
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (8): 811-824.DOI: 10.15541/jim20170529
• Orginal Article • Next Articles
HE Qian-Jun, CHEN Dan-Yang, FAN Ming-Jian
Received:2017-11-09
Revised:2017-12-27
Published:2018-08-28
Online:2018-07-17
Supported by:CLC Number:
HE Qian-Jun, CHEN Dan-Yang, FAN Ming-Jian. Progress of Precision Nanomedicine-mediated Gas Therapy[J]. Journal of Inorganic Materials, 2018, 33(8): 811-824.
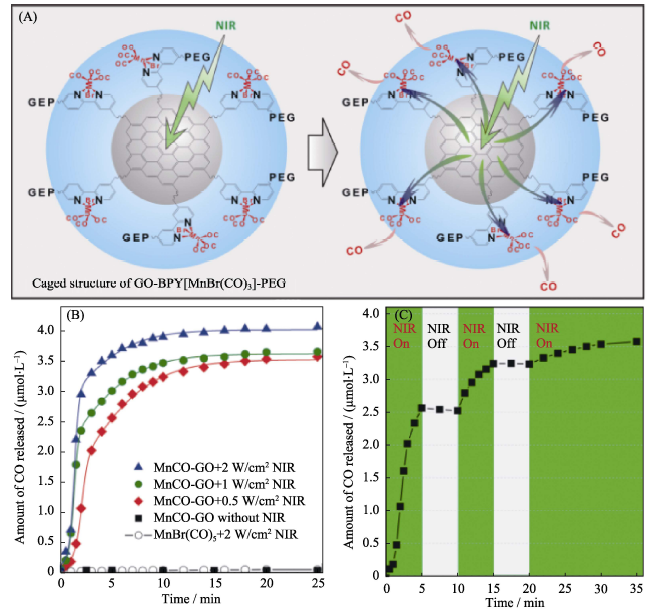
Fig. 1 (A) NIR-responsive CO release mechanism of the MnCO-GON nanomedicine with a caged structure, (B) NIR responsive for CO release profiles of MnCO-GON, and (C) NIR-controllability of MnCO-GON for CO release[27] GON: Graphene Oxide Nanosheet
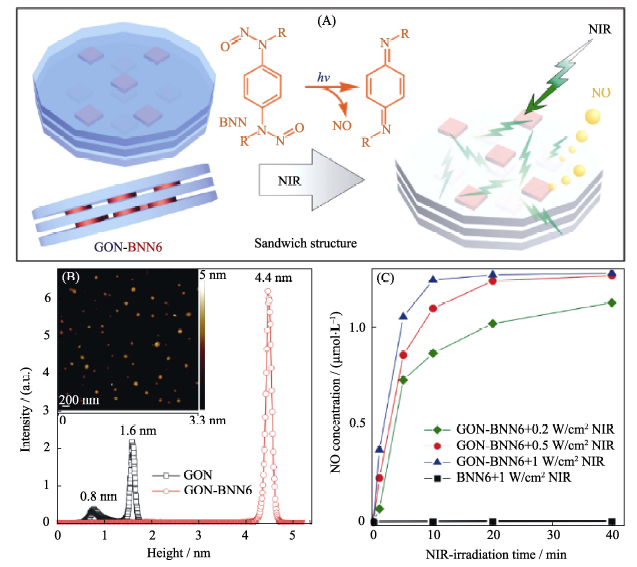
Fig. 2 (A) The sandwich structure of GO-BNN6 self-assembled by GO nanosheets and BNN6 molecules through the π-π stacking, and the mechanism of NIR-responsive NO release; (B) AFM data of GO-BNN6 and (C) NIR-controlled NO release profiles of the GO-BNN6 nanomedicine[28] BNN: bis-N-nitroso
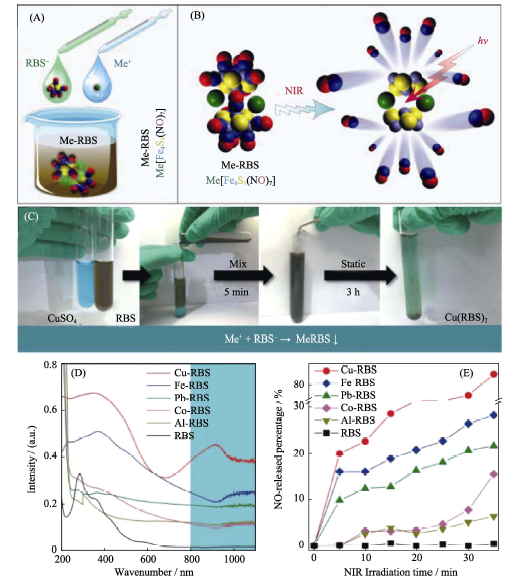
