
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (9): 991-1000.DOI: 10.15541/jim20210638
• RESEARCH ARTICLE • Previous Articles Next Articles
WANG Hongning1( ), HUANG Li1, QING Jiang3, MA Tengzhou3(
), HUANG Li1, QING Jiang3, MA Tengzhou3( ), HUANG Weiqiu2, CHEN Ruoyu1(
), HUANG Weiqiu2, CHEN Ruoyu1( )
)
Received:2021-10-18
Revised:2022-02-18
Published:2022-09-20
Online:2022-03-15
Contact:
CHEN Ruoyu, professor. E-mail: chry@cczu.edu.cn;About author:WANG Hongning (1980-), female, PhD candidate. E-mail: 444873772@qq.com
Supported by:CLC Number:
WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, HUANG Weiqiu, CHEN Ruoyu. Mesoporous Organic-inorganic Hybrid Siliceous Hollow Spheres: Synthesis and VOCs Adsorption[J]. Journal of Inorganic Materials, 2022, 37(9): 991-1000.
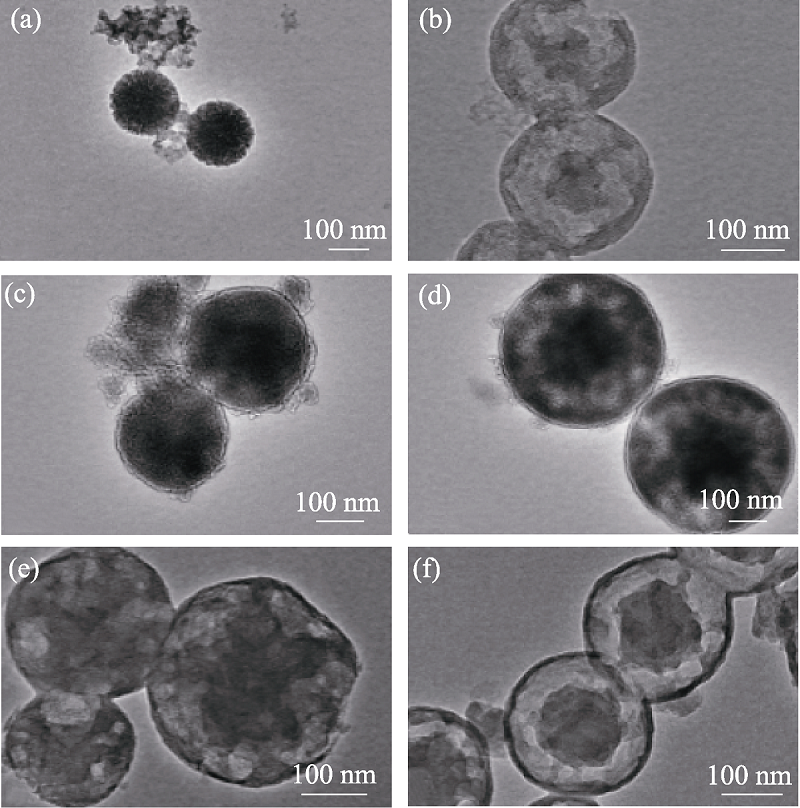
Fig. 1 TEM images of MOSs with different initial BTSE/ (BTSE+TEOS) molar ratios (a) MOS-0; (b) MOS-5%; (c) MOS-7.5%; (d) MOS-10%; (e) MOS-12.5%; (f) MOS-15%
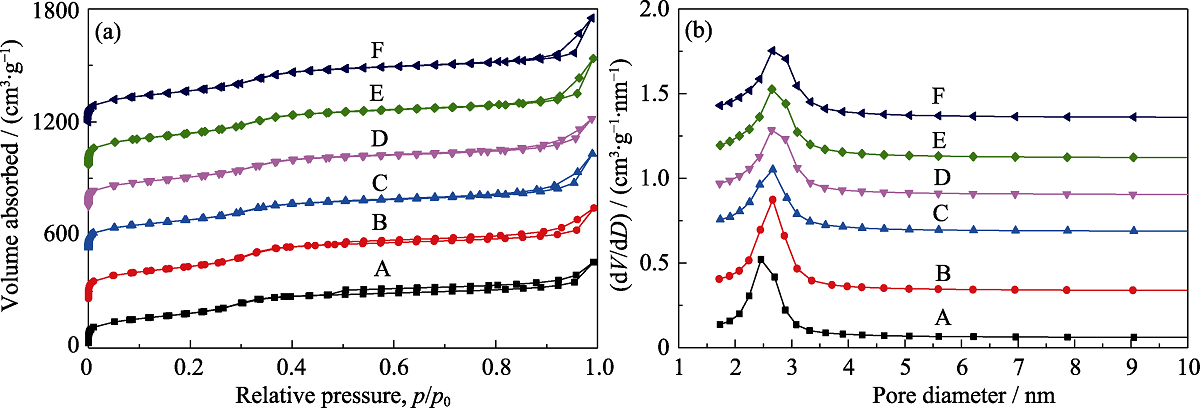
Fig. 2 N2 sorption isotherms (a) and pore size distributions (b) of MOSs with different initial BTSE/(BTSE+TEOS) molar ratios (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E)MOS-12.5%; (F) MOS-15%. In (a), the Y-axis values of (B-F) are 300, 600, 800, 1000, and 1300 m2·g-1, respectively. In (b), the Y-axis values of (B-F) are 0.1, 0.4, 0.8, 1.0, 1.2, and 1.4 cm3·g-1, respectively
| Sample | SBET/ (m2·g-1) | Sm/ (m2·g-1) | Vt/ (cm3·g-1) | Vm/ (cm3·g-1) | Pore size/ nm |
|---|---|---|---|---|---|
| MOS-0 | 591 | 0 | 0.721 | 0 | 2.5 |
| MOS-5% | 612 | 0 | 0.722 | 0 | 2.7 |
| MOS-7.5% | 612 | 0 | 0.776 | 0 | 2.8 |
| MOS-10% | 696 | 0 | 0.887 | 0 | 2.6 |
| MOS-12.5% | 655 | 0 | 0.873 | 0 | 2.7 |
| MOS-15% | 648 | 0 | 0.857 | 0 | 2.7 |
Table 1 Structural parameters of MOSs with different initial BTSE/(BTSE+TEOS) molar ratios
| Sample | SBET/ (m2·g-1) | Sm/ (m2·g-1) | Vt/ (cm3·g-1) | Vm/ (cm3·g-1) | Pore size/ nm |
|---|---|---|---|---|---|
| MOS-0 | 591 | 0 | 0.721 | 0 | 2.5 |
| MOS-5% | 612 | 0 | 0.722 | 0 | 2.7 |
| MOS-7.5% | 612 | 0 | 0.776 | 0 | 2.8 |
