
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (7): 769-780.DOI: 10.15541/jim20190433
Special Issue: 能源材料论文精选(二):超级电容器与储能电池(2020); 【虚拟专辑】超级电容器(2020~2021)
• REVIEW • Previous Articles Next Articles
LI Zehui1,TAN Meijuan2,ZHENG Yuanhao3,LUO Yuyang3,JING Qiushi3,JIANG Jingkun1,LI Mingjie4( )
)
Received:2019-08-16
Revised:2019-09-29
Published:2020-07-20
Online:2019-10-23
Supported by:CLC Number:
LI Zehui,TAN Meijuan,ZHENG Yuanhao,LUO Yuyang,JING Qiushi,JIANG Jingkun,LI Mingjie. Application of Conductive Metal Organic Frameworks in Supercapacitors[J]. Journal of Inorganic Materials, 2020, 35(7): 769-780.
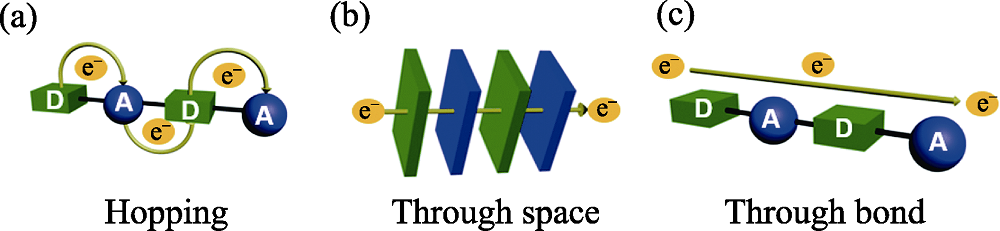
Fig. 1 Representation of possible modes of charge transport in MOFs: (a) hopping charge transport, (b) through-space charge transport, and (c) through-bond charge transport[22]
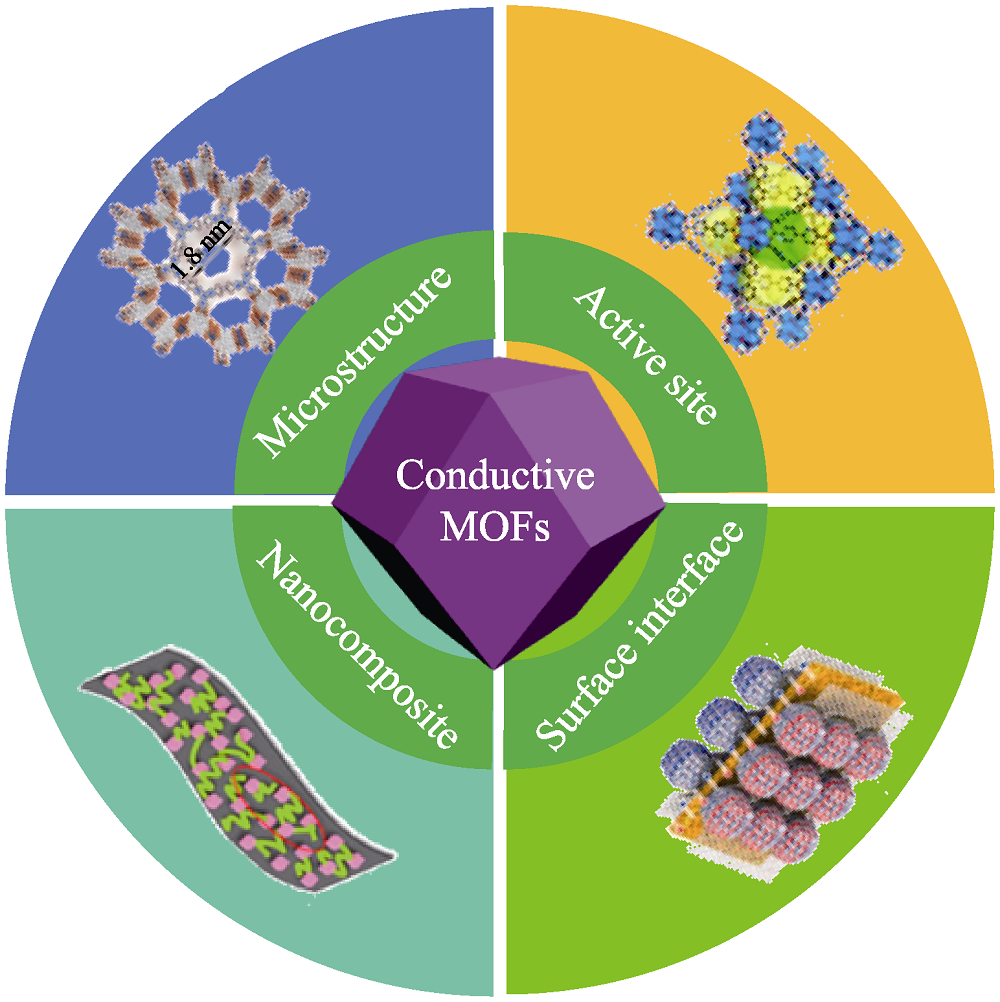
Fig. 2 Control strategy of conductive MOFs in supercapacitors From microstructure, active site, surface interface and nanocomposite of conductive MOFs to energy storage applications
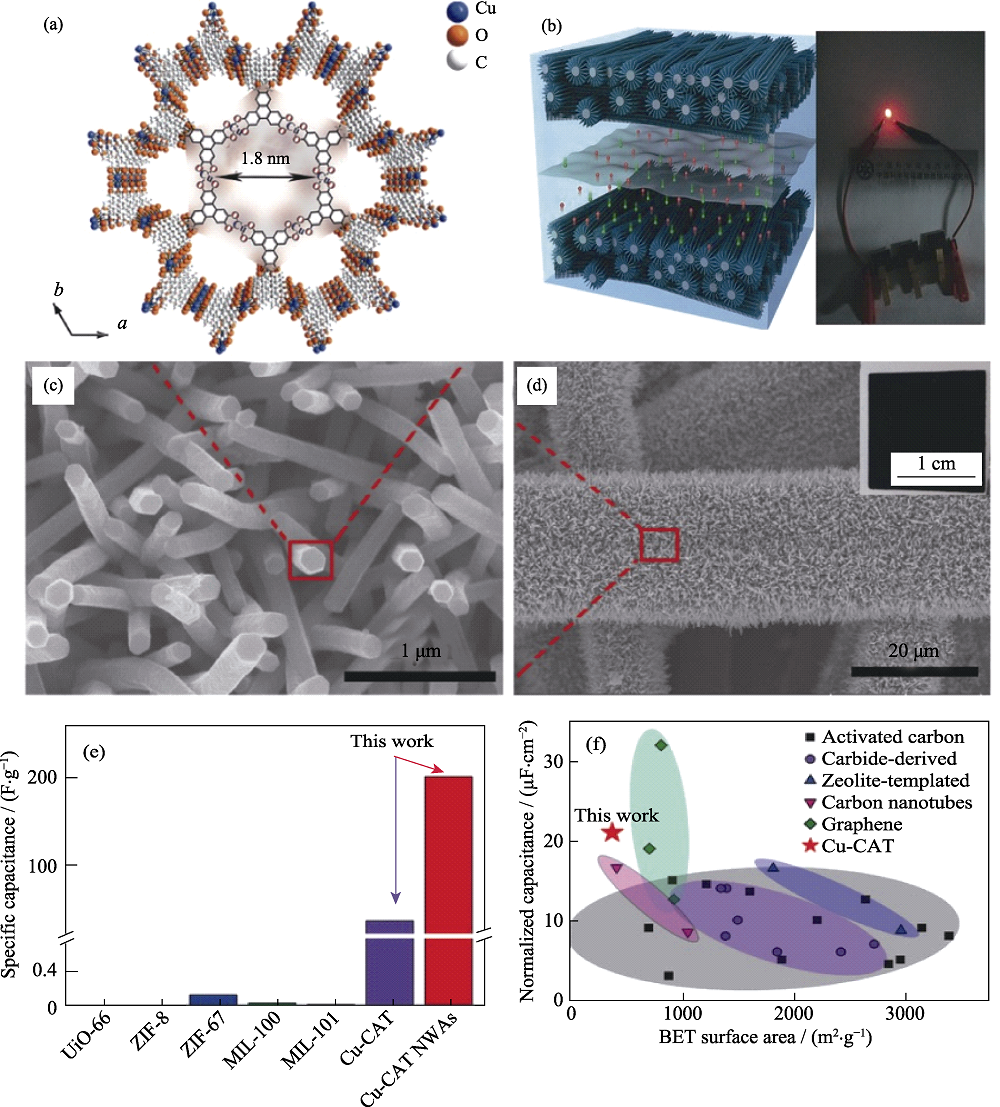
Fig. 3 (a) Crystal structure of Cu-CAT viewed along the c-axis; (b) Structure of the solid-state supercapacitor (left) and photograph of a red light-emitting-diode powered by the three supercapacitors connected in series (right); (c, d) SEM