
Journal of Inorganic Materials ›› 2017, Vol. 32 ›› Issue (6): 603-608.DOI: 10.15541/jim20160474
• Orginal Article • Previous Articles Next Articles
LI Xiao-Yu1, ZHANG Li2, TANG Xin-Feng3, ZHANG Qing-Jie4
Received:2016-08-17
Revised:2016-10-08
Published:2017-06-20
Online:2017-05-27
About author:LI Xiao-Yu.
Supported by:CLC Number:
LI Xiao-Yu, ZHANG Li, TANG Xin-Feng, ZHANG Qing-Jie. Preparation and Characterization of γ-NaxCoO2 by Sodium Polyacrylate Gel Method[J]. Journal of Inorganic Materials, 2017, 32(6): 603-608.
| Nominal Na content, x | Actual Na content, x |
|---|---|
| 0.8 | 0.784 |
| 0.9 | 0.878 |
| 1.0 | 0.977 |
Table 1 ICP-AES results of Na content
| Nominal Na content, x | Actual Na content, x |
|---|---|
| 0.8 | 0.784 |
| 0.9 | 0.878 |
| 1.0 | 0.977 |
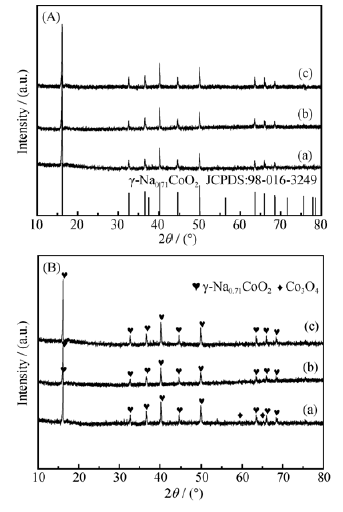
Fig. 3 XRD patterns of samples with different source concentrations(A) (a) 0.025 mol/L; (b) 0.020 mol/L; (c) 0.013 mol/L; (B) (a) 0.05 mol/L; (b) 0.033 mol/L; (c) 0.025 mol/L
| [1] | 卢喜凤, 郜超军, 郭娟, 等. 钴基氧化物热电材料掺杂研究进展. 材料导报, 2015, 29(13): 40-43. |
| [2] | VENGUST D, JANCAR B, SESTAN A, et al.Chemical decomposition as a likely source of ambient and thermal instabilities of layered sodium cobaltate.Chemistry of Materials, 2013, 25(23): 4791-4797. |
| [3] | VONESHEN D J, REFSON K, BORISSENKO E, et al.Suppression of thermal conductivity by rattling modes in thermoelectric sodium cobaltate.Nature Materials, 2013, 12(11): 1028-1032. |
| [4] | TIAN Z, WANG X, LIU J, et al.Power factor enhancement induced by Bi and Mn co-substitution in NaxCoO2 thermoelectric materials.Journal of Alloys and Compounds, 2016, 661: 161-167. |
| [5] | ASSADI M H N, KATAYAMA-YOSHIDA H. Dopant incorporation site in sodium cobaltate’s host lattice: a critical factor for thermoelectric performance.Journal of Physics: Condensed Matter, 2015, 27(17): 175504-175512. |
| [6] | LEI Y, LI X, LIU L, et al.Synthesis and stoichiometry of different layered sodium cobalt oxides. Chemistry of Materials, 2014, 26(18): 5288-5296. |
| [7] | OKANERDAL M, KOYUNCU M, USLU I.High thermoelectric performance of unsintered NaCo2O4 nanocrystal.International Journal of Scientific & Technology Research, 2015, 4(4): 37-39. |
| [8] | TERASAKI I.Layered cobalt oxides: correlated electrons for thermoelectrics.Thermoelectric Nanomaterials, 2013, 182: 51-70. |
| [9] | KRASUTSKAYA N S, KLYNDYUK A I, EVSEEVA L E, et al.Synthesis and properties of NaxCoO2 (x= 0.55, 0.89) oxide thermoelectrics.Inorganic Materials, 2016, 52(4): 393-399. |
| [10] | PANCHAKARLA L S, LAJAUNIE L, RAMASUBRAMANIAM A, et al.Nanotubes from oxide-based misfit family: the case of calcium cobalt oxide. ACS Nano, 2016, 10(6): 6248-6256. |
| [11] | ERDAL M O, KOYUNCU M, USLU I.The effect of synthesis technique on thermoelectric properties of nanocrystalline NaCo2O4 ceramics.Journal of Nanoparticle Research, 2014, 16(11): 1-8. |
| [12] | PRSIC S, SAVIC S M, BRANKOVIC Z, et al.Mechanochemically assisted solid-state and citric acid complex syntheses of Cu-doped sodium cobaltite ceramics.Journal of Alloys and Compounds, 2015, 640: 480-487. |
| [13] | HIROSHI ITAHARA, KENJIRO FUJITA, JUN SUGIYAMA, et al.Highly textured NaxCoO2 ceramics fabricated by both template grain growth and reactive template grain growth methods using single-crystalline particles as template.Journal of the Ceramic Society of Japan, 2003, 111: 227-231. |
| [14] | WU Y, WANG J, YAER X, et al. Effects of preferred orientation and crystal size on thermoelectric properties of sodium cobalt oxide. Functional Materials Letters, 2016, 9(01): 1650010-1-6. |