| MOS-10% | 696 | 0 | 0.887 | 0 | 2.6 |
| MOS-12.5% | 655 | 0 | 0.873 | 0 | 2.7 |
| MOS-15% | 648 | 0 | 0.857 | 0 | 2.7 |
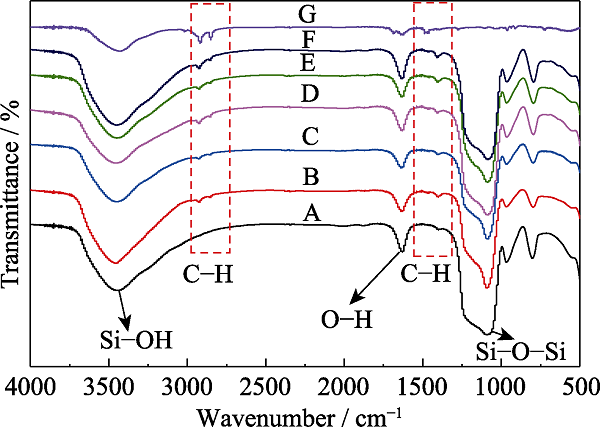
Fig. 3 FT-IR spectra of MOSs with different initial BTSE/ (BTSE+TEOS) molar ratios (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS- 12.5%; (F) MOS-15%; (G) CTAB
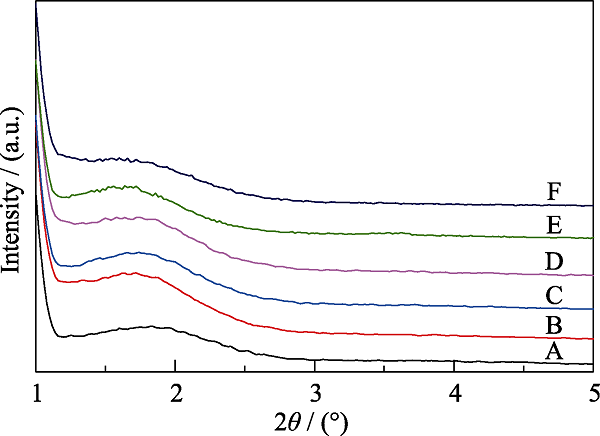
Fig. 4 XRD patterns of MOSs with different initial BTSE/ (BTSE+TEOS) molar ratios (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS- 12.5%; (F) MOS-15%
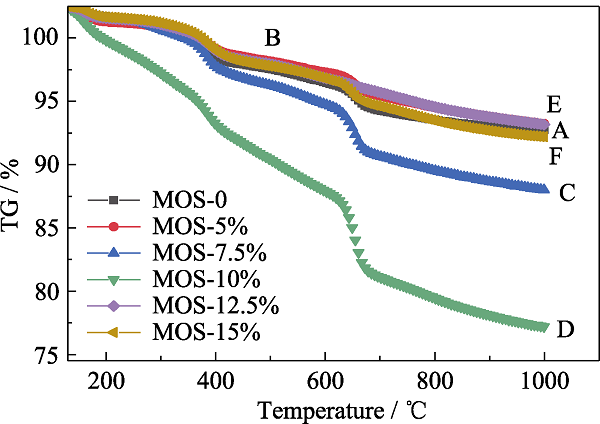
Fig. 5 TGA curves of MOSs with different initial BTSE/ (BTSE+TEOS) molar ratios (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS- 12.5%; (F) MOS-15%;

Fig. 6 Histograms of static VOCs (n-hexane, toluene and 92# gasoline) adsorption capacities (a, c, e) and desorption efficiencies (b, d, f) of different samples (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS-12.5%; (F) MOS-15%; (G) SG; (H) AC
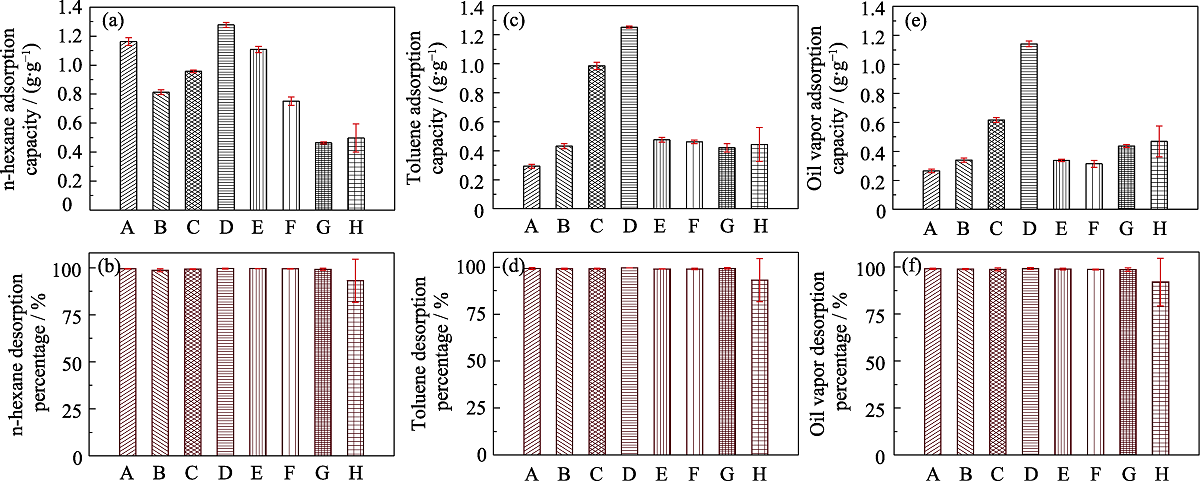
Fig. 6 Histograms of static VOCs (n-hexane, toluene and 92# gasoline) adsorption capacities (a, c, e) and desorption efficiencies (b, d, f) of different samples (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS-12.5%; (F) MOS-15%; (G) SG; (H) AC
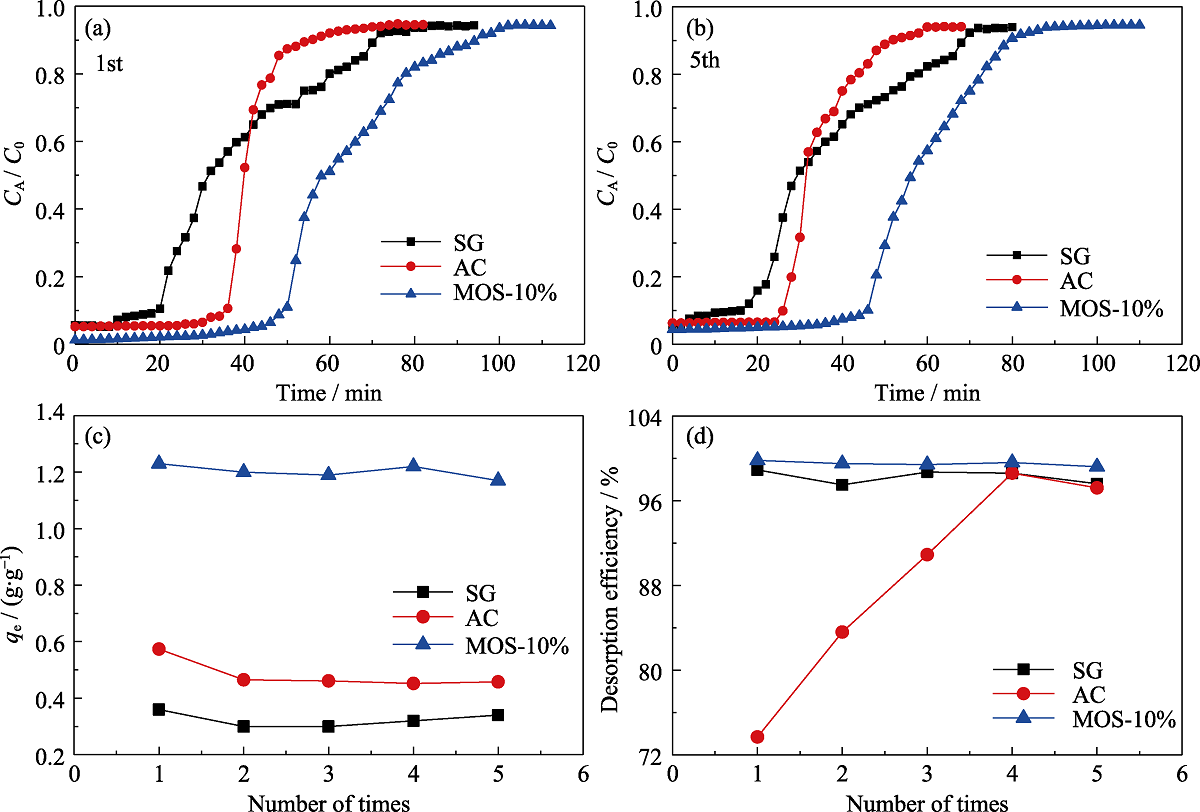
Fig. 8 Breakthrough curves for n-hexane of SG (■), AC (●) and MOS-10% (▲) under dry condition for the (a) first time and (b) fifth time and comparison of the qe (c) and desorption efficiency (d) of 5 times
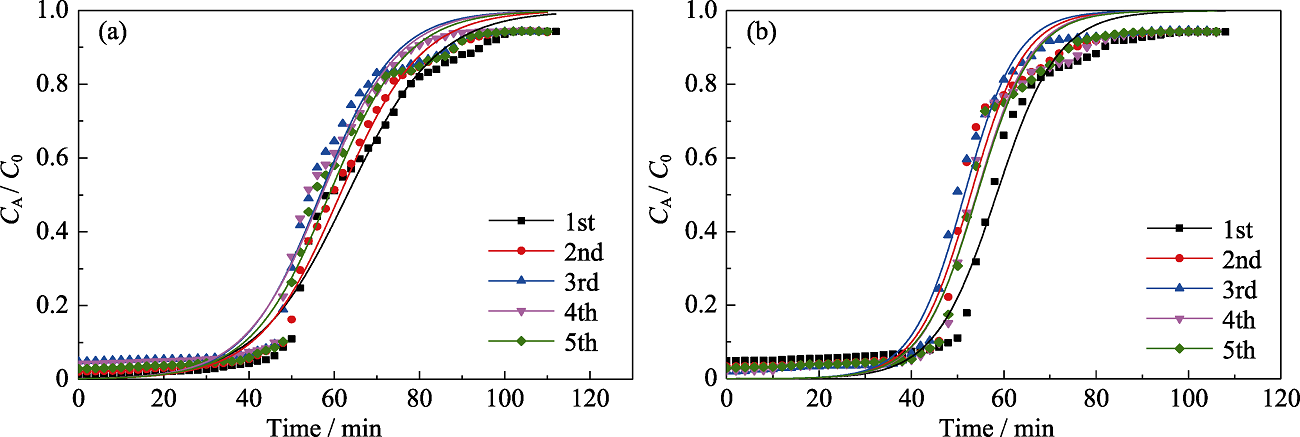
Fig. 10 Yoon and Nelson model fitting for 5 times adsorption of n-hexane (a) and toluene (b) on MOS-10% under dry condition Colorful figures are available on website
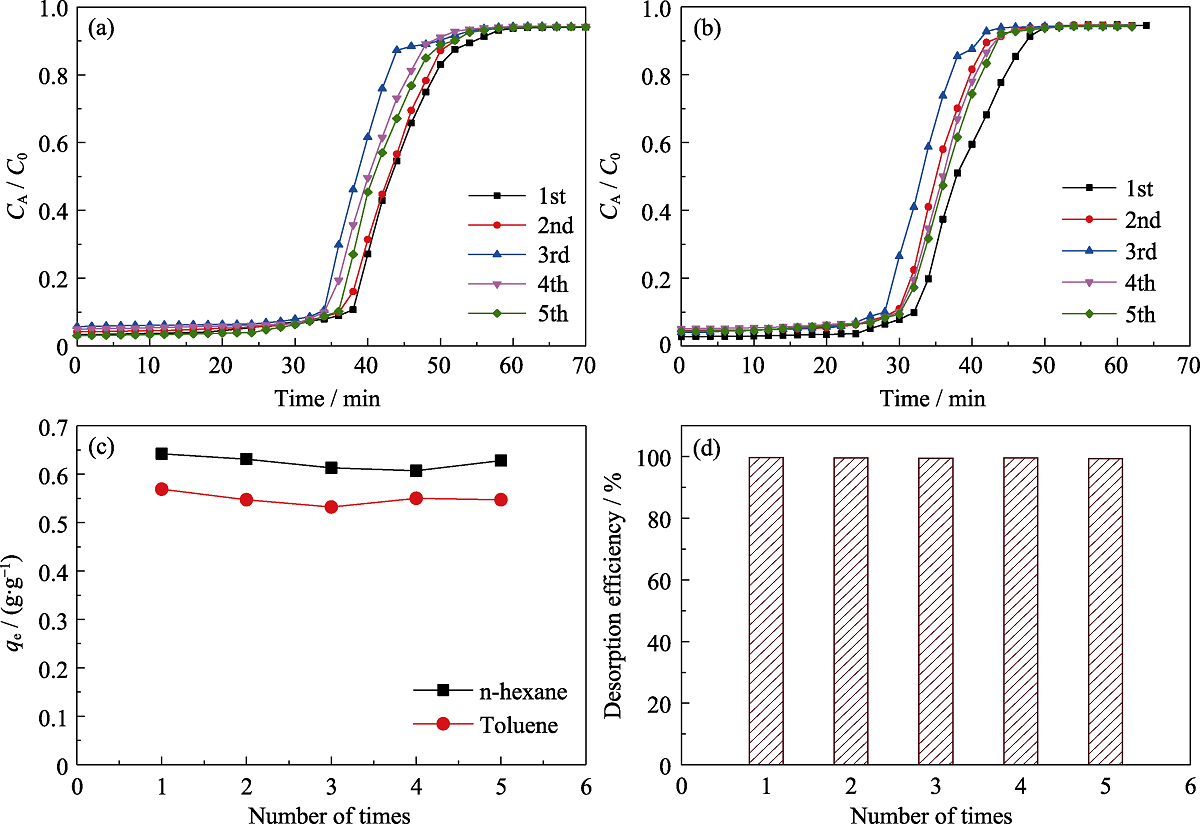
Fig. 12 Simultaneous breakthrough adsorption n-hexane (a) and toluene (b) on MOS-10% under dry condition, comparison of the equilibrium adsorption capacities (c) and desorption efficiency (d) for 5 times, respectively
| Sample | SBET/(m2 ·g-1) | Sm/(m2 ·g-1) | Vt/(cm3 ·g-1) | Vm/(cm3 ·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| AC | 1451 | 973 | 1.03 | 0.48 | 5.6 |