and photographic images (inset in (d)) of the Cu-CAT NWAs growing on carbon fiber paper; Performance comparison of (e)Cu-CAT NWAs and (f) MOF materials, and carbon materials based symmetric solid-state supercapacitors[29]
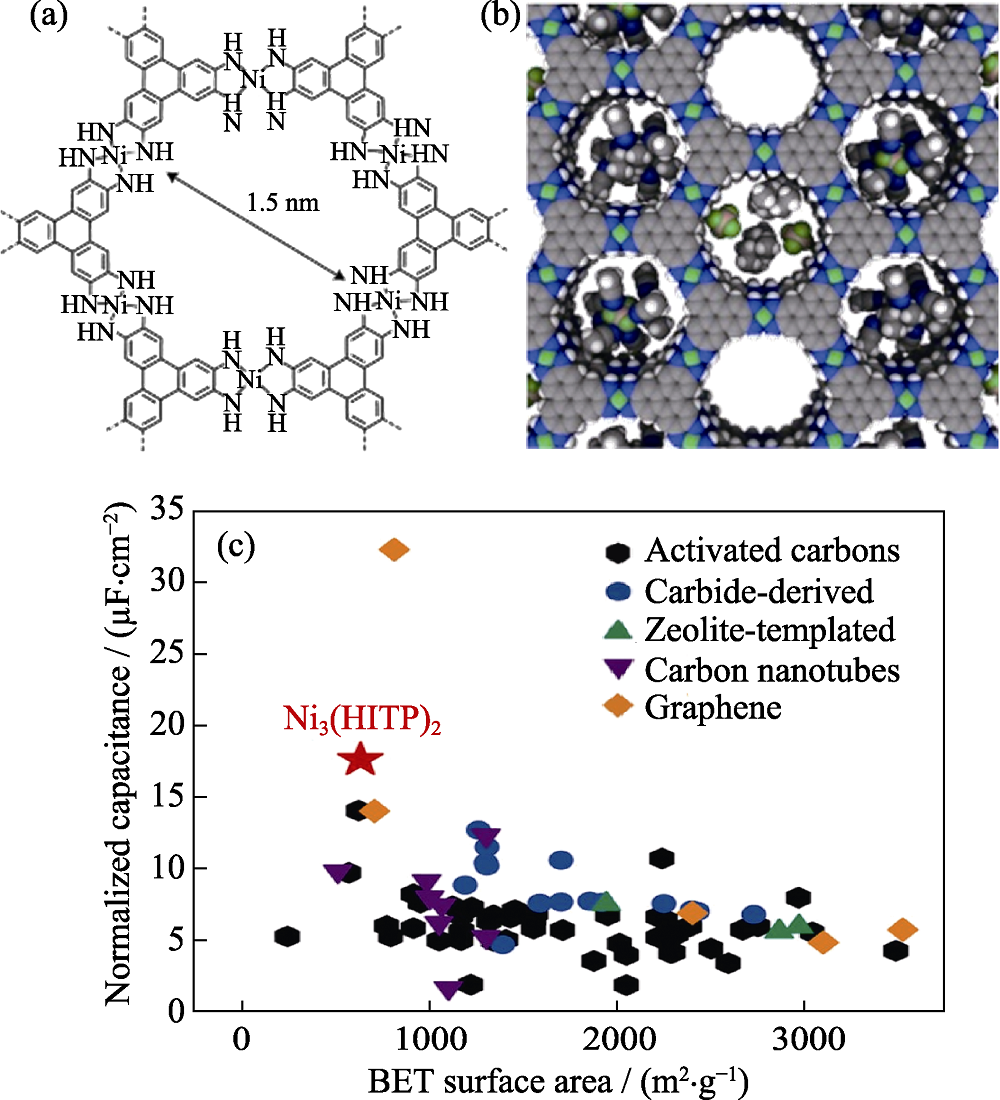
Fig. 4 (a) Molecular structure of Ni3(HITP)2; (b) Relative size of pores, electrolyte Et4N+ and BF4- ions, and acetonitrile solvent molecules showing in a space-filling diagram of idealized Ni3(HITP)2; (c) Comparison of areal capacitance for various materials normalized relative to their BET surface areas[30]
| Metal | Organic ligand | MOFs | Conductivity /(S?m-1) | SBET /(m2·g-1) | Specific capacitance | Energy density | Power density | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe | 1,3,5-Benzenetricarboxylic acid (H3BT)C | MIL-100 | - | - | 39 F·g-1 | - | - | [ |
| Co | Polyethylene glycol (PEG) | Co-MOF-71 | - | - | 206.76 F·g-1 | 7.18 Wh·kg-1 | - | [ |
| Co | Benzendicarboxylic (BDC) acid | Co-BDC | - | 9.09 | 131.8 F·g-1 | 20.7 Wh·kg-1 | 3880 W·kg-1 | [ |
| Co | 2,6-Naphthalenedicarboxylic (NDC) acid | Co-NDC | - | 20.29 | 147.3 F·g-1 | 23.1 Wh·kg-1 | 5490 W·kg-1 | [ |
| Co | 4,4-Biphenyldicarboxylic (BPDC) acid | Co-BPDC | - | 138.35 | 179.2 F·g-1 | 31.4 Wh·kg-1 | 5640 W·kg-1 | [ |
| Ni | Isonicotinic acid | Ni-MOF | - | 148 | 634 F·g-1 | - | - | [ |
