
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (10): 1070-1076.DOI: 10.15541/jim20180032
Special Issue: 二维材料
• RESEARCH PAPER • Previous Articles Next Articles
ZENG Yan-Fei, XIN Guo-Xiang, BULIN Chao-Ke, ZHANG Bang-Wen
Received:2018-01-29
Revised:2018-04-16
Published:2018-10-20
Online:2018-09-25
About author:ZENG Yan-Fei. E-mail: 610032926@qq.com
Supported by:CLC Number:
ZENG Yan-Fei, XIN Guo-Xiang, BULIN Chao-Ke, ZHANG Bang-Wen. One-step Preparation and Electrochemical Performance of 3D Reduced Graphene Oxide/NiO as Supercapacitor Electrodes Materials[J]. Journal of Inorganic Materials, 2018, 33(10): 1070-1076.
| Sample | BET surface area/(m2∙g-1) | Total pore volume/ (cm3∙g-1) |
|---|---|---|
| 3D rGO | 239.0 | 0.383 |
| 3D rGO/NiO-1 | 227.8 | 0.351 |
| 3D rGO/NiO-2 | 222.0 | 0.306 |
| 3D rGO/NiO-3 | 190.7 | 0.293 |
Table 1 Surface properties of 3D rGO, 3D rGO/NiO-1, 3D rGO/NiO-2 and 3D rGO/NiO-3
| Sample | BET surface area/(m2∙g-1) | Total pore volume/ (cm3∙g-1) |
|---|---|---|
| 3D rGO | 239.0 | 0.383 |
| 3D rGO/NiO-1 | 227.8 | 0.351 |
| 3D rGO/NiO-2 | 222.0 | 0.306 |
| 3D rGO/NiO-3 | 190.7 | 0.293 |
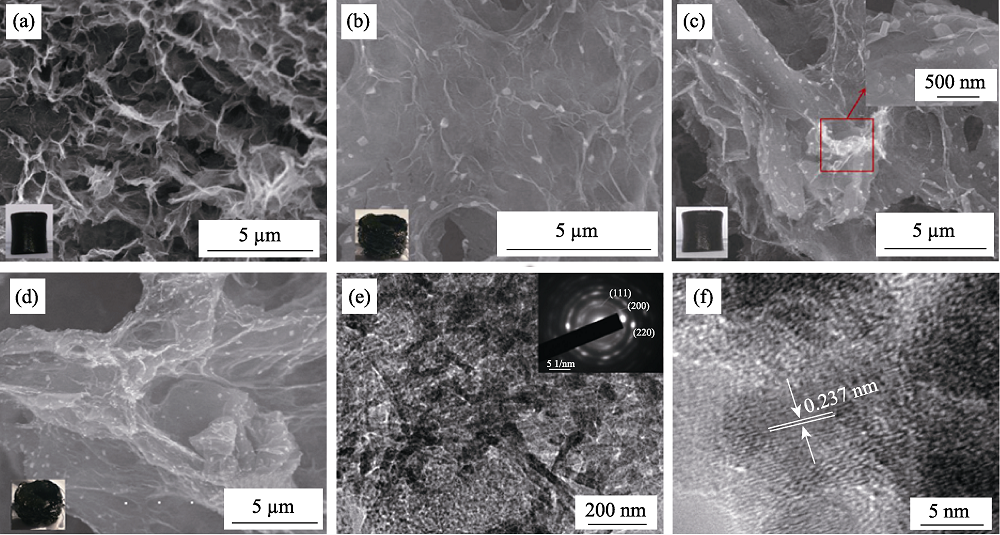
Fig. 5 SEM images of 3D rGO (a), 3D rGO/NiO-1(b), 3D rGO/NiO-2(c), 3D rGO/NiO-3(d) with the inserts (left bottom) showing the samples photographs and the right top one in (c) showing the magnified image in red square, and TEM (e) and HRTEM (f) images of 3D rGO/NiO-2 with the insert in (e) showing SAED image
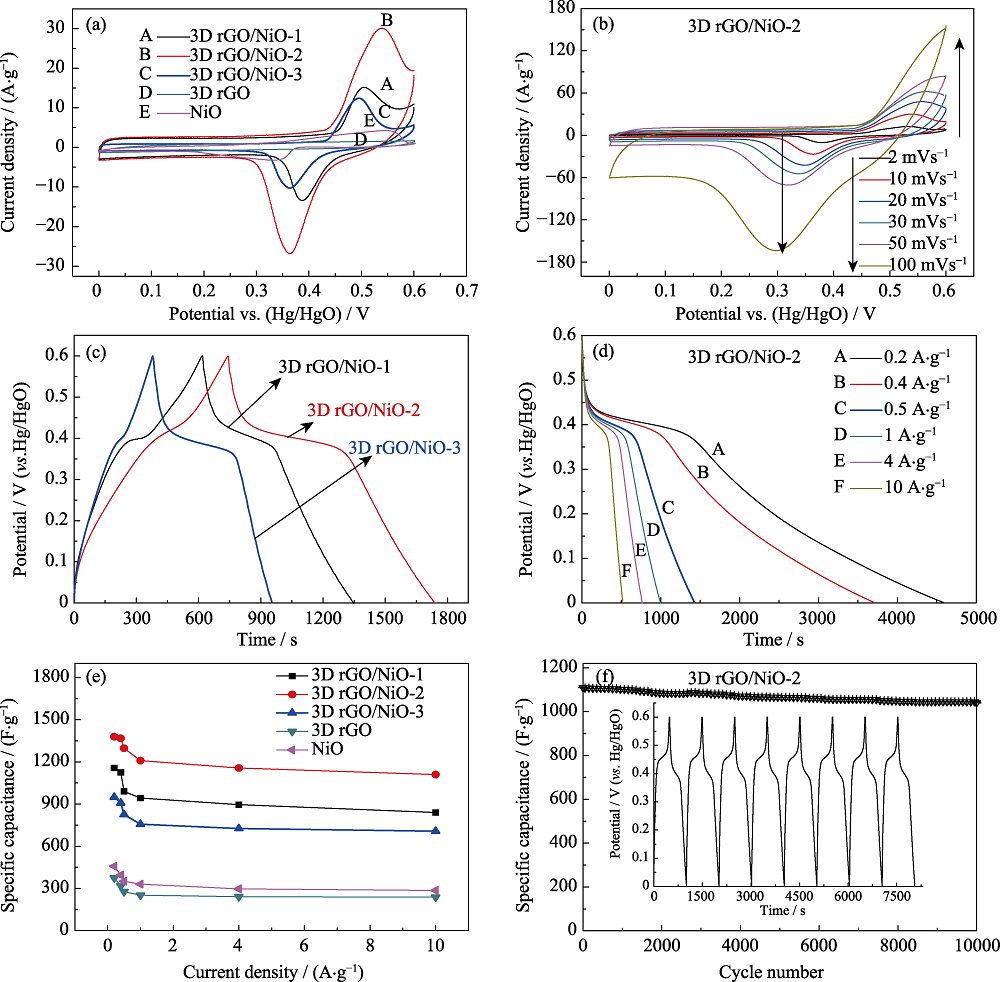
Fig. 6 CV curves of 3D rGO, 3D rGO/NiO and NiO at a scan rate of 10 mV·s-1 (a), 3D rGO/NiO-2 at different scan rates (b), GCD curves of 3D rGO/NiO-1, 3D rGO/NiO-2 and 3D rGO/NiO-3 at a current density of 1 A·g-1 (c), GCD curves of 3D rGO/NiO-2 at different current density (d), specific capacitance of 3D rGO, 3D rGO/NiO-1, 3D rGO/NiO-2, 3D rGO/NiO-3 and NiO at different current densities (e), and cycling performance of 3D rGO/NiO-2 at a current density of 10 A·g-1 after 10000 cycles (f)
| [1] | YUAN C Z, LONG Y, HOU L,et al. Flexible hybrid paper made of monolayer Co3O4 microsphere arrays on rGO/CNTs and their application in electrochemical capacitors. Adv. Funct. Mater., 2012, 22(12): 2560-2566. |
| [2] | ZHANG K, LI H, XU X,et al. Synthesis of reduced graphene oxide/ NiO nanocomposites for the removal of Cr(VI) from aqueous water by adsorption. Microporous Mesoporous Mater., 2018, 255: 7-14. |