| SG | 430 | 15 | 0.710 | 0.010 | 6.9 |
Table S1 Structural parameters of AC and SG
| Sample | SBET/(m2 ·g-1) | Sm/(m2 ·g-1) | Vt/(cm3 ·g-1) | Vm/(cm3 ·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| AC | 1451 | 973 | 1.03 | 0.48 | 5.6 |
| SG | 430 | 15 | 0.710 | 0.010 | 6.9 |
| Sample | First event | Second event | Residual mass /% | ||
|---|---|---|---|---|---|
| (Tons-Tf )/℃ | Δm/% | (Tons-Tf )/℃ | Δm/% | ||
| MOS-0 | 30-200 | 1.7 | 200-900 | 8.1 | 90.2 |
| MOS-5% | 30-200 | 1.7 | 200-900 | 7.6 | 90.7 |
| MOS-7.5% | 30-200 | 1.9 | 200-900 | 12.6 | 85.5 |
| MOS-10% | 30-200 | 5.3 | 200-900 | 20.1 | 74.6 |
| MOS-12.5% | 30-200 | 1.5 | 200-900 | 7.9 | 90.6 |
| MOS-15% | 30-200 | 1.4 | 200-900 | 9.0 | 89.6 |
Table S2 TGA results of different MOSs under nitrogen atmosphere in the range of 30-900 ℃ (10 ℃/min)
| Sample | First event | Second event | Residual mass /% | ||
|---|---|---|---|---|---|
| (Tons-Tf )/℃ | Δm/% | (Tons-Tf )/℃ | Δm/% | ||
| MOS-0 | 30-200 | 1.7 | 200-900 | 8.1 | 90.2 |
| MOS-5% | 30-200 | 1.7 | 200-900 | 7.6 | 90.7 |
| MOS-7.5% | 30-200 | 1.9 | 200-900 | 12.6 | 85.5 |
| MOS-10% | 30-200 | 5.3 | 200-900 | 20.1 | 74.6 |
| MOS-12.5% | 30-200 | 1.5 | 200-900 | 7.9 | 90.6 |
| MOS-15% | 30-200 | 1.4 | 200-900 | 9.0 | 89.6 |
| Sample | Adsorption | Desorption | -OH/(×1020, g-1) | ||
|---|---|---|---|---|---|
| Average/(g·g-1) | STDEa/% | Average/% | STDEa/% | ||
| MOS-0% | 0.903 | 0.0168 | 99.4 | 0.205 | 3.11 |
| MOS-5% | 0.667 | 0.0177 | 99.3 | 0.329 | 2.35 |
| MOS-7.5% | 0.656 | 0.0165 | 99.4 | 0.283 | 2.31 |
| MOS-10% | 0.630 | 0.0137 | 99.4 | 0.134 | 2.23 |
| MOS-12.5% | 0.697 | 0.0156 | 99.4 | 0.230 | 2.44 |
| MOS-15% | 0.697 | 0.0136 | 99.4 | 0.365 | 2.45 |
| SG | 0.433 | 0.0984 | 97.7 | 0.867 | 1.72 |
| AC | 0.482 | 0.0305 | 93.5 | 11.1 | 1.77 |
Table S3 Water vapor adsorption capacities, desorption efficiencies and the densities of surface hydroxyl groups of different samples
| Sample | Adsorption | Desorption | -OH/(×1020, g-1) | ||
|---|---|---|---|---|---|
| Average/(g·g-1) | STDEa/% | Average/% | STDEa/% | ||