Fig. 3 (A) Schematic illustration of the coordination-precipitation process of insoluble Me-RBS, (B) the NIR-responsive NO release mechanism of Me-RBS, (C) comparison of UV absorption behaviors of Me-RBS and (D) comparison of NO release behaviors of Me-RBS[38] RBS: Rosen Black Salt
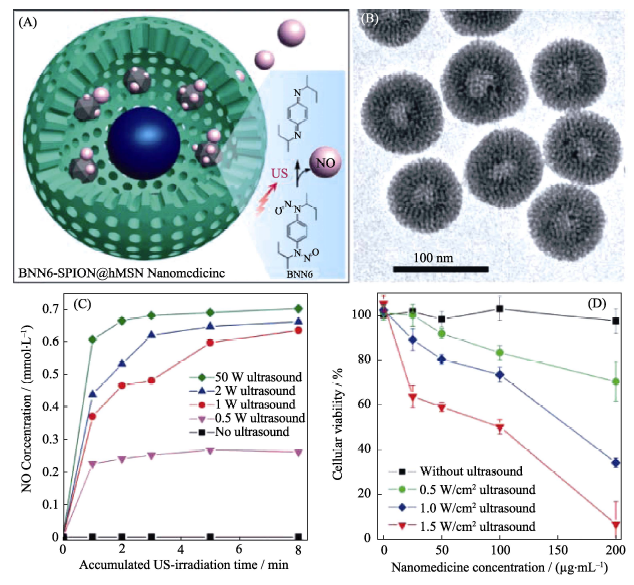
Fig. 4 (A) The mechanism of ultrasound-responsive NO release from the rattle-structured BNN6-SPION@hMSN nanomedicine, (B) TEM image of the nanomedicine, (C) ultrasound-responsive NO release behavior of the nanomedicine and (D) ultrasound-induced cytotoxicity of the nanomedicine[41] BNN: bis-N-nitroso; SPION: Superparamagnetic Iron Oxide-encapsulated; hMSN: hollow Mesoporous Silica Nanoparticles
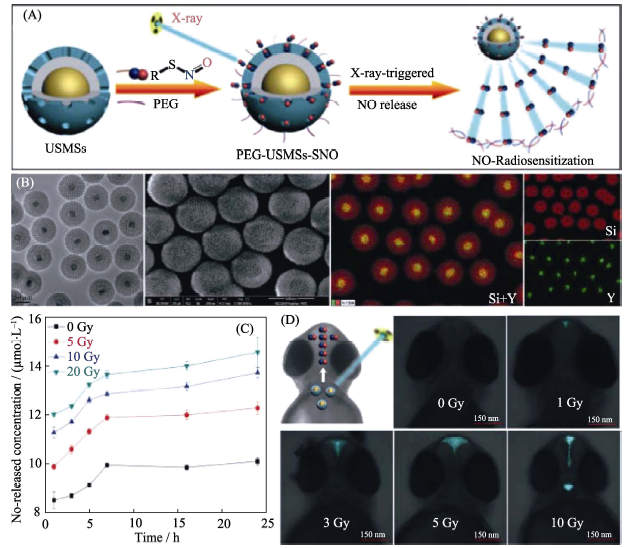
Fig. 5 (A) The mechanism of X-ray responsive NO release from the PEG-USMSs-SNO nanomedicine with the core-shell structure, (B) TEM images and elementary mapping of the nanomedicine, (C) X-ray controlled NO release behavior of the nanomedicine in vitro, and (D) X-ray controlled NO release behavior of the nanomedicine on zebrafish[42] USMSs: Upconversion nano-theranostic system; SNO: S-nitrosothiol.
