
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (9): 1005-1010.DOI: 10.15541/jim20190444
Special Issue: 环境材料论文精选(2020); 【虚拟专辑】污染物吸附水处理(2020~2021)
• RESEARCH PAPER • Previous Articles Next Articles
LI Jing1,2( ),LIU Xiaoyue2,QIU Qianfeng2,LI Ling2,CAO Xiaoyan1,2,3(
),LIU Xiaoyue2,QIU Qianfeng2,LI Ling2,CAO Xiaoyan1,2,3( )
)
Received:2019-08-28
Revised:2020-01-13
Published:2020-09-20
Online:2020-03-03
Supported by:CLC Number:
LI Jing,LIU Xiaoyue,QIU Qianfeng,LI Ling,CAO Xiaoyan. Phosphorus Sorption Characteristics on Aluminum Oxides with Different Structures[J]. Journal of Inorganic Materials, 2020, 35(9): 1005-1010.
| NaNO3 concentration/ (mol·L-1) | HS concentration/(mol·L-1) | TOTHpH=8/(mmol·L-1) | pHPZNPC* | |||
|---|---|---|---|---|---|---|
| γ-Al2O3 | Amorphous alumina | γ-Al2O3 | Amorphous alumina | γ-Al2O3 | Amorphous alumina | |
| 0.01 | 3.35×10-3 | 2.69×10-3 | - | - | 4.52 | 4.25 |
| 0.10 | 2.94×10-3 | 1.79×10-3 | - | - | ||
| 0.70 | 2.75×10-3 | 1.09×10-3 | -0.809 | -1.380 | ||
Table 1 Surface acid-base properties of two aluminum oxides in different ionic strength media
| NaNO3 concentration/ (mol·L-1) | HS concentration/(mol·L-1) | TOTHpH=8/(mmol·L-1) | pHPZNPC* | |||
|---|---|---|---|---|---|---|
| γ-Al2O3 | Amorphous alumina | γ-Al2O3 | Amorphous alumina | γ-Al2O3 | Amorphous alumina | |
| 0.01 | 3.35×10-3 | 2.69×10-3 | - | - | 4.52 | 4.25 |
| 0.10 | 2.94×10-3 | 1.79×10-3 | - | - | ||
| 0.70 | 2.75×10-3 | 1.09×10-3 | -0.809 | -1.380 | ||
| Medium | Frap | Fslow | kslow/h-1 | krap/h-1 | Qe/(mg·g-1) | r2 |
|---|---|---|---|---|---|---|
| 0.7 mol·L-1 NaNO3 | 0.813 | 0.187 | 0.977 | 26.32 | 21.42 | 0.977 |
| NSW | 0.740 | 0.260 | 0.193 | 20.23 | 16.56 | 0.988 |
Table 2 Sorption kinetic parameters of phosphorus on amorphous alumina
| Medium | Frap | Fslow | kslow/h-1 | krap/h-1 | Qe/(mg·g-1) | r2 |
|---|---|---|---|---|---|---|
| 0.7 mol·L-1 NaNO3 | 0.813 | 0.187 | 0.977 | 26.32 | 21.42 | 0.977 |
| NSW | 0.740 | 0.260 | 0.193 | 20.23 | 16.56 | 0.988 |
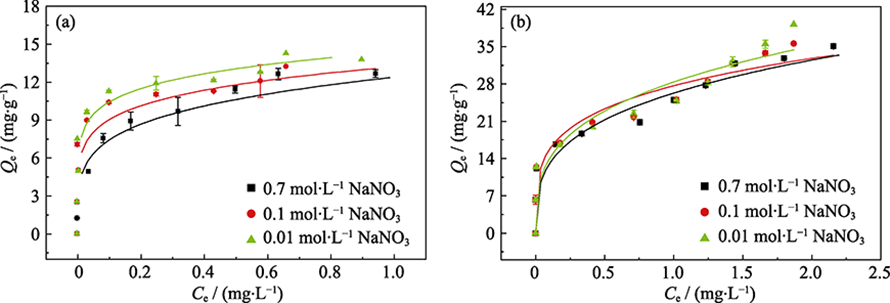
Fig. 5 Sorption isotherms of phosphate on γ-Al2O3 (a) and amorphous alumina (b) in NaNO3 with different concentrations Dot: experimental data; Line: Freundlich model fitting
| Sorbent | I/(mol·L-1) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|---|
| KF/(mg·g-1) (L·mg-1)1/n | n | r2 | Qm/(mg·g-1) | KL/(L·mg-1) | r2 | ||
