
Journal of Inorganic Materials ›› 2017, Vol. 32 ›› Issue (6): 609-614.DOI: 10.15541/jim20160518
• Orginal Article • Previous Articles Next Articles
MENG Fan-Bin1, MA Xiao-Fan1, ZHANG Wei1, WU Guang-Heng2, ZHANG Yu-Jie1
Received:2016-09-18
Revised:2016-11-30
Published:2017-06-20
Online:2017-05-27
Supported by:CLC Number:
MENG Fan-Bin, MA Xiao-Fan, ZHANG Wei, WU Guang-Heng, ZHANG Yu-Jie. Structure and Magnetic Property of Fe and Mn Doped Spinel Co2MnO4[J]. Journal of Inorganic Materials, 2017, 32(6): 609-614.
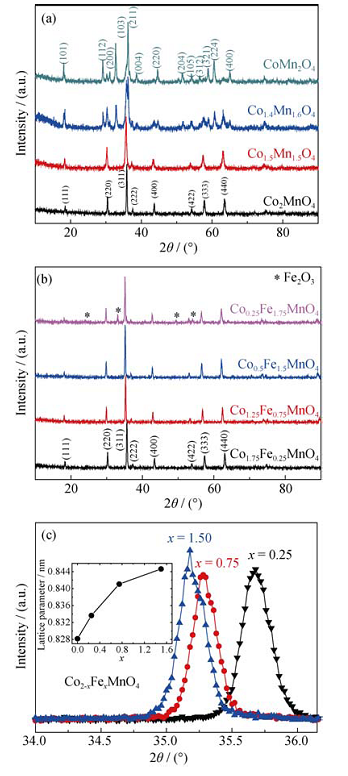
Fig. 1 XRD patterns for Co2-xMn1+xO4 (x= 0, 0.5, 0.6, 1) (a); XRD patterns for Co2-xFexMnO4 (x= 0.25, 0.75, 1.50, 1.75), the impure phase peaks are marked with asterisks; (b) Detailed XRD patterns for Co2-xFexMnO4 (x= 0.25, 0.75, 1.50) showing the peak of (311) reflection (c). Inset shows the lattice parameter as a function of Fe content
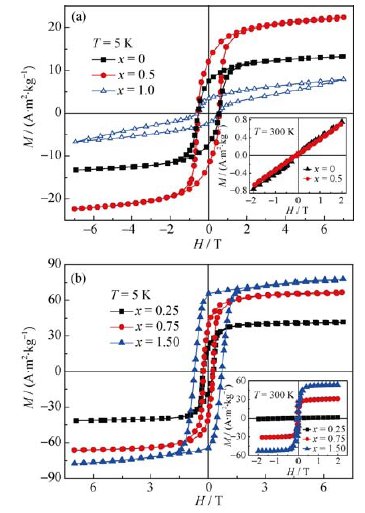
Fig. 3 Hysteresis loop for Co2-xMn1+xO4 (x=0, 0.5, 1.0) (a) and Co2-xFexMnO4 (x=0.25, 0.75, 1.50) (b) at the temperature of 5 K. Inset of them are the loops tested at room temperature
| Co2-xMn1+xO4 | MS/(A∙m2∙kg-1) | Mr/(A∙m2∙kg-1) | HC/T |
|---|---|---|---|
| x = 0 | 13.23 | 7.32 | 0.5421 |
| x = 0.5 | 22.41 | 12.16 | 0.5427 |
| Co2-xFexMnO4 | Ms/(A∙m2∙kg-1) | Mr/(A∙m2∙kg-1) | HC/T |
| x = 0 | 13.23 | 7.32 | 0.5421 |
| x = 0.25 | 41.61 | 19.91 | 0.2763 |
| x = 0.75 | 66.78 | 37.36 | 0.2507 |
| x = 1.50 | 77.97 | 64.83 | 0.7118 |
Table 1 Magnetic parameters for Co2-xMn1+xO4 and Co2-xFexMnO4 tested at 5 K
| Co2-xMn1+xO4 | MS/(A∙m2∙kg-1) | Mr/(A∙m2∙kg-1) | HC/T |
|---|---|---|---|
| x = 0 | 13.23 | 7.32 | 0.5421 |
| x = 0.5 | 22.41 | 12.16 | 0.5427 |
| Co2-xFexMnO4 | Ms/(A∙m2∙kg-1) | Mr/(A∙m2∙kg-1) | HC/T |
| x = 0 | 13.23 | 7.32 | 0.5421 |
| x = 0.25 | 41.61 | 19.91 | 0.2763 |
| x = 0.75 | 66.78 | 37.36 | 0.2507 |
| x = 1.50 | 77.97 | 64.83 | 0.7118 |
| [1] | JIN F H, KNEZ M, SCHOLZ R, et al.Monocrystalline spinel nanotube fabrication based on the Kirkendall effect.Nature Materials, 2006, 5(8): 627-631. |
| [2] | LIANG Y, WANG H, ZHOU J, et al.Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts.Journal of the American Chemical Society, 2012, 134(7): 3517-3523. |
| [3] | CHENG F Y, SHEN J, PENG B, et al.Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts.Nature Chemistry, 2011, 3(1): 79-84. |
| [4] | YAMASAKI Y, MIYASAKA S, KANEKO Y, et al.Magnetic reversal of the FerrGslectric polarization in a multiferroic spinel oxide.Physical Review Letters, 2006, 96(20): 207204. |
| [5] | YU L, ZHANG L, WU H B, et al.Controlled synthesis of hierarchical CoxMn3-xO4 array micro-/nanostructures with tunable morphology and composition as integrated electrodes for lithium-ion batteries.Energy & Environmental Science, 2013, 6(9): 2664-2671. |
| [6] | RAJEEVAN N E, KUMAR R, SHUKLA D K, et al.Structural, electrical and magnetic properties of Bi-substituted Co2MnO4.Materials Science and Engineering: B, 2009, 163(1): 48-56. |
| [7] | ROUSSET A.Reactivity of solids and new metastable phases: examples of mixed valence defect spinel ferrites and manganites.Solid State Ionics, 1994, 25(11): 236-242. |