| Ni | Salicylate ion | 1D Ni-MOF | - | 186.8 | 1698 F·g-1 | - | - | [ |
| Ni | p-Benzenedicarboxylic acid (PTA) | Ni-MOF-24 | - | - | 1127 F·g-1 | 19.17 Wh·kg-1 | 1750 W·kg-1 | [ |
| Ni | 1,3,5-Benzenetricarboxylic (btc) acid (H3BT)C | Ni3(btc)2·12H2O | - | - | 726 F·g-1 | 16.5 Wh·kg-1 | 2078 W·kg-1 | [ |
| Ni | 9,10-Anthracenedicarboxylic acid (ADC) | Ni-DMOF-ADC | - | 783 | 552 F·g-1 | - | - | [ |
| Ni | 2,3,6,7,10,11-Hexaiminotriphenylene (HITP) | Ni3(HITP)2 | >5000 | 630 | 111 F·g-1 | - | - | [ |
| Zn | 4,4’-Biphenyldicarboxylic acid (H2BPC) | Zn6(BPC)6(L)3· 9DMF | - | - | 23 F·g-1 | 1.9 Wh·kg-1 | 3 W·kg-1 | [ |
| Zn | p-Phenylenediamine (pPDA) | Zn-(pPDA)MOF | 0.1~1.05 | 200.86 F·g-1 | 62.8 Wh·kg-1 | 4500 W·kg-1 | [ | |
| Cd | 2,5-Thiophenedicarboxylic acid (H2TDC) | Cd2(TDC)2(L)2· 2H2O | - | - | 22 F·g-1 | 2.1 Wh·kg-1 | 3.3 W·kg-1 | [ |
| Zr | 2,2’-Bipyridine-5,5’-dicarboxylate (BPYDC) | nMOF-867 | - | - | 5.085 mF·cm-2 | 6.04× 10-4 Wh?cm-3stack | 1.097 W·cm-3stack | [ |
| Zn, Ni | p-Benzenedicarboxylic acid (PTA) | Zn-doped Ni-MOF | - | - | 1620 F·g-1 | 27.56 Wh·kg-1 | 1750 W·kg-1 | [ |
| Co, Zn | Benzendicarboxylic acid | Co8-MOF-5 | - | 2900 | 0.49 F·g-1 | - | - | [ |
| Zn, Zr | Terephthalic acid | HP-UiO-66 | - | - | 849 F·g-1 | 32 Wh·kg-1 | 240 W·kg-1 | [ |