| [3] | FOO C Y, SUMBOJA A, TAN D J H,et al. Flexible and highly scalable V2O5-rGO electrodes in an organic electrolyte for supercapacitor devices. Adv. Eng. Mater., 2015, 4(12): 1-7. |
| [4] | QORBANI M, NASERI N, MOSHFEGH A Z.Hierarchical Co3O4/Co(OH)2 nanoflakes as a supercapacitor electrode: experimental and semi-empirical model. ACS Appl. Mater. Interfaces, 2015, 7(21): 11172-11179. |
| [5] | HAN Y, ZHANG S, SHEN N,et al. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of medicago sativa. New Phytologist, 2008, 177(1): 155-166. |
| [6] | LI Q, LU C, CHEN C,et al. Layered NiCo2O4/reduced graphene oxide composite as an advanced electrode for supercapacitor. Energy Storage Materials, 2017, 8: 59-67. |
| [7] | JIANG N, HUANG F, XIA W,et al. Facile fabrication of rGO/CNT hybrid fibers for high-performance flexible supercapacitors. J. Mater. Sci.: Mater. Electron., 2017, 28(16): 12147-12157. |
| [8] | XIONG C, LI T, ZHAO T,et al. Reduced graphene oxide-carbon nanotube grown on carbon fiber as binder-free electrode for flexible high-performance fiber supercapacitors. Composites Part B Engineering, 2017, 116: 7-15. |
| [9] | LI Y, CHEN J, HUANG L, et al. Highly compressible macroporous graphene monoliths via an improved hydrothermal process. Adv. Mater., 2014, 26(28): 4789-4793. |
| [10] | LIANG Y, WANG H, ZHOU J,et al. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc., 2016, 134(7): 3517-3523. |
| [11] | ZHANG Z, ZHAO J, ZHOU J,et al. Interfacial engineering of metal oxide/graphene nanoscrolls with remarkable performance for lithium ion batteries. Energy Storage Materials, 2017, 8: 35-41. |
| [12] | ZHOU G, WANG D W, YIN L C,et al. Oxygen bridges between NiO nanosheets and graphene for improvement of lithium storage. ACS Nano, 2012, 6(4): 3214-3223. |
| [13] | LOPEZSSLAS N, GUTIERREZ M C, ANIA C O,et al. Nitrogen- doped carbons prepared from eutectic mixtures as metal-free oxygen reduction catalysts. J. Mater. Chem. A, 2016, 4(2): 478-488. |
| [14] | LI D, CHENG H C, WANG Y,et al. The effect of thermal annealing on charge transport in organolead halide perovskite microplate field-effect transistors. Adv. Mater., 2017, 29(4): 1-7. |
| [15] | PING H, BOLUN W, LI Q M,et al. Copper silicate hydrate hollow spheres constructed by nanotubes encapsulated in reduced graphene oxide as long-life lithium-ion battery anode. ACS Appl. Mater. Interfaces, 2015, 7(48): 26572-26578. |