| MOS-0% | 0.903 | 0.0168 | 99.4 | 0.205 | 3.11 |
| MOS-5% | 0.667 | 0.0177 | 99.3 | 0.329 | 2.35 |
| MOS-7.5% | 0.656 | 0.0165 | 99.4 | 0.283 | 2.31 |
| MOS-10% | 0.630 | 0.0137 | 99.4 | 0.134 | 2.23 |
| MOS-12.5% | 0.697 | 0.0156 | 99.4 | 0.230 | 2.44 |
| MOS-15% | 0.697 | 0.0136 | 99.4 | 0.365 | 2.45 |
| SG | 0.433 | 0.0984 | 97.7 | 0.867 | 1.72 |
| AC | 0.482 | 0.0305 | 93.5 | 11.1 | 1.77 |
| Sample | tb/min | te/min | qe/(g·g-1) | Desorption efficiency/% |
|---|---|---|---|---|
| MOS-10%-1st | 50 | 104 | 1.23 | 99.8 |
| MOS-10%-2nd | 48 | 102 | 1.20 | 99.5 |
| MOS-10%-3rd | 46 | 100 | 1.19 | 99.4 |
| MOS-10%-4th | 48 | 102 | 1.22 | 99.6 |
| MOS-10%-5th | 48 | 102 | 1.21 | 99.2 |
| SG-1st | 16 | 72 | 0.361 | 98.9 |
| SG-2nd | 14 | 70 | 0.321 | 97.5 |
| SG-3rd | 14 | 70 | 0.334 | 98.7 |
| SG-4th | 12 | 72 | 0.321 | 98.6 |
| SG-5th | 10 | 72 | 0.343 | 97.6 |
| AC-1st | 38 | 50 | 0.574 | 73.7 |
| AC-2nd | 28 | 44 | 0.465 | 83.6 |
| AC-3rd | 28 | 42 | 0.461 | 90.9 |
| AC-4th | 26 | 44 | 0.452 | 98.6 |
| AC-5th | 24 | 42 | 0.458 | 97.2 |
Table S4 Comparison of dynamic n-hexane adsorption parameters on different samples for 5 times under dry condition
| Sample | tb/min | te/min | qe/(g·g-1) | Desorption efficiency/% |
|---|---|---|---|---|
| MOS-10%-1st | 50 | 104 | 1.23 | 99.8 |
| MOS-10%-2nd | 48 | 102 | 1.20 | 99.5 |
| MOS-10%-3rd | 46 | 100 | 1.19 | 99.4 |
| MOS-10%-4th | 48 | 102 | 1.22 | 99.6 |
| MOS-10%-5th | 48 | 102 | 1.21 | 99.2 |
| SG-1st | 16 | 72 | 0.361 | 98.9 |
| SG-2nd | 14 | 70 | 0.321 | 97.5 |
| SG-3rd | 14 | 70 | 0.334 | 98.7 |
| SG-4th | 12 | 72 | 0.321 | 98.6 |
| SG-5th | 10 | 72 | 0.343 | 97.6 |
| AC-1st | 38 | 50 | 0.574 | 73.7 |
| AC-2nd | 28 | 44 | 0.465 | 83.6 |
| AC-3rd | 28 | 42 | 0.461 | 90.9 |
| AC-4th | 26 | 44 | 0.452 | 98.6 |
| AC-5th | 24 | 42 | 0.458 | 97.2 |
| tb/min | te/min | qe/(g·g-1) | Desorption efficiency/% | |
|---|---|---|---|---|
| 1st | 48 | 100 | 1.21 | 99.7 |
| 2nd | 46 | 98 | 1.18 | 99.5 |
| 3rd | 44 | 96 | 1.17 | 99.3 |
| 4th | 46 | 98 | 1.18 | 99.4 |
| 5th | 46 | 98 | 1.18 | 99.1 |
