
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (8): 839-844.DOI: 10.15541/jim20130602
• Orginal Article • Previous Articles Next Articles
CHEN Jun-Jun, WANG Jing, HUI Xiang
Received:2013-11-18
Revised:2014-01-06
Published:2014-08-20
Online:2014-07-15
About author:CHEN Jun-Jun. E-mail: gunchen@126.com
CLC Number:
CHEN Jun-Jun, WANG Jing, HUI Xiang. Preparation and Characterization of Sodium Zirconium Phosphate Powder with Peculiar Morphology[J]. Journal of Inorganic Materials, 2014, 29(8): 839-844.

Fig. 2 SEM images of products obtained at different molar ratios of raw materials (a) 1:2.4; (b) 1:2.0; (c) 1:1.8; (d) 1:1.6. Insets are enlarged images of part area in related images
| Molar ratio | 1:2.4 | 1:2.0 | 1:1.8 | 1:1.6 | 1:1.4 | 1:1.2 |
|---|---|---|---|---|---|---|
| Initial pH value | 7.24 | 7.00 | 6.72 | 6.57 | 6.43 | 6.06 |
| Final pH value | 5.72 | 5.66 | 5.58 | 5.49 | 5.38 | 5.32 |
Table 1 pH value of the solution with different molar ratio of raw materials before and after hydrothermal treatment
| Molar ratio | 1:2.4 | 1:2.0 | 1:1.8 | 1:1.6 | 1:1.4 | 1:1.2 |
|---|---|---|---|---|---|---|
| Initial pH value | 7.24 | 7.00 | 6.72 | 6.57 | 6.43 | 6.06 |
| Final pH value | 5.72 | 5.66 | 5.58 | 5.49 | 5.38 | 5.32 |
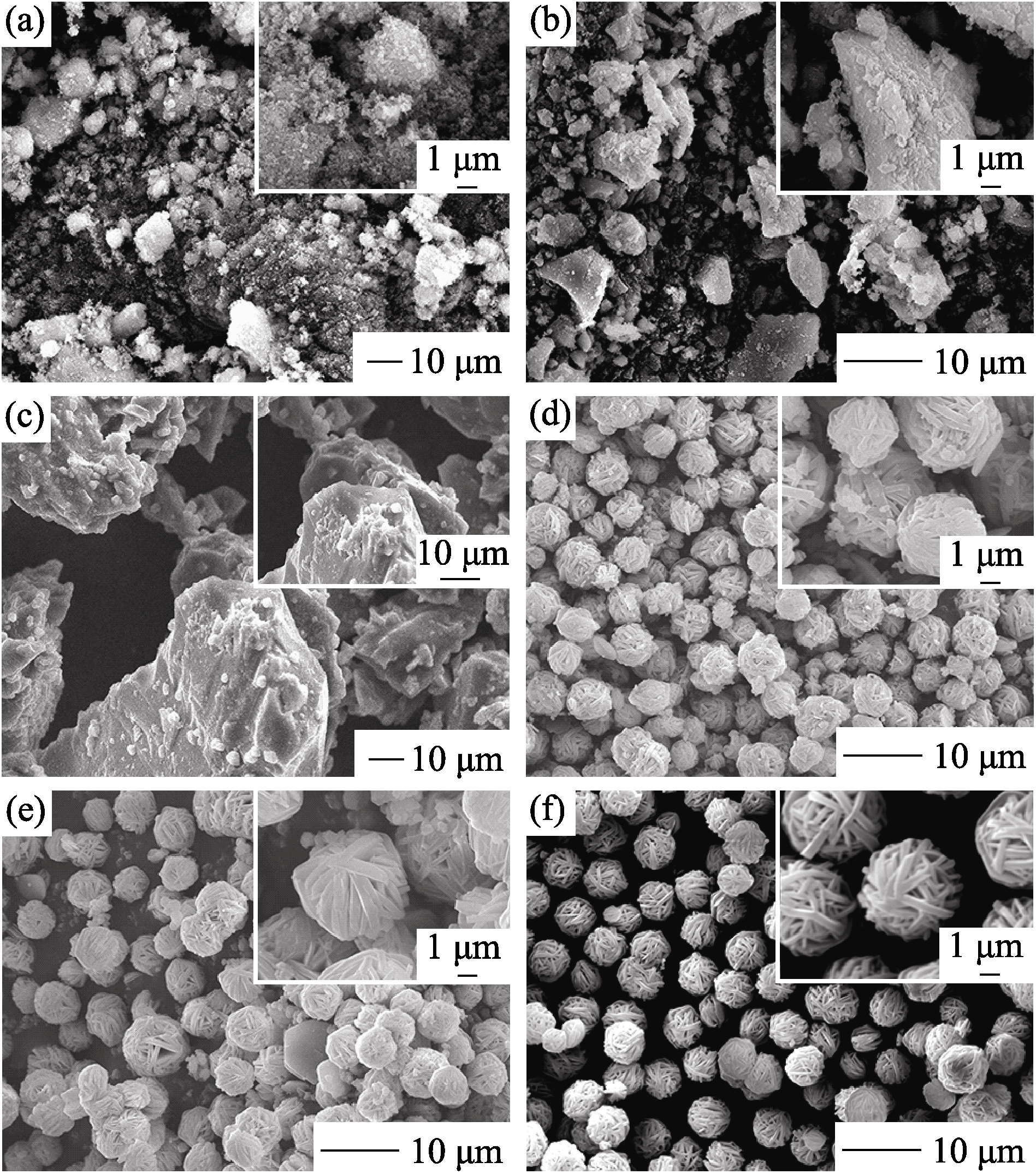
Fig. 5 SEM images of products obtained at different hydrothermal temperatures (a)100℃; (b)120℃; (c) 140℃; (d)160℃; (e)180℃; (f)200℃. Insets are enlarged images of part area in related images

Fig. 7 SEM images of products obtained under different hydrothermal time (a) 4 h; (b) 6 h; (c) 8 h; (d) 10 h; (e)12 h; (f) 14 h. Insets are enlarged images of part area in related images
| [1] | PET'KOV V I. Complex phosphates formed by metal cations in oxidation states Ⅰ and Ⅳ. Russian Chemical Reviews, 2012, 81(1): 606-637. |
| [2] | ZHANG BIAO, GUO JING-KUN, HUANG XIAO-XIAN, et al. Properties of [NZP] structure-functional materials. Journal of Inorganic Materials, 1996, 11(1): 14-19. |
| [3] | SCHEETZ B E, AGRAWAL D K, BREVAL E, et al. Sodium zirconium phosphate(NZP) as a host structure for nuclear waste immobilization: a review. Waste Management, 1994, 14(6): 489-505. |
| [4] | BURDA C, CHEN X, NARAYANAN R, et al. Chemistry and properties of nanocrystals of different shapes. Chemical Reviews, 2005, 105(4): 1025-1102. |
| [5] | ZHANG Q, LIU S J, YU S H. Recent advances in oriented attachment growth and synthesis of functional materials: concept, evidence mechanism, and future. Journal of Material Chemistry, 2009, 19(1): 191-207. |
| [6] | PET'KOV V I, ASABINA E A. Complex phosphates, containing elements with oxidation degree +1 and +4. Physics Procedia, 2013, 44: 166-176. |
| [7] | ZHU LIN-HUA, LIAO XUE-PIN, LANG XIAO-CHUAN, et al. Synthesis of Ca1-xBaxZr4(PO4)6 phosphate ceramic and its thermal shock resistance performance of near-zero thermal expansion composition. Journal of Inorganic Materials, 2001, 16(3): 452-458. |