Table 1 MOFs with different center metal atoms for SCs
| Metal | Organic ligand | MOFs | Conductivity /(S?m-1) | SBET /(m2·g-1) | Specific capacitance | Energy density | Power density | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe | 1,3,5-Benzenetricarboxylic acid (H3BT)C | MIL-100 | - | - | 39 F·g-1 | - | - | [ |
| Co | Polyethylene glycol (PEG) | Co-MOF-71 | - | - | 206.76 F·g-1 | 7.18 Wh·kg-1 | - | [ |
| Co | Benzendicarboxylic (BDC) acid | Co-BDC | - | 9.09 | 131.8 F·g-1 | 20.7 Wh·kg-1 | 3880 W·kg-1 | [ |
| Co | 2,6-Naphthalenedicarboxylic (NDC) acid | Co-NDC | - | 20.29 | 147.3 F·g-1 | 23.1 Wh·kg-1 | 5490 W·kg-1 | [ |
| Co | 4,4-Biphenyldicarboxylic (BPDC) acid | Co-BPDC | - | 138.35 | 179.2 F·g-1 | 31.4 Wh·kg-1 | 5640 W·kg-1 | [ |
| Ni | Isonicotinic acid | Ni-MOF | - | 148 | 634 F·g-1 | - | - | [ |
| Ni | Salicylate ion | 1D Ni-MOF | - | 186.8 | 1698 F·g-1 | - | - | [ |
| Ni | p-Benzenedicarboxylic acid (PTA) | Ni-MOF-24 | - | - | 1127 F·g-1 | 19.17 Wh·kg-1 | 1750 W·kg-1 | [ |
| Ni | 1,3,5-Benzenetricarboxylic (btc) acid (H3BT)C | Ni3(btc)2·12H2O | - | - | 726 F·g-1 | 16.5 Wh·kg-1 | 2078 W·kg-1 | [ |
| Ni | 9,10-Anthracenedicarboxylic acid (ADC) | Ni-DMOF-ADC | - | 783 | 552 F·g-1 | - | - | [ |
| Ni | 2,3,6,7,10,11-Hexaiminotriphenylene (HITP) | Ni3(HITP)2 | >5000 | 630 | 111 F·g-1 | - | - | [ |