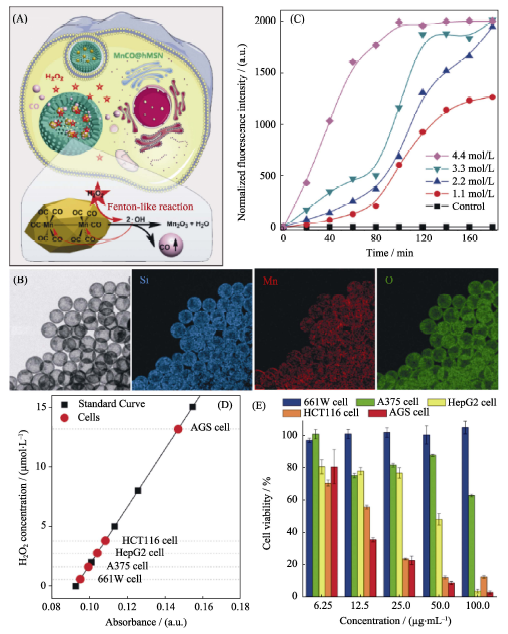
Fig. 6 (A) The H2O2-triggered CO release mechanism of the MnCO@hMSN nanomedicine constructed by hMSN and manganese carbonyl prodrug, (B) TEM image and elementary mapping of the nanomedicine, (C) H2O2-triggered CO release behavior of the nanomedicine in vitro, (D) comparison of H2O2 levels in various cells and (E) comparison of cytotoxicity against various cells[43]hMSN: hollow Mesoporous Silica Nanoparticles
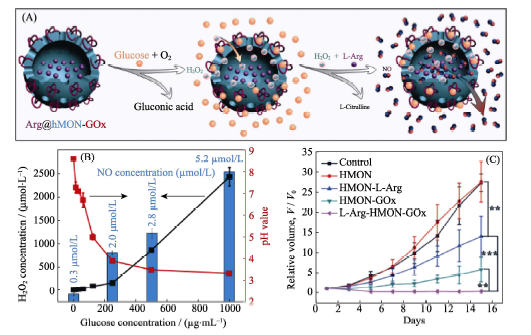
Fig. 7 (A) Construction and SEM image of the Arg@hMON-GOx nanomedicine based on the hMON carrier and the Arginie/GOx prodrugs, and the mechanism of glucose-responsive release of NO, (B) effects of glucose concentration on hydrogen peroxide concentration, pH and NO concentration, and (C) in vivo outcome of gas therapy by nanomedicine[44]hMON: hollow Mesoporous Organosilica Nanoparticle
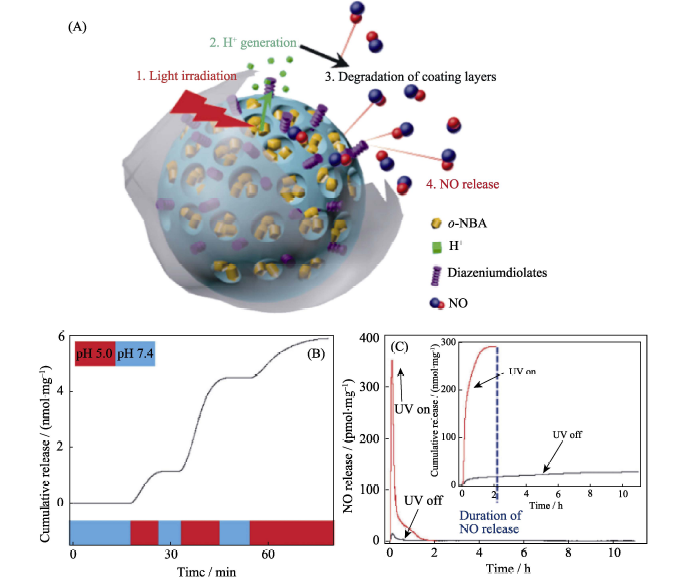
Fig. 8 (A) The construction of the MSN-CaP-NO nanomedicine and its controlled NO release mechanism, (B) acid-responsive NO release behavior of the nanomedicine, and (C) light-controlled NO release profile of the nanomedicine[32]MSN: Mesoporous Silica Nanoparticles
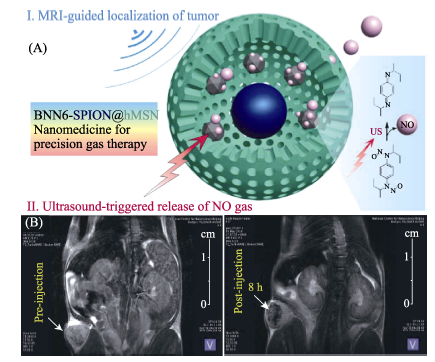
Fig. 9 (A) The MRI-guided ultrasound-triggered NO release mechanism of BNN6-SPION@hMSN nanomedicine, and (B) tumor-targeting property and the corresponding MRI profile[41]BNN: bis-N-nitroso; SPION: Superparamagnetic Iron Oxide-encapsulated; hMSN: hollow Mesoporous Silica Nanoparticles
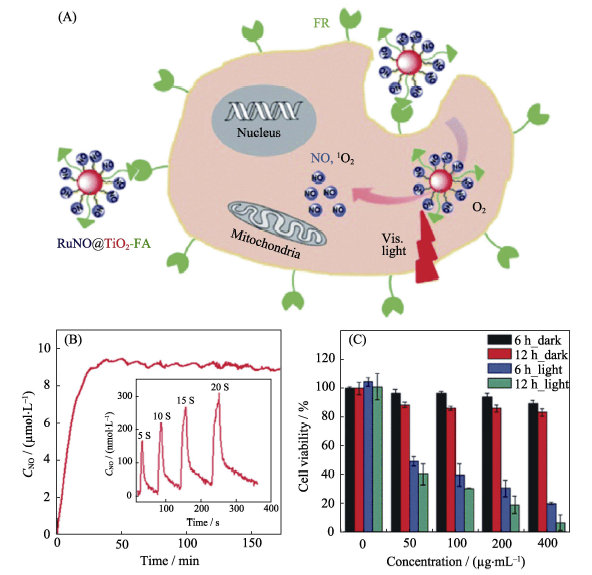
Fig. 10 (A) The construction of the RuNO@TiO2NPs nanomedicine and its light-controlled ROS/NO co-release mechanism, (B) behavior of light-responsive release of NO and (C) cytotoxicity profile of the nanmedicine[48]
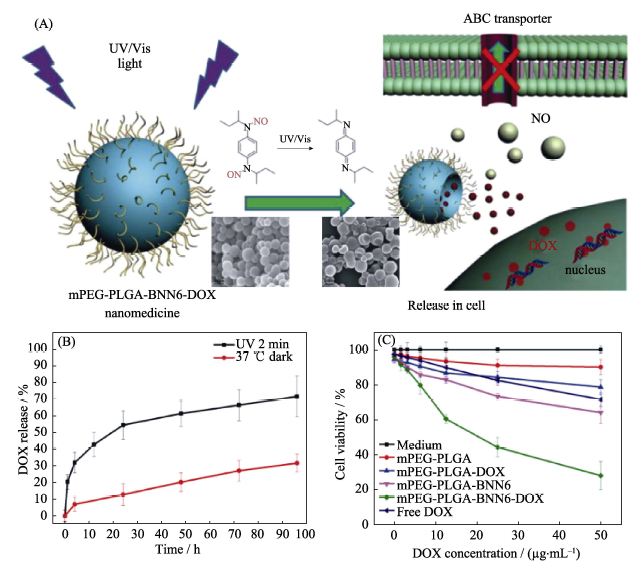
Fig. 11 (A) The mechanism of light-controlled multidrug co-release from the mPEG-BNN6-DOX nanomedicine, (B) behavior of UV-controlled NO release of the nanomedicine, and (C) cytotobxicity profile of the nanomedicine[31]BNN: bis-N-nitroso; DOX: Doxorubicin
| [1] | SZABÓ C.Gasotransmitters in cancer: from pathophysiology to experimental therapy.Nat. Rev. Drug Discov., 2016, 15(3): 185-203. |
| [2] | SZABÓ C.Hydrogen sulphide and its therapeutic potential.Nat. Rev. Drug Discov., 2007, 6(11): 917-935. |
| [3] | FUKUMURA D, KASHIWAGI S, JAIN R K.The role of nitric oxide in tumour progression.Nat. Rev. Cancer., 2006, 6(7): 521-534. |
