力。
在液相还原法制备纳米Cu2O/BNNSs-OH过程中, 不同pH环境下, 产物的组成与形貌有所不同。前驱体阶段(图5(a)), 当混合溶液pH在5~7区间, Cu2+转变为Cu(OH)2形式, 随着pH升至9~11区间, 则以CuO形式为主。抗坏血酸还原阶段(图5(b)), 当反应体系pH分别为3、5时, 以载体BNNSs-OH的XRD图谱为参照, 图谱中其余三个尖锐的衍射峰分别对应Cu的(111)、(200)、(220)晶面, 与Cu标准卡片(JCPDS 04-0836)相对应, 且图中未出现Cu2O和CuO的衍射峰。当反应体系pH为7时, 除了Cu以外, 产物开始出现Cu2O物相(JCPDS 77-0199), 但Cu仍为主要产物。随着反应体系pH升高至9, Cu2O(111)衍射峰的强度逐渐增强, 而Cu(111)衍射峰的强度大幅降低, 说明Cu2O的含量随着反应体系pH的升高而增加, 产物物相的组成发生剧烈变化, 主导相变为Cu2O。当反应体系pH持续升高至11时, 反应产物为Cu2O, 其特征衍射峰归属于Cu2O的(110)、(111)、(200)、(220)、(311)晶面, 且没有其他杂质峰。发生上述物相变化的原因是,抗坏血酸的还原能力与反应体系的pH有关。在强碱性条件下, VC还原能力降低, CuO作为反应前驱体仅能被还原为Cu2O[19] (2CuO+C6H8O6→Cu2O+ C6H6O6+H2O); 在酸性或弱碱性条件下, VC的共轭体系能够提供足够的电子将前驱体Cu(OH)2还原为单质Cu(Cu(OH)2+C6H8O6→Cu2O+C6H6O6+H2O, Cu2O+C6H8O6→Cu+C6H6O6+H2O); 在液相还原过程中, VC的存在可以抑制Cu及Cu2O被氧化成CuO[24]。
为了进一步了解催化剂的成分和元素价态, 采用XPS对样品进行表征, 结果如图6所示。B1s和N1s谱图中190.3和398.0 eV与文献报道中h-BN的出峰位置一致[25], O1s图谱中530.1 eV信号对应Cu2O中晶格氧O1s的电子结合能[26], Cu2p轨道能谱图中位于932.3 (FWHM=1.8 eV)和952.1 eV的强峰分别对应Cu2p3/2和Cu2p1/2的电子结合能, 均属于Cu2O中Cu(I)的特征峰, 且Cu2p3/2出峰位置与块状Cu2O(932.6 eV)[27]相比向低电子结合能方向移动, 说明Cu2O以纳米晶体形式负载在BNNSs-OH表面上[28]。Cu2p谱图中并未出现任何卫星峰和伴峰, 表明抗坏血酸液相还原及气氛焙烧法成功制备了高纯度的纳米级Cu2O/BNNSs-OH催化剂。
Other Images/Table from this Article
|