| Zn | 4,4’-Biphenyldicarboxylic acid (H2BPC) | Zn6(BPC)6(L)3· 9DMF | - | - | 23 F·g-1 | 1.9 Wh·kg-1 | 3 W·kg-1 | [ |
| Zn | p-Phenylenediamine (pPDA) | Zn-(pPDA)MOF | 0.1~1.05 | 200.86 F·g-1 | 62.8 Wh·kg-1 | 4500 W·kg-1 | [ | |
| Cd | 2,5-Thiophenedicarboxylic acid (H2TDC) | Cd2(TDC)2(L)2· 2H2O | - | - | 22 F·g-1 | 2.1 Wh·kg-1 | 3.3 W·kg-1 | [ |
| Zr | 2,2’-Bipyridine-5,5’-dicarboxylate (BPYDC) | nMOF-867 | - | - | 5.085 mF·cm-2 | 6.04× 10-4 Wh?cm-3stack | 1.097 W·cm-3stack | [ |
| Zn, Ni | p-Benzenedicarboxylic acid (PTA) | Zn-doped Ni-MOF | - | - | 1620 F·g-1 | 27.56 Wh·kg-1 | 1750 W·kg-1 | [ |
| Co, Zn | Benzendicarboxylic acid | Co8-MOF-5 | - | 2900 | 0.49 F·g-1 | - | - | [ |
| Zn, Zr | Terephthalic acid | HP-UiO-66 | - | - | 849 F·g-1 | 32 Wh·kg-1 | 240 W·kg-1 | [ |
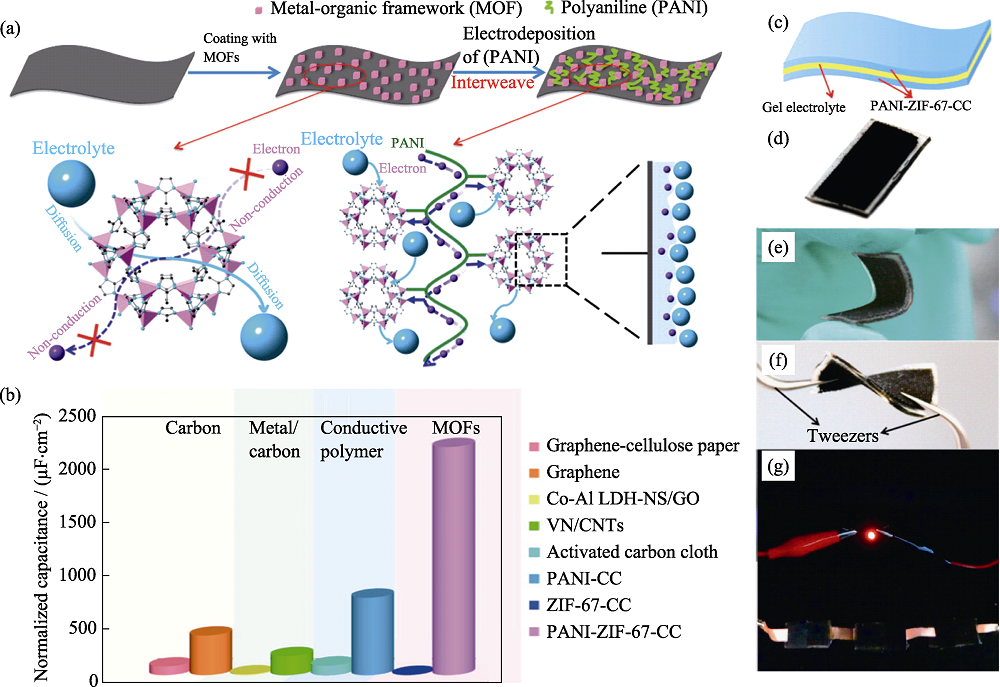
Fig. 8 (a) Illustration of the synthesis methodology for polyaniline-ZIF-67 on carbon cloth; (b) Areal capacitances of the PANI-ZIF-67-CC and other electrode materials; (c-f) Schematic illustrations of PANI-ZIF-67-CC flexible solid-state SC device; (g) Photograph of a red light-emitting-diode (LED) powered by the three SCs connected in series[52]
| [1] |
MEHTAB T, YASIN G, ARIF M, et al. Metal-organic frameworks for energy storage devices: batteries and supercapacitors. J. Energy Storage, 2019,21:632-646.