| [4] | MOTTERLINI R, OTTERBEIN L E.The therapeutic potential of carbon monoxide. Nat. Rev. Drug. Discov., 2010, 9(9): 728-743. |
| [5] | GALLEGO S G,BERNARDES G J L. Carbon-monoxide-releasing molecules for the delivery of therapeutic CO in vivo. Angew. Chem. Int. Ed., 2014, 53(37): 9712-9721. |
| [6] | BIBHUTI B M, VIJAY A K R, GREGORY W M,et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β Christian Bogdan. .Nat. Immunol., 2013, 14(1): 52-60. |
| [7] | LALA P K, CHAKRABORTY C.Role of nitric oxide in carcinogenesis and tumour progression.Lancet Oncol., 2001, 2(3): 149-156. |
| [8] | CHIN B Y, JIANG G, WEGIEL B,et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning.Proc. Natl. Acad. Sci. USA, 2007, 104(12): 5109-5114. |
| [9] | OTTERBEIN L E, ZUCKERBRAUN B S, HAGA M,et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury.Nat. Med., 2003, 9(2): 183-190. |
| [10] | OTTERBEIN L E, BACH F H, ALAM J,et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med., 2000, 6(4): 422-428. |
| [11] | NASSOUR I, KAUTZA B, RUBIN M,et al. Carbon monoxide protects against hemorrhagic shock and resuscitation-induced microcirculatory injury and tissue injury.Shock, 2015, 43(2): 166-171. |
| [12] | CARPENTER A W, SCHOENFISCH M H.Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev., 2012, 41(10): 3742-3752. |
| [13] | DIRING S, WANG D O, KIM C,et al. Localized cell stimulation by nitric oxide using a photoactive porous coordination polymer platform. Nat. Commun., 2013, 4(10): 2684. |
| [14] | KIM J, SARAVANAKUMAR G, CHOI H W,et al. A platform for nitric oxide delivery.J. Mater. Chem., 2014, 2(4): 341-356. |
| [15] | RAJU T N.The Nobel chronicles. 1998: Robert Francis Furchgott (b 1911), Louis J Ignarro (b 1941), and Ferid Murad (b 1936).Lancet, 2000, 356(9226): 346. |
| [16] | WEGIEL B, GALLO D, CSIZMADIA E,et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res., 2013, 73(23): 7009-7021. |
| [17] | MONCADA S, ERUSALIMSKY J D.Does nitric oxide modulate mitochondrial energy generation and apoptosis?.Nat. Rev. Mol. Cell Biol., 2002, 3(4): 214-220. |
| [18] | MÓDIS K, BOS E M, CALZIA E,et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects.Br. J. Pharmacol., 2014, 171(8): 2123-2146. |
| [19] | HE Q J.Precision gas therapy using intelligent nanomedicine.Biomater. Sci., 2017, 5(11): 2226-2230. |
| [20] | ERNST A, ZIBRAK J D.Carbon monoxide poisoning.N. Engl. J. Med., 1998, 339(22): 1603-1608. |