| [16] | LEE K, LEE J, KWON K W,et al. 3D graphene-Ni foam as an advanced electrode for high-performance nonaqueous redox flow batteries. ACS Appl. Mater. Interfaces, 2017, 9(27): 22502-22508. |
| [17] | DAM D T, WANG X, LEE J M.Graphene/NiO nanowires: controllable one-pot synthesis and enhanced pseudocapacitive behavior. ACS Appl. Mater. Interfaces, 2014, 6(11): 8246-8256. |
| [18] | PECK M A, LANGELL M A.Comparison of nanoscaled and bulk NiO structural and environmental characteristics by XRD, XAFS, and XPS.Chem. Mater, 2013, 24(23): 4483-4490. |
| [19] | JI Z, SHEN X, XU Y,et al. A facile and general route for the synthesis of semiconductor quantum dots on reduced graphene oxide sheets. RSC. Adv, 2014, 4(26): 13601-13609. |
| [20] | UHLENBROCK S, SCHARFSCHWERDT C, NEUMANN M,et al. The influence of defects on the Ni 2p and O 1s XPS of NiO. J. Phys.: Condens. Matter, 1992, 4(40): 7973-7978. |
| [21] | XU Y, WANG L, CAO P,et al. Mesoporous composite nickel cobalt oxide/graphene oxide synthesized via a template-assistant co-precipitation route as electrode material for supercapacitors. Electrochimica Acta, 2016, 306: 742-752. |
| [22] | MEHER S K, JUSTIN P, RAO G R.Microwave-mediated synthesis for improved morphology and pseudocapacitance performance of nickel oxide.ACS Appl. Mater. Interfaces, 2011, 3(6): 2063-2073. |
| [23] | ZOU F, CHEN Y M, LIU K,et al. Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano, 2016, 10(1): 377-386. |
| [1] | YANG Endong, LI Baole, ZHANG Ke, TAN Lu, LOU Yongbing. ZnCo2O4-ZnO@C@CoS Core-shell Composite: Preparation and Application in Supercapacitors [J]. Journal of Inorganic Materials, 2024, 39(5): 485-493. |
| [2] | CHAO Shaofei, XUE Yanhui, WU Qiong, WU Fufa, MUHAMMAD Sufyan Javed, ZHANG Wei. Efficient Potassium Storage through Ti-O-H-O Electron Fast Track of MXene Heterojunction [J]. Journal of Inorganic Materials, 2024, 39(11): 1212-1220. |
| [3] | LI Yuejun, CAO Tieping, SUN Dawei. Bi4O5Br2/CeO2 Composite with S-scheme Heterojunction: Construction and CO2 Reduction Performance [J]. Journal of Inorganic Materials, 2023, 38(8): 963-970. |
| [4] | DING Ling, JIANG Rui, TANG Zilong, YANG Yunqiong. MXene: Nanoengineering and Application as Electrode Materials for Supercapacitors [J]. Journal of Inorganic Materials, 2023, 38(6): 619-633. |
| [5] | NIU Haibin, HUANG Jiahui, LI Qianwen, MA Dongyun, WANG Jinmin. Directly Hydrothermal Growth and Electrochromic Properties of Porous NiMoO4 Nanosheet Films [J]. Journal of Inorganic Materials, 2023, 38(12): 1427-1433. |