DOI URL |
| [2] |
FAN Z, YAN J, TONG W, et al. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater., 2011,21(12):2366-2375.
DOI URL |
| [3] |
CHEN W, YU H, LEE S Y, et al. Nanocellulose: a promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev., 2018,47(8):2837-2872.
DOI URL PMID |
| [4] |
CHENG J, CHEN S, CHEN D, et al. Editable asymmetric all-solid-state supercapacitors based on high-strength, flexible, and programmable 2D-metal-organic framework/reduced graphene oxide self-assembled papers. J. Mater. Chem. A, 2018,6(41):20254-20266.
DOI URL |
| [5] |
XUAN W, ZHU C, LIU Y, et al. Mesoporous metal-organic framework materials. Chem. Soc. Rev., 2012,41(5):1677-1695.
DOI URL PMID |
| [6] |
ZHU Q L, XU Q. Metal-organic framework composites. Chem. Soc. Rev., 2014,43(16):5468-5512.
DOI URL PMID |
| [7] |
ZHENG S, LI X, YAN B, et al. Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage. Adv. Energy Mater., 2017,7(18):1602733.
DOI URL |
| [8] |
JIANG L, SHENG L, LONG C, et al. Densely packed graphene nanomesh-carbon nanotube hybrid film for ultra-high volumetric performance supercapacitors. Nano Energy, 2015,11(21):471-480.
DOI URL |
| [9] |
SUNDRIYAL S, KAUR H, BHARDWAJ S K, et al. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev., 2018,369:15-38.
DOI URL |
| [10] | LI L X, TAO J, GENG X, et al. Preparation and supercapacitor performance of nitrogen-doped carbon nanotubes from polyaniline modification. Acta Phys-Chim. Sin., 2013,29(1):924-929. |
| [11] |
YAN J, TONG W, BO S, et al. Preparation of a graphene nanosheet/ polyaniline composite with high specific capacitance. Carbon, 2010,48(2):487-493.
DOI URL |
| [12] | SUBRAMANIAN V, ZHU H, VAJTAI R, et al. Hydrothermal synthesis and pseudocapacitance properties of MnO2 nanostructures. J. Physi. Chem. B, 2005,109(43):20207-20214. |
| [13] |
WANG Q, WEN Z, JINGHONG L. A hybrid supercapacitor fabricated with a carbon nanotube cathode and a TiO2-B nanowire anode. Adv. Funct. Mater., 2010,16:2141-2146.
DOI URL |
| [14] |
ROWSELL J L C, YAGHI O M. Metal-organic frameworks: a new class of porous materials. Micropor. Mesopor. Mat., 2004,73(1):3-14.
DOI URL |
| [15] |
HENDON C H, TIANA D, WALSH A. Conductive metal-organic frameworks and networks: fact or fantasy? Phys. Chem. Chem. Phys., 2012,14(38):13120-13132.
DOI URL PMID |
| [16] |
KOBAYASHI Y, JACOBS B, ALLENDORF M D, et al. Conductivity, doping, and redox chemistry of a microporous dithiolene-based metal-organic framework. Chem. Mater., 2010,22(14):4120-4122.
DOI URL |
| [17] |
GÁNDARA F, URIBE-ROMO F J, BRITT D K, et al. Porous, conductive metal-triazolates and their structural elucidation by the charge-flipping method. Chem. Eur-J., 2012,18(34):10595-10601.
DOI URL PMID |
| [18] |
LI R, WANG S H, CHEN X X, et al. Highly anisotropic and water molecule-dependent proton conductivity in a 2D homochiral copper (II) metal-organic framework. Chem. Mater., 2017,29(5):2321-2331.
DOI URL |
| [19] |
SADAKIYO M, YAMADA T, KITAGAWA H. Rational designs for highly proton-conductive metal-organic frameworks. J. Am. Chem. Soc., 2009,131(29):9906-9007.
DOI URL PMID |
| [20] |
SADAKIYO M, YAMADA T, KITAGAWA H. Proton conductivity control by ion substitution in a highly proton-conductive metal-organic framework. J. Am. Chem. Soc., 2014,136(38):13166-13169.
DOI URL |
| [21] |
TAYLOR J M, DEKURA S, IKEDA R, et al. Defect control to enhance proton conductivity in a metal-organic framework. Chem. Mater., 2015,27(7):2286-2289.
DOI URL |
| [22] |
KO M, MENDECKI L, MIRICA K A. Conductive two-dimensional metal-organic frameworks as multifunctional materials. Chem. Commun., 2018,54(57):7873-7891.