| γ-Al2O3 | 0.7 | 12.39 | 4.52 | 0.942 | 14.93 | 5.288 | 0.848 |
| 0.1 | 13.20 | 6.08 | 0.912 | 13.47 | 18.29 | 0.702 | |
| 0.01 | 14.37 | 7.20 | 0.896 | 14.28 | 27.90 | 0.485 | |
| Amorphous alumina | 0.7 | 26.28 | 3.27 | 0.933 | 34.70 | 3.828 | 0.838 |
| 0.1 | 27.66 | 4.09 | 0.933 | 34.60 | 4.888 | 0.820 | |
| 0.01 | 28.36 | 3.24 | 0.907 | 43.22 | 2.046 | 0.817 | |
Table 3 Isothermal sorption parameters of phosphates on γ-Al2O3 and amorphous alumina
| Sorbent | I/(mol·L-1) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|---|
| KF/(mg·g-1) (L·mg-1)1/n | n | r2 | Qm/(mg·g-1) | KL/(L·mg-1) | r2 | ||
| γ-Al2O3 | 0.7 | 12.39 | 4.52 | 0.942 | 14.93 | 5.288 | 0.848 |
| 0.1 | 13.20 | 6.08 | 0.912 | 13.47 | 18.29 | 0.702 | |
| 0.01 | 14.37 | 7.20 | 0.896 | 14.28 | 27.90 | 0.485 | |
| Amorphous alumina | 0.7 | 26.28 | 3.27 | 0.933 | 34.70 | 3.828 | 0.838 |
| 0.1 | 27.66 | 4.09 | 0.933 | 34.60 | 4.888 | 0.820 | |
| 0.01 | 28.36 | 3.24 | 0.907 | 43.22 | 2.046 | 0.817 | |
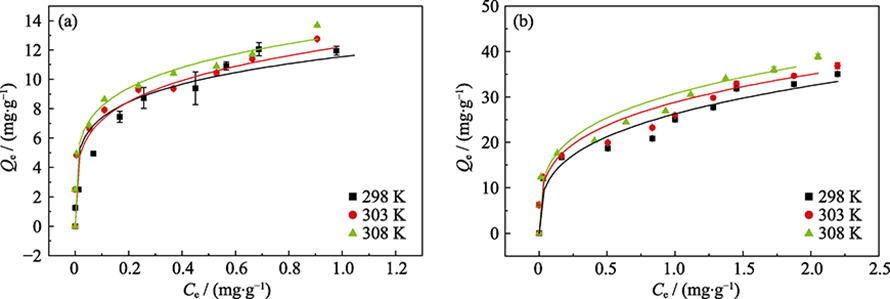
Fig. 6 Sorption isotherms of phosphate on γ-Al2O3 (a) and amorphous alumina (b) at different femperatures Dot: experimental data; Line: Freundlich model fitting
| Adsorbent | T/K | KF/(mg·g-1) (L·mg-1)1/n | n | r2 | ΔGθ/(kJ·mol-1) | ΔHθ/(kJ·mol-1) | ΔSθ/(J·mol-1·K-1) |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | 298 | 11.55 | 5.18 | 0.938 | -23.18 | 9.007 | 108.0 |
| 303 | 12.22 | 4.40 | 0.948 | -23.71 | |||
| 308 | 12.99 | 4.98 | 0.952 | -24.26 | |||
| Amorphous alumina | 298 | 26.28 | 3.27 | 0.933 | -25.21 | 11.96 | 124.8 |
| 303 | 28.84 | 3.60 | 0.947 | -25.87 | |||
| 308 | 30.73 | 3.64 | 0.951 | -26.46 |
Table 4 Sorption thermodynamic parameters of phosphate on γ-Al2O3 and amorphous alumina
| Adsorbent | T/K | KF/(mg·g-1) (L·mg-1)1/n | n | r2 | ΔGθ/(kJ·mol-1) | ΔHθ/(kJ·mol-1) | ΔSθ/(J·mol-1·K-1) |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | 298 | 11.55 | 5.18 | 0.938 | -23.18 | 9.007 | 108.0 |
| 303 | 12.22 | 4.40 | 0.948 | -23.71 | |||
| 308 | 12.99 | 4.98 | 0.952 | -24.26 | |||
| Amorphous alumina | 298 | 26.28 | 3.27 | 0.933 | -25.21 | 11.96 | 124.8 |
| 303 | 28.84 | 3.60 | 0.947 | -25.87 | |||
| 308 | 30.73 | 3.64 | 0.951 | -26.46 |
| [1] |
JALALI M, PEIKAM E N. Phosphorus sorption-desorption behaviour of river bed sediments in the Abshineh river, Hamedan, Iran, related to their composition. Environmental Monitoring and Assessment, 2013,185(1):537-552.