| [21] | SULLIVAN J B, KRIEGER G B, THOMAS R J.Methemoglobin-forming chemicals in hazardous materials toxicology: clinical principals of environmental health.J. Occup. Environ. Med., 1992, 34(4): 365-371. |
| [22] | RIDNOUR L A, THOMAS D D, DONZELLI S,et al. The biphasic nature of nitric oxide responses in tumor biology.Antioxid. Redox. Sign., 2006, 8(7/8): 1329-1337. |
| [23] | WANG L Z, SHI J L, YUN J,et al. Research progress of mesoporous silicon materials.J. Inorg. Mater., 1999, 14(3): 333-342. |
| [24] | SHI J L, CHEN Y, CHEN H R.Research progress of multifunctional mesoporous silica based nanoparticles for diagnosis and treatment.J. Inorg. Mater., 2013, 28(1): 1-11. |
| [25] | HE Q J, SHI J L.MSN anti-cancer nanomedicines: chemotherapy enhancement, overcoming of drug resistance and metastasis inhibition.Adv. Mater., 2014, 26(3): 391-411. |
| [26] | ZHOU Y L, FENG X X, ZHAI W Y.Research on loading and release of Epirubicin with mesoporous bioactive glass.J. Inorg. Mater., 2011, 26(1): 68-72. |
| [27] | HE Q, KIESEWETTER D O, QU Y,et al. NIR-responsive on-demand release of CO from metal carbonyl-caged graphene oxide nanomedicine.Adv. Mater., 2015, 27(42): 6741-6746. |
| [28] | FAN J, HE N Y, HE Q J,et al. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO.Nanoscale, 2015, 7(47): 20055-20062. |
| [29] | GARCIA J V, YANG J, SHEN D,et al. NIR-triggered release of caged nitric oxide using upconverting nanostructured materials.Small, 2012, 8(24): 3800-3805. |
| [30] | ZHANG X, TIAN G, YIN W,et al. Controllable generation of nitric oxide by near-infrared-sensitized upconversion nanoparticles for tumor therapy. Adv. Funct. Mater., 2015, 25(20): 3049-3055. |
| [31] | FAN J, HE Q, LIU Y,et al. Light-responsive biodegradable nanomedicine overcomes multidrug resistanc.via NO-enhanced chemosensitization. ACS Appl. Mater. Interfaces, 2016, 8(22): 13804-1381. |
| [32] | WOO C H, JIHOON K, JINHWAN K,et al. Light-induced acid generation on a gatekeeper for smart nitric oxide delivery.ACS Nano, 2016, 10(4): 4199-4208. |
| [33] | OSTROWSKI D, LIN B F, TIRRELL M V,et al. Liposome encapsulation of a photochemical NO precursor for controlled nitric oxide release and simultaneous fluorescence imaging.Mol. Pharm., 2012, 9(10): 2950-2955. |
| [34] | WANG P G, XIAN M, TANG X P,et al. Nitric oxide donors: Chemical activities and biological applications.Chem. Rev., 2002, 102(4): 1091-1134. |