| [8] | LAARJ M, KACIM S, GILLOT B, et al.Cationic distribution and oxidation mechanism of trivalent manganese ions in submicrometer MnxCoFe2-xO4 spinel ferrites.Journal of Solid State Chemistry, 1996, 125(1): 67-74. |
| [9] | DOS SANTOS M E, FERREIRA R A, LISBOA-FILHO P N, et al. Cation distribution and magnetic characterization of the multiferroic cobalt manganese Co2MnO4 spinel doped with bismuth.Journal of Magnetism and Magnetic Materials, 2013, 329(3): 53-58. |
| [10] | KANG S H, KIM I W, JEONG Y H, et al.Crystal growth and magnetic properties of spinel (Co,Mn)3O4.Journal of Crystal Growth, 2012, 344(1): 65-68. |
| [11] | RAJEEVAN N E, PRADYUMNAN P P, KUMAR R, et al.MagnetGslectric properties of BixCo2-xMnO4 (0≤x≤0.3).Applied Physics Letters, 2008, 92(10): 102910. |
| [12] | JING M J, HOU H S, YANG Y C, et al.Electrochemically alternating voltage tuned Co2MnO4/Co hydroxide chloride for an asymmetric supercapacitor.Electrochimica Acta, 2015, 165: 198-205. |
| [13] | HE H Q, ZHANG L, BABAEI A, et al.Co2MnO4 spinel-palladium co-infiltrated La0.7Ca0.3Cr0.5Mn0.5O3-δ cathodes for intermediate temperature solid oxide fuel cells.Journal of Alloys and Compounds, 2011, 509(40): 9708-9717. |
| [14] | RAJEEVAN N E, RAVI K, SHUKLA D K, et al.Bi-substitution-induced magnetic moment distribution in spinel BixCo2- xMnO4multiferroic.Journal of Physics: Condensed Matter, 2009, 21(40): 3173-3178. |
| [15] | RIOS E, LARA P, SERAFINI D, et al.Synthesis and characterization of manganese- cobalt solid solutions prepared at low temperature.Journal of the Chilean Chemical Society, 2010, 55(2): 261-265. |
| [16] | HAKIM M A, KUMAR N S, SIKDER S S, et al.Cation distribution and electromagnetic properties of spinel type Ni-Cd ferrites.Journal of Physics and Chemistry of Solids, 2013, 74(9): 1316-1321. |
| [17] | BENNER J, THOLKAPPIYAN R, VISHISTA K, et al.Attestation in self-propagating combustion approach of spinel AFe2O4 (A=Co, Mg and Mn) complexes bearing mixed oxidation states: Magnetostructural properties.Applied Surface Science, 2016, 383: 113-125. |
| [18] | MANIKANDAN A, DURKA M, AMUTHA S M, et al.Sesamum indicum plant extracted microwave combustion synthesis and opto-magnetic properties of spinel MnxCo1-xAl2O4 nano-catalysts.Journal of Nanoscience and Nanotechnology, 2016, 16(1): 448-456. |
| [19] | 彭文世, 刘高魁. 矿物红外光谱图集. 贵阳: 科学出版社, 1982: 98-104. |
| [20] | KURTAN U, GÜNGÜNEŞ H, SÖZERI H, et al. Synthesis and characterization of monodisperse NiFe2O4 nanoparticles.Ceramics International, 2016, 42(7): 7987-7992. |
| [21] | 张伟. 尖晶石型铁酸锰材料制备及性能研究. 南京: 南京理工大学硕士学位论文, 2010. |
| [22] | 雷中伟. 过渡金属氧化物系列红外辐射材料研究. 苏州: 苏州大学硕士学位论文, 2007. |
| [23] | WU C, WANG Z X, WU F, et al.Spectroscopic studies on cation-doped spinel LiMn2O4 for lithium ion batteries.Solid State Ionics, 2001, 144(3/4): 277-285. |
| [24] | ZHANG S T, LU M H, WU D, et al. Larger polarization and weak ferromagnetism in quenched BiFeO3 ceramics with a distorted rhombohedral crystal structure. Applied Physics Letters, 2005, 87(26): 262907-1-3. |
| [25] | RAJEEVAN N E, KUMAR R, SHUKLA D K, et al.MagnetGslectric behavior of ferrimagnetic BixCo2-xMnO4 (x=0, 0.1 and 0.3) thin films.Journal of Magnetism and Magnetic Materials, 2011, 323(13): 1760-1765. |