Table S5 Comparison of dynamic toluene adsorption parameters on MOS-10% for 5 times under dry condition
| tb/min | te/min | qe/(g·g-1) | Desorption efficiency/% | |
|---|---|---|---|---|
| 1st | 48 | 100 | 1.21 | 99.7 |
| 2nd | 46 | 98 | 1.18 | 99.5 |
| 3rd | 44 | 96 | 1.17 | 99.3 |
| 4th | 46 | 98 | 1.18 | 99.4 |
| 5th | 46 | 98 | 1.18 | 99.1 |
| Sample | SBET/(m2·g-1) | Sm/(m2·g-1) | Vt/(cm3·g-1) | Vm/(cm3·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| MOS-10% | 696 | 0 | 0.887 | 0 | 2.64 |
| MOS-10%-5th | 638 | 0 | 0.830 | 0 | 2.64 |
| AC | 1654 | 652 | 1.06 | 0.48 | 5.58 |
| AC-5th | 1310 | 395 | 0.880 | 0.38 | 5.51 |
Table S6 Structural parameters of MOS-10% and AC before and after 5 times dynamic n-hexane adsorption under dry condition
| Sample | SBET/(m2·g-1) | Sm/(m2·g-1) | Vt/(cm3·g-1) | Vm/(cm3·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| MOS-10% | 696 | 0 | 0.887 | 0 | 2.64 |
| MOS-10%-5th | 638 | 0 | 0.830 | 0 | 2.64 |
| AC | 1654 | 652 | 1.06 | 0.48 | 5.58 |
| AC-5th | 1310 | 395 | 0.880 | 0.38 | 5.51 |
| n-hexane | Toluene | |||||
|---|---|---|---|---|---|---|
| τ0/min | K׳/min-1 | R2 | τ0/min | K׳/min-1 | R2 | |
| 1st | 63.1 | 0.150 | 0.984 | 58.4 | 0.0948 | 0.979 |
| 2nd | 61.3 | 0.172 | 0.990 | 52.8 | 0.105 | 0.977 |
| 3rd | 56.7 | 0.174 | 0.979 | 51.5 | 0.114 | 0.987 |
| 4th | 57.1 | 0.169 | 0.986 | 54.1 | 0.111 | 0.978 |
| 5th | 58.9 | 0.165 | 0.984 | 54.2 | 0.110 | 0.982 |
Table S7 Simulation parameters of 5 times dynamic n-hexane and toluene adsorption on MOS-10% under dry condition
| n-hexane | Toluene | |||||
|---|---|---|---|---|---|---|
| τ0/min | K׳/min-1 | R2 | τ0/min | K׳/min-1 | R2 | |
| 1st | 63.1 | 0.150 | 0.984 | 58.4 | 0.0948 | 0.979 |
| 2nd | 61.3 | 0.172 | 0.990 | 52.8 | 0.105 | 0.977 |
| 3rd | 56.7 | 0.174 | 0.979 | 51.5 | 0.114 | 0.987 |
| 4th | 57.1 | 0.169 | 0.986 | 54.1 | 0.111 | 0.978 |
| 5th | 58.9 | 0.165 | 0.984 | 54.2 | 0.110 | 0.982 |
| tb/min | te/min | qe,hexane /g·g-1 | qe,t /g·g-1 adsorbent | qe,water /g·g-1 adsorbent | qe,hexane/ qe,water | Desorption efficiency /% | |
|---|---|---|---|---|---|---|---|