| [35] | RIMMER R D, PIERRI A E, FORD P C.Photochemically activated carbon monoxide release for biological targets. toward developing air-stable photoCORMs labilized by visible light.Coordin. Chem. Rev., 2012, 256(15/16): 1509-1519. |
| [36] | ZHENG D W, LI B, LI C X, et al. Photocatalyzing CO2 to CO for enhanced cancer therapy.Adv. Mater., 2017, 29(44): 1703822-1-8. |
| [37] | Reddy G U, AXTHELM J, HOFFMANN P,et al. Co-registered molecular logic gate with a CO-releasing molecule triggered by light and peroxide.J. Am. Chem. Soc., 2017, 139(14): 4991-4994. |
| [38] | CHEN L J, HE Q J, LEI M Y,et al. Facile coordination-precipitation route to insoluble metal roussin’s black salts for NIR-responsive release of NO for anti-metastasis.ACS Appl. Mater. Interfaces., 2017, 9(42): 36473-36477. |
| [39] | MARIN A, MUNIRUZZAMAN M, RAPOPORT N.Mechanism of the ultrasonic activation of micellar drug delivery. J. Control. Release, 2001, 75(1/2): 69-81. |
| [40] | POSTEMA M, BOUAKAZ A,CATE F J T,et al. Nitric oxide delivery by ultrasonic cracking: some limitations.Ultrasonics, 2006, 44(Suppl.): e109-e113. |
| [41] | JIN Z, WEN Y, HU Y,et al. MRI-guided and ultrasound-triggered release of NO by advanced nano-medicine.Nanoscale, 2017, 9(10): 3637-3645. |
| [42] | FAN W, BU W, HE Q,et al. X-ray radiation-controlled NO-release for on-demand depth-independent hypoxic radiosensitization.Angew. Chem. Int. Ed., 2015, 54(47): 14026-14030. |
| [43] | JIN Z, WEN Y, XIONG L,et al. Intratumoral H2O2-triggered release of CO from a metal carbonyl-based nanomedicine for efficient CO therapy. Chem. Commun., 2017, 53(40): 5557-5560. |
| [44] | FAN W, LU N, HUANG P,et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy.Angew. Chem. Int. Ed., 2016, 55(1): 1-6. |
| [45] | HE Q J, GUO S R, QIAN Z Y,et al. Development of individualized anti-metastasis strategies by engineering nanomedicines. Chem. Soc. Rev., 2015, 44(17): 6258-6286. |
| [46] | GUI R J, WAN A J, ZHANG Y L,et al. Light-triggered nitric oxide release and targeted fluorescence imaging in tumor cells developed from folic acid-graft-carboxymethyl chitosan nanospheres.RSC Adv., 2014, 4(57): 30129-30136. |
| [47] | ZHANG X F, MANSOURI S, MBEH D A,et al. Nitric oxide delivery by core/shell superparamagnetic nanoparticle vehicles with enhanced biocompatibility.Langmuir, 2012, 28(35): 12879-12885. |