DOI URL PMID |
| [2] |
COTOVICZ JUNIOR L C, MACHADO E D, BRANDINI N,et al. Distributions of total, inorganic and organic phosphorus in surface and recent sediments of the sub-tropical and semi-pristine Guaratuba Bay estuary, SE Brazil. Environmental Earth Sciences, 2014,72(2):373-386.
DOI URL |
| [3] |
HUANG S, HUANG H, ZHU H. Effects of the addition of iron and aluminum salt on phosphorus adsorption in wetland sediment. Environmental Science and Pollution Research, 2016,23(10):10022-10027.
URL PMID |
| [4] |
QIAN L, MA M, CHENG D. The effect of water chemistry on adsorption and desorption of U(VI) on nano-alumina. Journal of Molecular Liquids, 2014,197:295-300.
DOI URL |
| [5] | CUI Y, XIAO R, XIE Y,et al. Phosphorus fraction and phosphate sorption-release characteristics of the wetland sediments in the Yellow River Delta. Physics and Chemistry of the Earth, 2018,103:19-27. |
| [6] | 王赫, 贾永锋, 刘利. 沉积物中典型氧化物矿物吸附的镉对芦苇的生物有效性研究. 环境科学, 2009,30(6):215-220. |
| [7] |
LAING G D, RINKLEBE J, VANDECASTEELE B,et al. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Science of the Total Environment, 2009,407(13):3972-3985.
URL PMID |
| [8] | 杨晓芳, 王东升, 孙中溪. 三水铝石(γ-Al(OH)3)和α-Al2O3表面酸碱性质与磷酸根吸附研究. 环境科学学报, 2007,27(4):637-642. |
| [9] | 孟文娜, 谢杰, 吴德意. 活性氧化铝对水中磷的去除与回收研究. 环境科学, 2013,34(1):231-236. |
| [10] |
KIM Y, KIRKPATRICK R J. An investigation of phosphate adsorbed on aluminium oxyhydroxide and oxide phases by nuclear magnetic resonance. European Journal of Soil Science, 2004,55(2):243-251.
DOI URL |
| [11] |
JOHNSON B B, IVANOV A V, ANTZUTKIN O N,et al. 31P nuclear magnetic resonance study of the adsorption of phosphate and phenyl phosphates on γ-Al2O3. Langmuir, 2002,18(4):1104-1111.
DOI URL |
| [12] | 杨晓芳. 原位红外光谱研究含氧离子氧化物微界面吸附过程与机理. 北京: 中国科学院大学博士学位论文, 2010. |
| [13] |
邵兴华, 章永松, 林咸永. 三种铁氧化物的磷吸附解吸特性以及与磷吸附饱和度的关系. 植物营养与肥料学报, 2006,12(2):208-212.