| 1st | 46 | 100 | 1.23 | 1.23 | 0.006 | 205 | 99.6 |
| 2nd | 44 | 98 | 1.20 | 1.20 | 0.003 | 400 | 99.7 |
| 3rd | 42 | 96 | 1.19 | 1.19 | 0.006 | 199 | 99.6 |
| 4th | 42 | 96 | 1.21 | 1.21 | 0.003 | 404 | 99.4 |
| 5th | 44 | 98 | 1.17 | 1.20 | 0.008 | 147 | 99.2 |
Table S8 Dynamic n-hexane adsorption parameters on MOS-10% for 5 times under 95% RH
| tb/min | te/min | qe,hexane /g·g-1 | qe,t /g·g-1 adsorbent | qe,water /g·g-1 adsorbent | qe,hexane/ qe,water | Desorption efficiency /% | |
|---|---|---|---|---|---|---|---|
| 1st | 46 | 100 | 1.23 | 1.23 | 0.006 | 205 | 99.6 |
| 2nd | 44 | 98 | 1.20 | 1.20 | 0.003 | 400 | 99.7 |
| 3rd | 42 | 96 | 1.19 | 1.19 | 0.006 | 199 | 99.6 |
| 4th | 42 | 96 | 1.21 | 1.21 | 0.003 | 404 | 99.4 |
| 5th | 44 | 98 | 1.17 | 1.20 | 0.008 | 147 | 99.2 |
| Dynamic adsorption capacity, qe /(g·g-1) | |||||
|---|---|---|---|---|---|
| Single component | Bi-component | ||||
| n-hexane | Toluene | n-hexane | Toluene | Total VOCs | |
| 1st | 1.23 | 1.21 | 0.642 | 0.569 | 1.21 |
| 2nd | 1.20 | 1.18 | 0.631 | 0.547 | 1.18 |
| 3rd | 1.19 | 1.17 | 0.613 | 0.532 | 1.15 |
| 4th | 1.22 | 1.18 | 0.607 | 0.550 | 1.16 |
| 5th | 1.21 | 1.18 | 0.628 | 0.547 | 1.18 |
Table S9 Comparison of simultaneous adsorption n-hexane and toluene parameters on MOS-10% for 5 times under dry condition
| Dynamic adsorption capacity, qe /(g·g-1) | |||||
|---|---|---|---|---|---|
| Single component | Bi-component | ||||
| n-hexane | Toluene | n-hexane | Toluene | Total VOCs | |
| 1st | 1.23 | 1.21 | 0.642 | 0.569 | 1.21 |
| 2nd | 1.20 | 1.18 | 0.631 | 0.547 | 1.18 |
| 3rd | 1.19 | 1.17 | 0.613 | 0.532 | 1.15 |
| 4th | 1.22 | 1.18 | 0.607 | 0.550 | 1.16 |
| 5th | 1.21 | 1.18 | 0.628 | 0.547 | 1.18 |
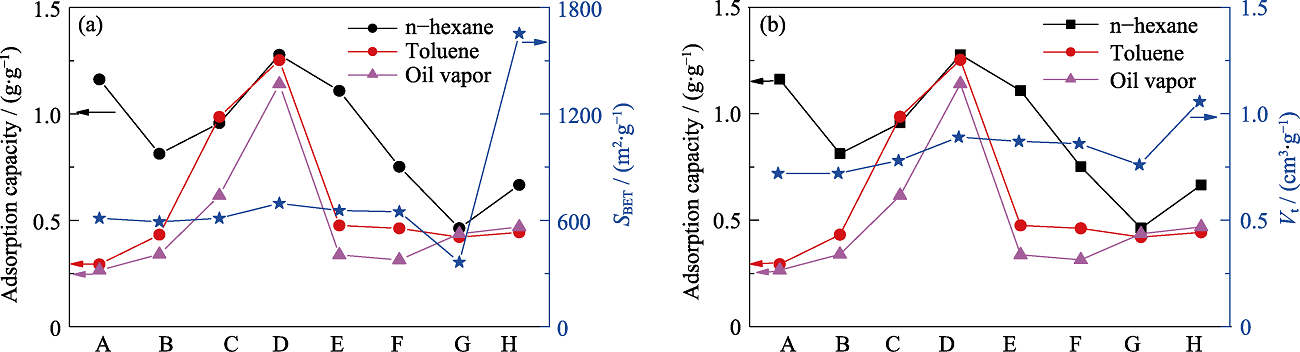
Fig. S2 Relationship between VOCs adsorption capacities and structure parameters of different samples (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS- 12.5%; (F) MOS-15%; (G) SG; (H) AC
| [1] |
ZHAO X S, MA Q, LU G Q M. VOC removal: comparison of MCM-41 with hydrophobic zeolites and activated carbon. Energ. Fuel, 1998, 12(6): 1051-1054.