| [15] | KOSHIBAE W, TSUTSUI K, MAEKAWA S. Thermopower in cobalt oxides.Phys. Rev. B, 2000(62): 6869-6872. |
| [1] | HE Danqi, WEI Mingxu, LIU Ruizhi, TANG Zhixin, ZHAI Pengcheng, ZHAO Wenyu. Heavy-Fermion YbAl3 Materials: One-step Synthesis and Enhanced Thermoelectric Performance [J]. Journal of Inorganic Materials, 2023, 38(5): 577-582. |
| [2] | CHENG Cheng, LI Jianbo, TIAN Zhen, WANG Pengjiang, KANG Huijun, WANG Tongmin. Thermoelectric Property of In2O3/InNbO4 Composites [J]. Journal of Inorganic Materials, 2022, 37(7): 724-730. |
| [3] | KANG Huijun,ZHANG Xiaoying,WANG Yanxia,LI Jianbo,YANG Xiong,LIU Daquan,YANG Zerong,WANG Tongmin. Effect of Rare-earth Variable-valence Element Eu doping on Thermoelectric Property of BiCuSeO [J]. Journal of Inorganic Materials, 2020, 35(9): 1041-1046. |
| [4] | QIU Xiaoxiao,ZHOU Xiying,FU Yuntian,SUN Xiaomeng,WANG Lianjun,JIANG Wan. Influence of Ge1-xInxTe Microstructure on Thermoelectric Properties [J]. Journal of Inorganic Materials, 2020, 35(8): 916-922. |
| [5] | YU Guan-Ting, XIN Jia-Zhan, ZHU Tie-Jun, ZHAO Xin-Bing. Thermoelectric Property of Zn-Sb Doped Mg2(Si,Sn) Alloys [J]. Journal of Inorganic Materials, 2019, 34(3): 310-314. |
| [6] | SHEN Jia-Jun, FANG Teng, FU Tie-Zheng, XIN Jia-Zhan, ZHAO Xin-Bing, ZHU Tie-Jun. Lattice Thermal Conductivity in Thermoelectric Materials [J]. Journal of Inorganic Materials, 2019, 34(3): 260-268. |
| [7] | LUO Jun, HE Shi-Yang, LI Zhi-Li, LI Yong-Bo, WANG Feng, ZHANG Ji-Ye. Progress on High-throughput Synthesis and Characterization Methods for Thermoelectric Materials [J]. Journal of Inorganic Materials, 2019, 34(3): 247-259. |
| [8] | LI Xin, XI Li-Li, YANG Jiong. First Principles High-throughput Research on Thermoelectric Materials: a Review [J]. Journal of Inorganic Materials, 2019, 34(3): 236-246. |
| [9] | ZONG Peng-An, CHEN Li-Dong. Preparation and Mechanical Properties of Ce0.85Fe3CoSb12/rGO Thermoelectric Nanocomposite [J]. Journal of Inorganic Materials, 2017, 32(1): 33-38. |
| [10] | XING Zhi-Bo, LI Jing-Feng. Powder Metallurgic Synthesis of Mid-temperature Lead-free AgSn18SbTe20 Thermoelectric Materials and Processing Influence on Thermoelectric Performance [J]. Journal of Inorganic Materials, 2015, 30(8): 872-876. |
| [11] | HAN Zhi-Ming, ZHANG Xin, LU Qing-Mei, ZHANG Jiu-Xing, ZHANG Fei-Peng. Preparation and Thermoelectric Properties of (Mg2Si1-xSbx)0.4-(Mg2Sn)0.6 Alloys [J]. Journal of Inorganic Materials, 2012, 27(8): 822-826. |
| [12] | MA Bing, CHENG Su-Dan, ZHAO Wen-Yu, ZHANG Qing-Jie. Preparation and Thermoelectric Transport Properties of In-doping β-Zn4Sb3 Bulk Thermoelectric Materials [J]. Journal of Inorganic Materials, 2010, 25(6): 598-602. |
| [13] | XU Jing-Jing, DU Bao-Li, ZHANG Wen-Hao, TANG Xin-Feng. Sonochemicial Synthesis of AgSbTe2 Thermoelectric Compounds [J]. Journal of Inorganic Materials, 2010, 25(6): 593-597. |
| [14] | SHEN Jun-Jie, ZHU Tie-Jun, YU Cui, YANG Sheng-Hui, ZHAO Xin-Bing. Influence of Ag2Te Doping on the Thermoelectric Properties of p-type Bi0.5Sb1.5Te3 Bulk Alloys [J]. Journal of Inorganic Materials, 2010, 25(6): 583-587. |
| [15] | QIN Bing-Ke,LI Xiao-Lei,LI Shang-Sheng,SU Tai-Chao,MA Hong-An,JIA Xiao-Peng. High Pressure Synthesis and Thermoelectric Properties of the Ba-filled Skutterudites [J]. Journal of Inorganic Materials, 2010, 25(1): 23-26. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||