DOI URL |
| [23] |
SUN L, HENDON C H, MINIER M A, et al. Million-fold electrical conductivity enhancement in Fe2(DEBDC) versus Mn2(DEBDC) (E=S, O). J. Am. Chem. Soc., 2015,137(19):6164-6167.
DOI URL PMID |
| [24] |
LIN S, USOV P M, MORRIS A J. The role of redox hopping in metal-organic framework electrocatalysis. Chem. Commun., 2018,54(51):6965-6974.
DOI URL |
| [25] |
LEE D Y, SHINDE D V, KIM E K, et al. Supercapacitive property of metal-organic-frameworks with different pore dimensions and morphology. Micropor. Mesopor. Mat., 2013,171(10):53-57.
DOI URL |
| [26] |
HUANG H, LI J R, WANG K, et al. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks. Nat. Commun., 2015,6:8847.
DOI URL PMID |
| [27] |
HOU J, CAO C, IDREES F, et al. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano, 2015,9(3):2556-2564.
DOI URL PMID |
| [28] |
TAN Y, ZHANG W, GAO Y, et al. Facile synthesis and supercapacitive properties of Zr-metal organic frameworks (UiO-66). RSC Adv., 2015,5(23):17601-17605.
DOI URL |
| [29] |
LI W H, KUI D, TIAN H R, et al. Conductive metal-organic framework nanowire array electrodes for high-performance solid-state supercapacitors. Adv. Funct. Mater., 2017,27(27):1702067.
DOI URL |
| [30] |
SHEBERLA D, BACHMAN J C, ELIAS J S, et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater., 2017,16(2):220-224.
DOI URL PMID |
| [31] |
WEI C, RAKHI R B, WANG Q, et al. Morphological and electrochemical cycling effects in MnO2 nanostructures by 3D electron tomography. Adv. Funct. Mater., 2014,24(21):3130-3143.
DOI URL |
| [32] |
CHEN Y, DAN N, YANG X, et al. Microwave-assisted synthesis of honeycomblike hierarchical spherical Zn-doped Ni-MOF as a high-performance battery-type supercapacitor electrode material. Electrochim. Acta, 2018,278:114-123.
DOI URL |
| [33] |
ZHANG J, HAN B. Supercritical or compressed CO2 as a stimulus for tuning surfactant aggregations. Accounts Chem. Res., 2013,46(2):425-433.
DOI URL |
| [34] |
YU H, XU D, XU Q. Dual template effect of supercritical CO2 in ionic liquid to fabricate a highly mesoporous cobalt metal-organic framework. Chem. Commun., 2015,51(67):13197-13200.
DOI URL |
| [35] |
CAMPAGNOL N, ROMERO-VARA R, DELEU W, et al. A hybrid supercapacitor based on porous carbon and the metal-organic framework MIL-100(Fe). ChemElectroChem, 2014,1(7):1182-1188.
DOI URL |
| [36] |
LEE D Y, YOON S J, SHRESTHA N K, et al. Unusual energy storage and charge retention in Co-based metal-organic-frameworks. Micropor. Mesopor. Mat., 2012,153(3):163-165.
DOI URL |
| [37] | LIAO C, ZUO Y, WEI Z, et al. Russ. Electrochemical performance of metal-organic framework synthesized by a solvothermal method for supercapacitors. J. Electrochem., 2013,49(10):983-986. |
| [38] | XU J, CHAO Y, XUE Y, et al. Facile synthesis of novel metal-organic nickel hydroxide nanorods for high performance supercapacitor. Electrochim. Acta, 2016,211:595-602. |
| [39] | YANG J, XIONG P, ZHENG C, et al. Metal-organic frameworks: a new promising class of material for high performances supercapacitor electrode. J. Mater. Chem. A, 2014,2(39):16640-16644. |
| [40] | KANG L, SUN S X, KONG L B, et al. Investigating metal-organic framework as a new pseudo-capacitive material for supercapacitors. Chinese Chem. Lett., 2014,25(6):957-961. |
| [41] | QU C, JIAO Y, ZHAO B, et al. Nickel-based pillared MOFs for high-performance supercapacitors: design, synthesis and stability study. Nano Energy, 2016,26:66-73. |
| [42] |
GONG Y, LI J, JIANG P, et al. Novel metal(II) coordination polymers based on N,N'-bis-(4-pyridyl)phthalamide as supercapacitor electrode materials in an aqueous electrolyte. Dalton Trans., 2013,42(5):1603-1611.
DOI URL PMID |
| [43] | KANNANGARA Y Y, RATHNAYAKE U A, SONG J K. Redox active multi-layered Zn-pPDA MOFs as high-performance supercapacitor electrode material. Electrochim. Acta, 2019,297:145-154. |
| [44] |
CHOI K M, JEONG H M, PARK J H, et al. Supercapacitors of nanocrystalline metal-organic frameworks. ACS Nano, 2014,8(7):7451-7457.