DOI URL |
| [2] | ZHANG G, FEIZBAKHSHAN M, ZHENG S, et al. Effects of properties of minerals adsorbents for the adsorption and desorption of volatile organic compounds (VOC). Appl. Clay Sci., 2019, 173: 88-96. |
| [3] | TRAN THANH T, MANH TRUNG T, FELLER J F, et al. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon, 2020, 162: 662-662. |
| [4] |
LIU S, PENG Y, YAN T, et al. Modified silica adsorbents for toluene adsorption under dry and humid conditions: impacts of pore size and surface chemistry. Langmuir, 2019, 35(27): 8927-8934.
DOI URL |
| [5] |
WANG X, HE Y, LIU C, et al. A controllable asymmetrical/ symmetrical coating strategy for architectural mesoporous organosilica nanostructures. Nanoscale, 2016, 8(28): 13581-13588.
DOI URL |
| [6] |
SUN Y, CHEN M, WU L. Controllable synthesis of hollow periodic mesoporous organosilica spheres with radial mesochannels and their degradable behavior. J. Mater. Chem. A, 2018, 6(26): 12323-12333.
DOI URL |
| [7] |
BATONNEAU-GENER I, YONLI A, TROUVE A, et al. Tailoring the hydrophobic character of mesoporous silica by silylation for VOC removal. Sep. Sci. Technol., 2010, 45(6): 768-775.
DOI URL |
| [8] |
DOU B, HU Q, LI J, et al. Adsorption performance of VOCs in ordered mesoporous silicas with different pore structures and surface chemistry. J. Hazard. Mater., 2011, 186(2/3): 1615-1624.
DOI URL |
| [9] |
ZHANG W, QU Z, LI X, et al. Comparison of dynamic adsorption/ desorption characteristics of toluene on different porous materials. J. Environ. Sci., 2012, 24(3): 520-528.
DOI URL |
| [10] |
HARTMANN M, BISCHOF C. Mechanical stability of mesoporous molecular sieve MCM-48 studied by adsorption of benzene, n-heptane, and cyclohexane. J. Phys. Chem. B, 1999, 103(30): 6230-6235.
DOI URL |
| [11] | YANG K, SUN Q, XUE F, et al. Adsorption of volatile organic compounds by metal-organic frameworks MIL-101: Influence of molecular size and shape. J. Hazard. Mater., 2011, 195: 124-131. |
| [12] |
HU Q, LI J J, HAO Z P, et al. Dynamic adsorption of volatile organic compounds on organofunctionalized SBA-15 materials. Chem. Eng. J., 2009, 149(1/2/3): 281-288.
DOI URL |
| [13] |
KUBO S, KOSUGE K. Salt-induced formation of uniform fiberlike SBA-15 mesoporous silica particles and application to toluene adsorption. Langmuir, 2007, 23(23): 11761-11768.
DOI URL |
| [14] | QIN Y, WANG Y, WANG H, et al. Effect of morphology and pore structure of SBA-15 on toluene dynamic adsorption/desorption performance. 4th International Symposium on Environmental Science and Technology (ISEST), Dalian, 2013:366-371. |
| [15] | LIU S, CHEN J, PENG Y, et al. Studies on toluene adsorption performance and hydrophobic property in phenyl functionalized KIT-6. Chem. Eng. J., 2018, 334: 191-197. |
| [16] |
DOU B, LI J, HU Q, et al. Hydrophobic micro/mesoporous silica spheres assembled from zeolite precursors in acidic media for aromatics adsorption. Micropor. Mesopor. Mat., 2010, 133(1/2/3): 115-123.
DOI URL |
| [17] |
WANG H, TANG M, HAN L, et al. Synthesis of hollow organosiliceous spheres for volatile organic compound removal. J. Mater. Chem. A, 2014, 2(45): 19298-19307.
DOI URL |
| [18] | WANG H, TANG M, ZHANG K, et al. Functionalized hollow siliceous spheres for VOCs removal with high efficiency and stability. J. Hazard. Mater., 2014, 268: 115-123. |
| [19] |
WANG H, RONG X, HAN L, et al. Controlled synthesis of hexagonal mesostructure silica and macroporous ordered siliceous foams for VOCs adsorption. RSC Adv., 2015, 5(8): 5695-5703.