| [8] | YIN Z, SAKAMOTO Y, YU J, et al. Microemulsion based synthesis of titanium phosphate nanotubes via amine extraction system. Journal of the American Chemical Society, 2004, 126(29):8882-8883 |
| [9] | LI Y H, LING Y H, BAI X D. Preparation and characterization of anisotropic ammonium titanium phosphate crystals via hydrothermal route. Key Engineering Materials, 2005, 280-283: 597-600. |
| [10] | PAVLOVA S N, SADYKOV V A, ZABOLOTNAYA G V, et al. The influence of solid precursors nature on structural, textural and surface properties of framework zirconium phosphates synthesized via mechanochemical activation. Solid State Ionics, 2001, 141-142: 683-688. |
| [11] | ZHANG XUE-HUA, LUO HAO-SU, ZHONG WEI-ZHUO. Anionic corrdination polyhedron growth units mode and its application in crystal growth. Science in China Series E, 2004, 34(3): 241-253. |
| [12] | PENN R L, BANFIELD J F. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: insights from titania. Geochimica et Cosmochimica Acta, 1999, 63(10): 1549-1557. |
| [13] | ECHEVERRÍA J, ALVAREZ S. Application of symmetry operation measures in structure inorganic chemistry. Inorganic Chemistry, 2008, 47(23): 10965-10970. |
| [14] | NAI J, WU J, GUO L, et al. Coordination polyhedra: a probable basic growth unit in solution for the crystal growth of inorganic nonmetallic nanomaterials?Crystal Growth Design, 2012, 12(5): 2653-2661. |
| [15] | AMBARISH D, AMIT D G, DEBRATA B, et al. A comparative study of conventionally sintered, microwave sintered and hot isostatic press sintered NZP and CZP structures interacted with fluoride. Ceramics International, 2013, 39(8): 9351-9359. |
| [16] | ORDÓÑEZ-REGIL E, CONTRERAS-RAMÍREZ A, FERNÁNDEZ- VALVERDE S M, et al. Crystal growth and thermoluminescence response of NaZr2(PO4)3 at high gamma radiation doses. Journal of Nuclear Materials, 2013, 443(1/2/3): 417-423. |
| [17] | GEORGE A, SEENA P T. Thermal studies on zirconium hydroxide gel formed by aqueous gelation. Journal of Thermal Analysis and Calorimetry, 2012, 110(3): 1037-1041. |
| [18] | BANFIELD J F, WELCH S A, ZHANG H, et al. Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science, 2000, 289(5480): 751-754. |
| [1] | LI Yuejun, CAO Tieping, SUN Dawei. Bi4O5Br2/CeO2 Composite with S-scheme Heterojunction: Construction and CO2 Reduction Performance [J]. Journal of Inorganic Materials, 2023, 38(8): 963-970. |
| [2] | NIU Haibin, HUANG Jiahui, LI Qianwen, MA Dongyun, WANG Jinmin. Directly Hydrothermal Growth and Electrochromic Properties of Porous NiMoO4 Nanosheet Films [J]. Journal of Inorganic Materials, 2023, 38(12): 1427-1433. |
| [3] | YAO Yishuai, GUO Ruihua, AN Shengli, ZHANG Jieyu, CHOU Kuochih, ZHANG Guofang, HUANG Yarong, PAN Gaofei. In-situ Loaded Pt-Co High Index Facets Catalysts: Preparation and Electrocatalytic Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 71-78. |