| [26] | ZHANG H G, WANG Z, LIU E K, et al. Site preference and compensation behavior in Co(Cr, Mn)2O4 system. Journal of Applied Physics, 2015, 117(17): 17B735-1-4. |
| [1] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [2] | HUANG Zipeng, JIA Wenxiao, LI Lingxia. Crystal Structure and Terahertz Dielectric Properties of (Ti0.5W0.5)5+ Doped MgNb2O6 Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 647-655. |
| [3] | ZHAO Kaixuan, LIU Wenpeng, DING Shoujun, DOU Renqin, LUO Jianqiao, GAO Jinyun, SUN Guihua, REN Hao, ZHANG Qingli. Nd:YLF Crystal Growth: Raw Materials Preparation by Melting Method and Property [J]. Journal of Inorganic Materials, 2025, 40(5): 529-535. |
| [4] | HUANG Jianfeng, LIANG Ruihong, ZHOU Zhiyong. Effects of W/Cr Co-doping on the Crystal Structure and Electric Properties of CaBi2Nb2O9 Piezoceramics [J]. Journal of Inorganic Materials, 2024, 39(8): 887-894. |
| [5] | SONG Yunxia, HAN Yinglei, YAN Tao, LUO Min. New Ultraviolet Nonlinear Optical Crystal Rb3Hg2(SO4)3Cl [J]. Journal of Inorganic Materials, 2023, 38(7): 778-784. |
| [6] | ZHAO Wei, XU Yang, WAN Yingjie, CAI Tianxun, MU Jinxiao, HUANG Fuqiang. Metal Cyanamides/Carbodiimides: Structure, Synthesis and Electrochemical Energy Storage Performance [J]. Journal of Inorganic Materials, 2022, 37(2): 140-151. |
| [7] | PENG Fan, ZENG Yi. Method of Crystal Structure Identification by Using Kikuchi Diffraction Patterns [J]. Journal of Inorganic Materials, 2021, 36(11): 1193-1198. |
| [8] | LI Shufang,ZHAO Shuang,ZHOU Xiao,LI Manrong. Crystal Structures, Optical, and Magnetic Properties of Zn3-xMnxTeO6 [J]. Journal of Inorganic Materials, 2020, 35(8): 895-901. |
| [9] | LI Shufang, ZHAO Shuang, LI Manrong. Flux Growth of Tungsten Oxychloride Li23CuW10O40Cl5 [J]. Journal of Inorganic Materials, 2020, 35(7): 834-838. |
| [10] | HUANG Chong,ZHAO Wei,WANG Dong,BU Kejun,WANG Sishun,HUANG Fuqiang. Synthesis, Crystal Structure, and Electrical Conductivity of Pd-intercalated NbSe2 [J]. Journal of Inorganic Materials, 2020, 35(4): 505-510. |
| [11] | Xiang-Xiong ZENG, Jin-Chao YANG, Lian ZUO, Ben-Ben YANG, Jun QIN, Zhi-Hang PENG. Li/Ce/La Multidoping on Crystal Structure and Electric Properties of CaBi2Nb2O9 Piezoceramics [J]. Journal of Inorganic Materials, 2019, 34(4): 379-386. |
| [12] | HUANG Long, DING Shi-Hua, ZHANG Xiao-Yun, YAN Xin-Kan, LI Chao, ZHU Hui. Structure and Microwave Dielectric Property of BaAl2Si2O8 with Li2O-B2O3-SiO2 Glass Addition [J]. Journal of Inorganic Materials, 2019, 34(10): 1091-1096. |
| [13] | ZHOU Xin, MA Lei, LIU Tao, GUO Yong-Bin, WANG Dao, DONG Pei-Lin. Crystal Structure and Magnetic Property of Si3N4/FePd/Si3N4 Thin Films [J]. Journal of Inorganic Materials, 2018, 33(8): 909-913. |
| [14] | WANG Qing-Qing, SHI Jian, LI Huan-Ying, CHEN Xiao-Feng, PAN Shang-Ke, BIAN Jian-Jiang, REN Guo-Hao. Optical and Scintillation Properties of Cs2LiYCl6:Ce Crystal [J]. Journal of Inorganic Materials, 2017, 32(2): 175-179. |
| [15] | DAN Meng, ZHANG Qian, ZHONG Yun-Qian, ZHOU Ying. Preparation of MnS with Different Crystal Phases for Photocatalytic H2 Production from H2S [J]. Journal of Inorganic Materials, 2017, 32(12): 1308-1314. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||