DOI URL |
| [20] | WANG J, FENG S, SONG Y, et al. Synthesis of hierarchically porous carbon spheres with yolk-shell structure for high performance supercapacitors. Catal. Today, 2015, 243: 199-208. |
| [21] | ZHANG C, WU C, HAN W, et al. Controllable synthesis of multi-morphological hollow mesoporous SiO2 and adsorption reduction of Cu2+ by its composites. Chem. J. Chinese U., 2019, 40(11): 2412-2418. |
| [22] | 王小文, 胡芸, 黄晶. 等. 疏水性分子筛对焦化废水生物处理尾水的吸附过程解析. 环境科学学报, 2012, 3(2): 2058-2065. |
| [23] |
LIU W, MA N, LI S, et al. A one-step method for pore expansion and enlargement of hollow cavity of hollow periodic mesoporous organosilica spheres. J. Mater. Sci., 2017, 52(5): 2868-2878.
DOI URL |
| [24] |
GAO M, HAN S, HU Y, et al. A pH-driven molecular shuttle based on rotaxane-bridged periodic mesoporous organosilicas with responsive release of guests. RSC Adv., 2016, 6(33): 27922-27932.
DOI URL |
| [25] | MATYSIAK W, TANSKI T. Analysis of the morphology, structure and optical properties of 1D SiO2 nanostructures obtained with Sol-Gel and electrospinning methods. Appl. Surf. Sci., 2019, 489: 34-43. |
| [26] |
CHEN J, SUN C, HUANG Z, et al. Fabrication of functionalized porous silica nanocapsules with a hollow structure for high performance of toluene adsorption-desorption. ACS Omega, 2020, 5(11): 5805-5814.
DOI URL |
| [27] | YUAN W W, YUAN P, LIU D, et al. A hierarchically porous diatomite/silicalite-1 composite for benzene adsorption/desorption fabricated via a facile pre-modification in situ synthesis route. Chem. Eng. J., 2016, 294: 333-342. |
| [28] | RAJABI H, MOSLEH M H, PRAKOSO T, et al. Competitive adsorption of multicomponent volatile organic compounds on biochar. Chemosphere, 2021, 283: 131288. |
| [1] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [2] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [3] | PAN Zesheng, YOU Yaping, ZHENG Ya, CHEN Haijie, WANG Lianjun, JIANG Wan. Stability of Phosphors for White LED Excitable by Violet Light [J]. Journal of Inorganic Materials, 2025, 40(3): 314-322. |
| [4] | QU Mujing, ZHANG Shulan, ZHU Mengmeng, DING Haojie, DUAN Jiaxin, DAI Henglong, ZHOU Guohong, LI Huili. CsPbBr3@MIL-53 Nanocomposite Phosphors: Synthesis, Properties and Applications in White LEDs [J]. Journal of Inorganic Materials, 2024, 39(9): 1035-1043. |
| [5] | MIAO Xin, YAN Shiqiang, WEI Jindou, WU Chao, FAN Wenhao, CHEN Shaoping. Interface Layer of Te-based Thermoelectric Device: Abnormal Growth and Interface Stability [J]. Journal of Inorganic Materials, 2024, 39(8): 903-910. |
| [6] | CHEN Tian, LUO Yuan, ZHU Liu, GUO Xueyi, YANG Ying. Organic-inorganic Co-addition to Improve Mechanical Bending and Environmental Stability of Flexible Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2024, 39(5): 477-484. |
| [7] | YANG Bo, LÜ Gongxuan, MA Jiantai. Electrocatalytic Water Splitting over Nickel Iron Hydroxide-cobalt Phosphide Composite Electrode [J]. Journal of Inorganic Materials, 2024, 39(4): 374-382. |
| [8] | LIU Song, ZHANG Faqiang, LUO Jin, LIU Zhifu. 0.9BaTiO3-0.1Bi(Mg1/2Ti1/2)O3 Ferroelectric Thin Films: Preparation and Energy Storage [J]. Journal of Inorganic Materials, 2024, 39(3): 291-298. |
| [9] | ZHANG Yuchen, LU Zhiyao, HE Xiaodong, SONG Guangping, ZHU Chuncheng, ZHENG Yongting, BAI Yuelei. Predictions of Phase Stability and Properties of S-group Elements Containing MAX Borides [J]. Journal of Inorganic Materials, 2024, 39(2): 225-232. |
| [10] | WANG Yu, XIONG Hao, HUANG Xiaokun, JIANG Linqin, WU Bo, LI Jiansheng, YANG Aijun. Regulation of Low-dose Stannous Iso-octanoate for Two-step Prepared Sn-Pb Alloyed Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2024, 39(12): 1339-1347. |
| [11] | ZHOU Yunkai, DIAO Yaqi, WANG Minglei, ZHANG Yanhui, WANG Limin. First-principles Calculation Study of the Oxidation Resistance of PANI Modified Ti3C2(OH)2 [J]. Journal of Inorganic Materials, 2024, 39(10): 1151-1158. |
| [12] | FANG Wanli, SHEN Lili, LI Haiyan, CHEN Xinyu, CHEN Zongqi, SHOU Chunhui, ZHAO Bin, YANG Songwang. Effect of Film Formation Processes of NiOx Mesoporous Layer on Performance of Perovskite Solar Cells with Carbon Electrodes [J]. Journal of Inorganic Materials, 2023, 38(9): 1103-1109. |
| [13] | CHEN Yu, LIN Puan, CAI Bing, ZHANG Wenhua. Research Progress of Inorganic Hole Transport Materials in Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2023, 38(9): 991-1004. |
| [14] | HU Zhongliang, FU Yuntian, JIANG Meng, WANG Lianjun, JIANG Wan. Thermal Stability of Nb/Mg3SbBi Interface [J]. Journal of Inorganic Materials, 2023, 38(8): 931-937. |
| [15] | LIU Jian, WANG Lingkun, XU Baoliang, ZHAO Qian, WANG Yaoxuan, DING Yi, ZHANG Shengtai, DUAN Tao. Nd-doped ZrSiO4 Ceramics: Synthesis in Molten Salt at Low Temperature, Phase Evolution and Chemical Stability [J]. Journal of Inorganic Materials, 2023, 38(8): 910-916. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||