| [4] | ZHANG Xian, ZHANG Ce, JIANG Wenjun, FENG Deqiang, YAO Wei. Synthesis, Electronic Structure and Visible Light Photocatalytic Performance of Quaternary BiMnVO5 [J]. Journal of Inorganic Materials, 2022, 37(1): 58-64. |
| [5] | XIAO Yumin, Li Bin, QIN Lizhao, LIN Hua, LI Qing, LIAO Bin. Efficient Preparation of CuGeO3 with Controllable Morphology Using CuCl2 as Copper Source [J]. Journal of Inorganic Materials, 2021, 36(1): 69-74. |
| [6] | WANG Juhan,WEN Xiong,LIU Chengchao,ZHANG Yuhua,ZHAO Yanxi,LI Jinlin. Preparation and Fischer-Tropsch Synthesis Performance of Hierarchical Co/Al-SiO2 Catalyst [J]. Journal of Inorganic Materials, 2020, 35(9): 999-1004. |
| [7] | XU Yun-Qing,WANG Hai-Zeng. Sodium Magnesium Fluoride Particles of Different Morphologies: Prepared by EDTA-assisted Hydrothermal Method [J]. Journal of Inorganic Materials, 2019, 34(9): 933-937. |
| [8] | GOU Sheng-Lian, NAI Xue-Ying, XIAO Jian-Fei, YE Jun-Wei, DONG Ya-Ping, LI Wu. Preparation and Thermal Decomposition of Basic Magnesium Chloride Whiskers [J]. Journal of Inorganic Materials, 2019, 34(7): 781-785. |
| [9] | Wei LIU, Kai ZHENG, Dong-Hong WANG, Yi-San LEI, Huai-Lin FAN. Co3O4 Nanowire Arrays@Activated Carbon Fiber Composite Materials: Facile Hydrothermal Synthesis and Its Electrochemical Application [J]. Journal of Inorganic Materials, 2019, 34(5): 487-492. |
| [10] | WANG Wei, LUO Shi-Jie, XIAN Cong, XIAO Qun, YANG Yang, OU Yun, LIU Yun-Ya, XIE Shu-Hong. Enhanced Thermoelectric Properties of Hydrothermal Synthesized BiCl3/Bi2S3 Composites [J]. Journal of Inorganic Materials, 2019, 34(3): 328-334. |
| [11] | JIANG Hai-Yan, XIA Yun-Sheng, LI Yu-Zhen. Preparation and Visible-light-driven Photocatalytic Performance of Porous Rod-like FeVO4 [J]. Journal of Inorganic Materials, 2018, 33(9): 949-955. |
| [12] | ZENG Yan-Fei, XIN Guo-Xiang, BULIN Chao-Ke, ZHANG Bang-Wen. One-step Preparation and Electrochemical Performance of 3D Reduced Graphene Oxide/NiO as Supercapacitor Electrodes Materials [J]. Journal of Inorganic Materials, 2018, 33(10): 1070-1076. |
| [13] | LI Guo-Chang, WANG Ping, LIU Chang-Bo. Hydrothermal Synthesis of Whitlockite [J]. Journal of Inorganic Materials, 2017, 32(11): 1128-1132. |
| [14] | MA Fang, CUI Ming-Fang, ZHU Jian-Hua, LI Ya-Li. Porous Hydroxyapatite Microspheres Prepared by Using Poly (Allylamine Hydrochloride) and Its Application in Drug Delivery [J]. Journal of Inorganic Materials, 2017, 32(11): 1215-1222. |
| [15] | RAN Hui-Li, HUANG Hao, MA Meng-Jun, ZHAI Jin-Sheng, FAN Jia-Jie. Dye-sensitized Solar Cells Based on Double-layer Composite Film with Enhanced Photovoltaic Performance [J]. Journal of Inorganic Materials, 2017, 32(10): 1049-1054. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||