DOI URL PMID |
| [45] | YANG J, ZHENG C, XIONG P, et al. Zn-doped Ni-MOF material with a high supercapacitive performance. J. Mater. Chem. A, 2014,2(44):19005-19010. |
| [46] | DÍAZ R, ORCAJO M G, BOTAS J A, et al. Co8-MOF-5 as electrode for supercapacitors. Mater. Lett., 2012,68:126-128. |
| [47] | GAO W, CHEN D, QUAN H, et al. Fabrication of hierarchical porous metal-organic framework electrode for aqueous asymmetric supercapacitor. ACS Sustain. Chem. Eng., 2017,5(5):4144-4153. |
| [48] |
TALIN A A, CENTRONE A, FORD A C, et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science, 2014,343(6166):66-69.
DOI URL PMID |
| [49] |
CHUI S S Y, LO S M F, CHARMANT J P, et al. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science, 1999,283(5405):1148-1150.
DOI URL PMID |
| [50] | WANG K, WANG Z, XIN W, et al. Flexible long-chain-linker constructed Ni-based metal-organic frameworks with 1D helical channel and their pseudo-capacitor behavior studies. J. Power Sources, 2018,377:44-51. |
| [51] | SALUNKHE R R, KANETI Y V, KIM J, et al. Nanoarchitectures for metal-organic framework-derived nanoporous carbons toward supercapacitor applications. Accounts Chem. Res., 2016,49(12):2796-2806. |
| [52] |
WANG L, FENG X, REN L, et al. Flexible solid-state supercapacitor based on a metal-organic framework interwoven by electrochemically-deposited PANI. J. Am. Chem. Soc., 2015,137(15):4920-4923.
DOI URL PMID |
| [53] | YANG J, GANG C, CHEN D, et al. Bimetal-organic framework assisted polymerization of pyrrole involving air oxidant to prepare composite electrodes for portable energy storage. J. Mater. Chem. A, 2017,5(45):23744-23752. |
| [54] | WANG Z, GAO C, LIU Y, et al. Electrochemical performance and transformation of Co-MOF/reduced graphene oxide composite. Mater. Lett., 2017,193:216. |
| [55] | BENNETT T D, CHEETHAM A K. Amorphous metal-organic frameworks. Accounts Chem. Res., 2014,47(5):1555-1562. |
| [56] | YANG F, LI W, TANG B J. Facile synthesis of amorphous UiO-66 (Zr-MOF) for supercapacitor application. Joarnal of Alloys & Compounds, 2018,733:8-14. |
| [57] |
MCHUGH L N, MCPHERSON M J, MCCORMICK L J, et al. Hydrolytic stability in hemilabile metal-organic frameworks. Nat. Chem., 2018,10(11):1096-1102.
DOI URL PMID |
| [58] | LAN Y, LI Z, YU C, et al. Application of zeolitic imidazolate framework in supercapacitor. New Chem. Mater., 2017,45(8):8-10. |
| [59] |
LI Z, WANG W, CAO H, et al. Boron doped ZIF-67@graphene derived carbon electrocatalyst for highly efficient enzyme-free hydrogen peroxide biosensor. Adv. Mater. Tech., 2017,2(12):1700224.
DOI URL |
| [60] | LI Z, JIANG Y, WANG Z, et al. Nitrogen-rich core-shell structured particles consisting of carbonized zeolitic imidazolate frameworks and reduced graphene oxide for amperometric determination of hydrogen peroxide. Microchim. Acta, 2018,185(11):501. |
| [61] | LI Z, LAN Y, CAO H, et al. Carbon materials derived from chitosan/ cellulose cryogel-supported zeolite imidazole frameworks for potential supercapacitor application. Carbohyd. Polym., 2017,175(1):223-230. |
| [62] | LI Z, HE H, CAO H, et al. Atomic Co/Ni dual sites and Co/Ni alloy nanoparticles in N-doped porous Janus-like carbon frameworks for bifunctional oxygen electrocatalysis. Appl. Catal. B: Environ., 2019,240:112-121. |
| [63] |
GAILLAC R, PULLUMBI P, BEYER K A, et al. Liquid metal-organic frameworks. Nat. Mater., 2017,16(11):1149-1154.
DOI URL PMID |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [8] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [9] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [10] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [11] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [12] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [13] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [14] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| [15] | ZHANG Jinghui, LU Xiaotong, MAO Haiyan, TIAN Yazhou, ZHANG Shanlin. Effect of Sintering Additives on Sintering Behavior and Conductivity of BaZr0.1Ce0.7Y0.2O3-δ Electrolytes [J]. Journal of Inorganic Materials, 2025, 40(1): 84-90. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||