| [6] | YAO Yishuai, GUO Ruihua, AN Shengli, ZHANG Jieyu, CHOU Kuochih, ZHANG Guofang, HUANG Yarong, PAN Gaofei. In-situ Loaded Pt-Co High Index Facets Catalysts: Preparation and Electrocatalytic Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 71-78. |
| [7] | ZHANG Xian, ZHANG Ce, JIANG Wenjun, FENG Deqiang, YAO Wei. Synthesis, Electronic Structure and Visible Light Photocatalytic Performance of Quaternary BiMnVO5 [J]. Journal of Inorganic Materials, 2022, 37(1): 58-64. |
| [8] | CHU Yuxing, LIU Hairui, YAN Shuang. Preparation and Gas Sensing Properties of SnO2/NiO Composite Semiconductor Nanofibers [J]. Journal of Inorganic Materials, 2021, 36(9): 950-958. |
| [9] | SUN Peng, ZHANG Shaoning, BI Hui, DONG Wujie, HUANG Fuqiang. Tuning Nitrogen Species and Content in Carbon Materials through Constructing Variable Structures for Supercapacitors [J]. Journal of Inorganic Materials, 2021, 36(7): 766-772. |
| [10] | LIU Fangfang, CHUAN Xiuyun, YANG Yang, LI Aijun. Influence of N/S Co-doping on Electrochemical Property of Brucite Template Carbon Nanotubes [J]. Journal of Inorganic Materials, 2021, 36(7): 711-717. |
| [11] | WANG Yiliang, AI Yunlong, YANG Shuwei, LIANG Bingliang, ZHENG Zhenhuan, OUYANG Sheng, HE Wen, CHEN Weihua, LIU Changhong, ZHANG Jianjun, LIU Zhiyong. Facile Synthesis and Supercapacitor Performance of M3O4(M=FeCoCrMnMg) High Entropy Oxide Powders [J]. Journal of Inorganic Materials, 2021, 36(4): 425-430. |
| [12] | ZHONG Xiaolan, LIU Xueqing, DIAO Xungang. Electrochromic Devices Based on Tungsten Oxide and Nickel Oxide: a Review [J]. Journal of Inorganic Materials, 2021, 36(2): 128-139. |
| [13] | XIAO Yumin, Li Bin, QIN Lizhao, LIN Hua, LI Qing, LIAO Bin. Efficient Preparation of CuGeO3 with Controllable Morphology Using CuCl2 as Copper Source [J]. Journal of Inorganic Materials, 2021, 36(1): 69-74. |
| [14] | WANG Juhan,WEN Xiong,LIU Chengchao,ZHANG Yuhua,ZHAO Yanxi,LI Jinlin. Preparation and Fischer-Tropsch Synthesis Performance of Hierarchical Co/Al-SiO2 Catalyst [J]. Journal of Inorganic Materials, 2020, 35(9): 999-1004. |
| [15] | LI Zehui,TAN Meijuan,ZHENG Yuanhao,LUO Yuyang,JING Qiushi,JIANG Jingkun,LI Mingjie. Application of Conductive Metal Organic Frameworks in Supercapacitors [J]. Journal of Inorganic Materials, 2020, 35(7): 769-780. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||