| [48] | XIANG J, AN L, TANG W W,et al. Photo-controlled targeted intracellular delivery of both nitric oxide and singlet oxygen using a fluorescencence trackable ruthenium nitrosyl functional nanoplatform.Chem. Commun., 2015, 51(13): 2555-2558. |
| [49] | CHAKRABORTY I, JIMENEZ J, SAMEERA W M,et al. Luminescent Re(I) carbonyl complexes as trackable PhotoCORMs for CO delivery to cellular targets. Inorg. Chem., 2017, 56(5): 2863-2873. |
| [50] | JI X, ZHOU C, JI K,et al. Click and release: a chemical strategy toward developing gasotransmitter prodrugs by using an intramolecular Diels-Alder reaction.Angew. Chem. Int. Ed., 2016, 55(1): 1-7. |
| [51] | CARRINGTON S J, CHAKRABORTY I, BERNARD J M L,et al. A theranostic two-tone luminescent PhotoCORM derived from Re(I) and (2-pyridyl)-benzothiazole: trackable CO delivery to malignant cells.Inorg. Chem., 2016, 55(16): 7852-7858. |
| [52] | CHAKRABORTY I, CARRINGTON S J, HAUSER J,et al. Rapid eradication of human breast cancer cells through trackable light- triggered CO delivery by mesoporous silica nanoparticles packed with a designed photoCORM.Chem. Mater., 2015, 27(24): 8387-8397. |
| [53] | CARRINGTON S, CHAKRABORTY I, BERNARD J M L,et al. Synthesis and characterization of a “turn-on” photoCORM for trackable CO delivery to biological targets.ACS Med. Chem. Lett., 2014, 5(12): 1324-1328. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | YANG Mingkai, HUANG Zeai, ZHOU Yunxiao, LIU Tong, ZHANG Kuikui, TAN Hao, LIU Mengying, ZHAN Junjie, CHEN Guoxing, ZHOU Ying. Co-production of Few-layer Graphene and Hydrogen from Methane Pyrolysis Based on Cu and Metal Oxide-KCl Molten Medium [J]. Journal of Inorganic Materials, 2025, 40(5): 473-480. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [10] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [11] | GAO Chenguang, SUN Xiaoliang, CHEN Jun, LI Daxin, CHEN Qingqing, JIA Dechang, ZHOU Yu. SiBCN-rGO Ceramic Fibers Based on Wet Spinning Technology: Microstructure, Mechanical and Microwave-absorbing Properties [J]. Journal of Inorganic Materials, 2025, 40(3): 290-296. |
| [12] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [13] | WANG Yue, WANG Xin, YU Xianli. Room-temperature Ferromagnetic All-carbon Films Based on Reduced Graphene Oxide [J]. Journal of Inorganic Materials, 2025, 40(3): 305-313. |
| [14] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [15] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||