DOI URL |
| [14] | CHATMAN S, ZARZYCKI P, PREOANIN T,et al. Effect of surface site interactions on potentiometric titration of hematite (α-Fe2O3) crystal faces. Journal of Colloid and Interface Science, 2013,39(1):125-134. |
| [15] | 严玉鹏. 几种土壤有机磷在铁铝氧化物表面的吸附、解吸与沉淀. 武汉: 华中农业大学硕士学位论文, 2015. |
| [16] | 杜淼, 李斌, 任海萍, 等. 纳米介孔氧化铝制备及表征. 无机盐工业, 2008,40(5):34-35. |
| [17] | DIZ P, MENA A, NOMBELA MIGUEL ÁNGEL, et al. Description of an experimental set up for the culture of benthic foraminifera in controlled pH conditions. Thalassas: An International Journal of Marine Sciences, 2015,31(1):23-32. |
| [18] | 张学清, 项金钟, 胡永茂. 纳米Al2O3的制备及红外吸收研究. 中国陶瓷, 2004,40(1):24-27. |
| [19] |
AZIZIAN S. Kinetic models of sorption: a theoretical analysis. Journal of Colloid and Interface Science, 2004,276(1):47-52.
DOI URL PMID |
| [20] | 李显波, 马力, 刘志红, 等. 活性氧化铝对废水中磷酸根离子的吸附特性研究. 非金属矿, 2017,40(4):4-7. |
| [21] | 张炳慧. 天然水中磷酸盐存在状态与pH值的关系. 地质实验室, 1992,8(2):98-100. |
| [22] | CHUBAR N I, KANIBOLOTSKYY V A, STRELKO V V,et al. Adsorption of phosphate ions on novel inorganic ion exchangers. Colloids and Surfaces A (Physicochemical and Engineering Aspects), 2005,255(1/2/3):55-63. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [3] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [4] | WEI Zhifan, CHEN Guoqing, ZU Yufei, LIU Yuan, LI Minghao, FU Xuesong, ZHOU Wenlong. ZrB2-HfSi2 Ceramics: Microstructure and Formation Mechanism of Core-rim Structure [J]. Journal of Inorganic Materials, 2025, 40(7): 817-825. |
| [5] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [6] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [7] | ZHOU Yangyang, ZHANG Yanyan, YU Ziyi, FU Zhengqian, XU Fangfang, LIANG Ruihong, ZHOU Zhiyong. Enhancement of Piezoelectric Properties in CaBi4Ti4O15-based Ceramics through Bi3+ Self-doping Strategy [J]. Journal of Inorganic Materials, 2025, 40(6): 719-728. |
| [8] | HUANG Zipeng, JIA Wenxiao, LI Lingxia. Crystal Structure and Terahertz Dielectric Properties of (Ti0.5W0.5)5+ Doped MgNb2O6 Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 647-655. |
| [9] | AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao. Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance [J]. Journal of Inorganic Materials, 2025, 40(5): 457-465. |
| [10] | ZHAO Kaixuan, LIU Wenpeng, DING Shoujun, DOU Renqin, LUO Jianqiao, GAO Jinyun, SUN Guihua, REN Hao, ZHANG Qingli. Nd:YLF Crystal Growth: Raw Materials Preparation by Melting Method and Property [J]. Journal of Inorganic Materials, 2025, 40(5): 529-535. |
| [11] | GUO Ziyu, ZHU Yunzhou, WANG Li, CHEN Jian, LI Hong, HUANG Zhengren. Effect of Zn2+ Catalyst on Microporous Structure of Porous Carbon Prepared from Phenolic Resin/Ethylene Glycol [J]. Journal of Inorganic Materials, 2025, 40(5): 466-472. |
| [12] | HONG Peiping, LIANG Long, WU Lian, MA Yingkang, PANG Hao. Structure Regulation of ZIF-67 and Adsorption Properties for Chlortetracycline Hydrochloride [J]. Journal of Inorganic Materials, 2025, 40(4): 388-396. |
| [13] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [14] | MU Haojie, ZHANG Yuanjiang, YU Bin, FU Xiumei, ZHOU Shibin, LI Xiaodong. Preparation and Properties of ZrO2 Doped Y2O3-MgO Nanocomposite Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 281-289. |
| [15] | BAO Weichao, GUO Xiaojie, XIN Xiaoting, PENG Pai, WANG Xingang, LIU Jixuan, ZHANG Guojun, XU Fangfang. Establishment of Symbiotic Structure with Metal Atomic-layer Phase-separation in Carbide Ceramics [J]. Journal of Inorganic Materials, 2025, 40(1): 17-22. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||